ABSTRACT
Circular RNA hsa_circ_0073748 (circ_0073748) is upregulated in patients with acute pancreatitis (AP), a clinically common sudden inflammatory response. MicroRNA (miR)-132-3p is a stress-induced factor with high conservation between species. Herein, expression and role of circ_0073748 and miR-132-3p in caerulein-induced pancreatitis were studied. Expression levels of circ_0073748, miR-132-3p, TNF receptor associated factor 3 (TRAF3), Bcl-2 and Bcl-2-associated X protein (Bax) were examined by reverse transcription-quantitative PCR and Western blotting. Cell proliferation was measured by MTS and EdU assays. Flow cytometry and assay kits detected apoptosis, inflammatory, and oxidative responses. Western blotting detected nuclear factor (NF)-κB signaling pathway. Circ_0073748 was upregulated and miR-132-3p was downregulated in AP patients’ plasma and human pancreatic ductal HPDE6-C7 cells with caerulein induction. Interfering circ_0073748 and reinforcing miR-132-3p improved cell viability, EdU incorporation, and superoxide dismutase (SOD) activity of caerulein-treated HPDE6-C7 cells but suppressed malonaldehyde (MDA), IL-6 and TNF-α levels and apoptosis rate. Moreover, TRAF3 downregulation was allied with circ_0073748 silencing and miR-132-3p overexpression in caerulein-induced HPDE6-C7 cells. Mechanically, circ_0073748 was identified as a sponge for miR-132-3p to modulate TRAF3 expression, thus establishing a competitive endogenous RNA (ceRNA) regulation model. Notably, circ_0073748 blockage could suppress expressions of phosphorylated P65 (p-P65) and p-IκB in caerulein-induced HPDE6-C7 cells by promoting miR-132-3p and inhibiting TRAF3. Silencing circ_0073748 and upregulating miR-132-3p could alleviate caerulein-induced HPDE6-C7 injury and inactivate canonical NF-κB signal by inhibiting TRAF3. Circ_0073748/miR-132-3p/TRAF3 ceRNA pathway might be one underlying mechanism and therapeutic target of caerulein-induced AP.
Introduction
Acute pancreatitis (AP) is a clinically common sudden inflammatory attack of the pancreas and only last for a short time but might lead to a life-threatening emergency [Citation1]. AP is characterized by local pancreatic inflammation with mild form in a majority of patients, whereas systemic inflammation with moderate and severe forms in around 25% of patients [Citation2]. Caerulein stimulation is the well-standardized model of experimental AP both in vitro and in vivo [Citation3], and oxidative stress and inflammatory signaling are essential to the pathogenesis and development of caerulein-induced pancreatitis [Citation4]. Canonical nuclear factor (NF)-κB signaling pathway plays an important role in inflammatory diseases [Citation5,Citation6], such as caerulein-induced pancreatitis [Citation4]. TNF receptor associated factor 3 (TRAF3), belonging to TRAFs family that are major signal transducers for both TNF and IL-1/TLR receptor [Citation7], is required for the activation of canonical NF-κB pathway [Citation8]. Moreover, caerulein induces the stabilization of TRAF3 protein in the process of immune and inflammatory responses in AP [Citation9].
A preliminary evidence suggests that extracellular vesicle long RNA species including circular RNAs (circRNAs) and messenger RNAs (mRNAs) in human blood show a diagnostic signature in pancreatic ductal adenocarcinoma (PDAC), chronic pancreatitis (CP) and healthy subjects [Citation10]. A set of three circRNAs are suggested as novel diagnostic biomarkers for human AP at early stage [Citation11]. To unveil the pathogenesis of AP, several circRNA expression profiles in pancreatic tissues in AP mouse model have been identified [Citation12,Citation13]. Notably, circRNAs role in rat pancreata acinar cells has been emerging via the competitive endogenous RNA (ceRNA) network [Citation11–14]. Hsa_circ_0073748 (circ_0073748), derived from lamin B1 (LMNB1), is one of the 25 upregulated circRNAs in mild AP patients according to circRNA microarray [Citation11]; however, whether it could exert biological functions in pancreatic ductal cells was unclear.
MicroRNAs (miRNAs) are a group of single-stranded non-coding RNAs that control the expression of the majority of genes through either cleavage or translational repression [Citation15], and targeting miRNAs have the potential to act as biomarkers for the diagnosis and treatment targets for AP [Citation16]. MiRNA (miR)-132-3p is a stress-induced evolutionarily conserved miRNA, and probably operates in stress-related, inflammatory, and metabolic pathways in mouse and human [Citation17]. Expression of miR-132-3p is altered in stress models in mice for gastrointestinal diseases, including caerulein-induced mild AP and CP [Citation18,Citation19]. Even though, the link between miR-132-3p role and pancreatitis was little researched.
Herein, we explored expressions of circ_0073748, miR-132-3p and TRAF3 in patients with AP and human pancreatic ductal HPDE6-C7 cells induced by caerulein, and their roles in caerulein-induced HPDE6-C7 cell injury and canonical NF-κB signaling pathway. Strikingly, we confirmed the potential relation among circ_0073748, miR-132-3p and TRAF3 in ceRNA regulatory model.
Materials and methods
Blood plasma samples
A total of 29 patients with AP and 19 healthy volunteers were included in this study, which was authorized by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University. Fasting venous blood was collected from each subject in the early morning after these subjects signed the written informed consent forms. Blood plasma samples were isolated from the bloods immediately by centrifugation at 3000 g for 15 min under 4°C. Inclusion criteria of AP patients were diagnosed depending on the consensus of integrative diagnosis and treatment of AP [Citation20], and age above 18 years. Exclusion criteria were patients with hematological malignancies, with solid tumors, with chronic inflammatory diseases, or with recurrent AP or chronic pancreatitis.
Caerulein-induced AP cell model
Human pancreatic ductal HPDE6-C7 cells (Procell, Wuhan, China) were cultured in DMEM/F12 (R&D systems, Minneapolis, MN, USA) plus 2 μg/mL Insulin, 5 μg/mL Transferrin, 40 ng/mL Hydrocortisone, 10 ng/ml EGF, and 5% fetal bovine serum (R&D systems). The culture condition was sterile humidified air plus 5% CO2 at 37°C. To establish experimental AP model, HPDE6-C7 cells were exposed to caerulein (Sigma-Aldrich, St. Louis, MO, USA) ranging from 0 to 15 nmol/L for 12 h. Additionally, 10 nmol/L caerulein-treated HPDE6-C7 cells were collected for further functional experiments.
RNA isolation and RNase R treatment
Total RNA from plasmas and cells was extracted in TRIzolTM LS Reagent (Invitrogen, Carlsbad, CA, USA), and cytoplasmic & nuclear RNAs were separately isolated using the RNA Purification Kit (Norgen Biotek, Thorold, Canada). Both kits were used in accordance with the manufacturers’ protocols, and isolated RNAs were subjected to further reverse transcription-quantitative PCR (qPCR) analysis.
An aliquot of total RNA from HPDE6-C7 cells was treated with 4 U/μg RNA RNase R GENESEED, Guangzhou, China) for 30 min at 37°C; RNase R-treated and mock-treated RNAs were purified using RNA Clean-Up and Concentration Kit (Norgen Biotek) for further qPCR analysis.
qPCR
RNA was the template for reverse transcription with cDNA Synthesis SuperMix kit (Donghuan Biotech, Shanghai, China), and the later cDNA was amplified and analyzed using SYBR Green array (Donghuan Biotech) and special primer sets for circ_0073748, LMNB1, miR-132-3p, TRAF3, GAPDH, and U6. The cDNA synthesis procedures were 30 min at 16°C, 30 min at 42°C, and 15 min at 70°C, and the qPCR amplification protocols were 94°C for 5 min, and 35 cycles of 94°C for 15 s and 60°C for 60 s. Primers for qPCR were listed in Supplementary file. The system of qPCR was 1 μL cDNA, 5 μL SYBR Green Master Mix, 0.5 μL each specific forward, and 0.5 μL paired reverse primer, and 3 μL ddH2O. The relative expression of target genes was calculated by the 2–ΔΔCt method and normalized to internal control (GAPDH or U6).
Cell transfection
HPDE6-C7 cells were transiently transfected with exogenous nucleotides for 48 h using X-tremeGENE siRNA Transfection Reagent (Roche Applied Science, Mannheim, Germany). Transfected HPDE6-C7 cells were following exposed to 10 nmol/L caerulein for 12 h. These exogenous nucleotides included circ_0073748 siRNA (si-circ_0073748), miR-132-3p mimic (miR-132-3p), miR-132-3p inhibitor (anti-miR-132-3p), and recombinant overexpression vectors including pCD5-ciR-circ_0073748 and pcDNA-TRAF3, as well as the negative controls (NC) and empty vectors. To construct overexpression vectors, the primary sequence of circ_0073748 was from circBank (ID: hsa_circLMNB1_010), and coding domain sequence of TRAF3 was from NCBI (NM_003300). Special siRNAs were shown in Supplementary file.
MTS and EdU assays
MTS Cell proliferation Colorimetric Assay Kit (Biovision, San Francisco, CA, USA) was used to monitor cell viability. In brief, control HPDE6-C7 and transfected HPDE6-C7 cells (1 × 104) were plated in 96-well plate with 5 biological replicates for 24 h. 10 nmol/L caerulein was added in these wells for 12 h, and the medium was transferred to serum-free medium plus 20 μL of MTS Reagent for another 4 h. Absorbance at 490 nm was read on microplate reader (SpectraMax M5/M5e; Molecular Devices, Shanghai, China).
iClickTM EdU Andy FluotTM 555 Imaging Kit (GeneCopoeia, Rockville, MD, USA) was used to measure cell proliferation. In short, 10 nmol/L caerulein-treated and – untreated HPDE6-C7 cells were labeled with 10 μM EdU which was reacted with Andy Fluor488 azide. Hoechst 33,342 counterstain was performed for the positive control. Stained cells were observed under fluorescence microscopy.
Enzyme-linked immunosorbent assay (ELISA)
Quantikine ELISA Kits (R&D system, Minneapolis, MN, USA) for human IL-6 immunoassay and TNF-α immunoassay were used to detect the concentrations of IL6 and TNF-α in cell culture supernatant after HPDE6-C7 cells were treated with 10 nmol/L caerulein or not. Readings were made at 450 nm and 570 nm on microplate reader (SpectraMax M5/M5e; Molecular Devices), and readings at 570 nm were used for wavelength correction.
Flow cytometry (FCM)
After 10 nmol/L caerulein treatment, HPDE6-C7 cells were subjected to the Annexin V-FITC/PI Double Staining Apoptosis Detection Kit (Bestbio, Beijing, China) and FCM analysis was on flow cytometer (CytoFLEX, Beckman-Coulter, Shanghai, China). Scatter diagram of PI/Annexin V gating was drawn and the percentage of scatters in Q1-UR and Q1-LR was calculated on CytExpert software (Beckman-Coulter).
Western blotting
Total protein was isolated from the whole bloods and cultured HPDE6-C7 cells by Whole Blood Protein Extraction Kit (Bestbio) and Whole Animal Protein Extraction Kit (Bestbio), respectively. The concentration of isolated protein samples was determined by BCA Protein Quantitativ Kit (Bestbio), and 30 μg proteins were used for Western blotting after proteins were mixed with 1 × SDS-PAGE Sample Loading Buffer (Beyotime, Shanghai, China) and boiled at 90°C for 5 min. The special primary antibodies for TRAF3, Bcl-2, Bcl-2-associated X protein (Bax), GAPDH, P65, IκB, phosphorylated p65 (p-P65), and phosphorylated IκB (p-IκB) were summarized in Supplementary file.
Malondialdehyde (MDA) assay and superoxide dismutase (SOD) activity assay
Cell culture extracts of HPDE6-C7 were collected after 10 nmol/L caerulein treatment in MDA Lysis Buffer (ab118970, Abcam, Cambridge, UK) supplemented with 1 × BHT (ab118970, Abcam), and centrifuged; the supernatant was added with TBA Solution (ab118970, Abcam) for 1 h at 95°C; eventually, absorbance at 532 nm was read. SOD Activity Assay Kit (ab65354, Abcam) was used to measure SOD activity in cell lysate with WST-1. The water-soluble formazan dye produced by superoxide anions and WST-1 was detected at 450 nm.
Dual-luciferase reporter assay
Starbase database was used to predict the binding site of miR-132-3p in circ_0073748 and TRAF3. Circ_0073748 and TRAF3 3ʹUTR were suffered mutations of the putative-binding sites using Hieff Mut™Site-Directed Mutagenesis Kit (Yeasen, Guanghzou, China). The wild type (WT) and mutant (MUT) of circ_0073748 and TRAF3 3ʹUTR were severally inserted into luciferase reporter plasmid psiCHECK-2 (Promega, Madison, WI, USA) at Xho I/Not I. HPDE6-C7 cells were co-transfected with these recombinant plasmids and miR-132-3p or miR-NC mimic for 48 h. Luciferase activities were determined by Dual-Glo® Luciferase Assay System (Promega), and normalized to the Renilla luciferase activity.
RNA immunoprecipitation (RIP) assay
RiboCluster ProfilerTM RIP-Assay Kit (MBL, Woburn, MA, USA) was used to measure enrichment of RNAs. HPDE6-C7 cells were lysed in the Lysis Buffer to collect the cytosolic fractions, which was later incubated with antibodies-immobilized Protein A/G Agarose beads (Pierce, Shanghai, China) for 1 h at 4°C. Immunoprecipitated RNA-protein complex was obtained in Lysis Buffer, and the RNAs in the complex was recovered and analyzed by qPCR.
Statistical analysis
Experiments were performed three times and each group was set at least three biological replicates. Data were displayed as mean ± standard deviation, and data normality was analyzed by Gaussian distribution and Shapiro–Wilk normality test. One-way analysis of variance and t test were performed for different comparisons between the two groups or among >2 groups. GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA) was used to draw graphs and analyze data. Statistical significance was set at *P < 0.05.
Results
Circ_0073748 was abnormally upregulated in patients and cells with AP
According to qPCR data, circ_0073748 expression leaped by more than 1.5-fold in plasma of patients with AP than control plasma (P < 0.0001;). Besides, its level showed gradually upward trend in HPDE6-C7 cells with the increase of caerulein concentration (0–15 nmol/L). Comparing to cytoplasmic control GAPDH, circ_0073748 expression was predominantly detected in the cytoplasm. Unexpectedly, circ_0073748 expression was unaffected by RNase R treatment, which was opposite to its linear counterpart LMNB1. These results showed that circ_0073748 was a stably upregulated circRNA in AP samples.
Silencing circ_0073748 alleviated caerulein-induced pancreatic ductal cell injury
10 nmol/L caerulein-treated HPDE6-C7 cells were harvested for functional assays, and caerulein upregulated the expression of circ_0073748. With caerulein induction, cell viability and EdU positive cell rate of HPDE6-C7 cells were synchronously decreased. Contrarily, IL-6 and TNF-α were increasingly secreted in response to caerulein treatment according to ELISA kits; apoptosis of caerulein-induced HPDE6-C7 cells was highly induced, as indicated by the promoted apoptosis rate and Bax expression, as well as the suppressed Bcl-2 expression. In addition, MDA level was boosted and SOD activity was depressed by caerulein. These outcomes demonstrated that caerulein-induced circ_0073748 upregulation in HPDE6-C7 cells allied with cell injury.
Next, loss-of-function assays of circ_0073748 were carried out, and circ_0073748 expression level was significantly silenced in HPDE6-C7 cells under caerulein treatment with si-circ_0073748 pre-transfection. Paralleled with this alteration, the lower levels of cell proliferation and inflammatory response in caerulein-treated HPDE6-C7 cells were significantly improved. Apoptosis induction mediated by caerulein was abated by si-circ_0073748. Consistently, caerulein-induced oxidative stress was also suppressed in condition of circ_0073748 silencing. These results depicted that silencing circ_0073748 could mitigate caerulein-induced HPDE6-C7 cell injury by enhancing cell proliferation and inhibiting apoptotic, inflammatory and oxidative responses.
MiR-132-3p was downregulated in AP and targeted by circ_0073748
Circ_0073748 showed computational miR-132-3p-binding sites, and these sites were mutated. Dual-luciferase reporter assay revealed that miR-132-3p overexpression via its mimic transfection could attenuate luciferase activity of reporter vector expressing WT-circ_0073748 in HPDE6-C7 cells, and could not deaden that of MUT-circ_0073748. Ago2 immunoprecipitation synchronously enriched circ_0073748 and miR-132-3p with normalization to IgG immunoprecipitation. Analysis of qPCR detected that miR-132-3p expression was downregulated in plasma samples of AP patients (P < 0.0001; and this expression was moderately correlated with circ_0073748. In HPDE6-C7 cells, expression of miR-132-3p was gradually but significantly decreased with the increase of caerulein concentration. Transient transfection of siRNA or overexpression vector could cause circ_0073748 silencing or overexpression in 10 nmol/L caerulein-treated HPDE6-C7 cellsand, allied with the upregulation or downregulation of miR-132-3p. These results demonstrated the target-binding relationship and negative regulation of circ_0073748 on miR-132-3p, indicating that circ_0073748 functioned as a sponge for miR-132-3p.
Exhausting miR-132-3p contributed to caerulein-induced injury in pancreatic ductal cells with circ_0073748 silencing
Either, miR-132-3p exhaustion could be mediated by its inhibitor transfection (P < 0.0001; , and this inhibitor could also exhaust miR-132-3p abundance in circ_0073748-interfered HPDE6-C7 cells under caerulein induction. Circ_0073748 silencing rescued cell viability and EdU positive cell rate of caerulein-induced HPDE6-C7 cells, both of which were further diminished by transfecting anti-miR-132-3p. IL-6, TNF-α and MDA levels were lessened and SOD activity was improved in caerulein-induced HPDE6-C7 cells with circ_0073748 blockage, and these effects were overall abated with miR-132-3p downregulation via anti-miR-132-3p. At last, circ_0073748 knockdown-mediated apoptosis inhibition in caerulein-induced HPDE6-C7 cells was distinctively diminished by miR-132-3p inhibitor, as evidenced by the increase of apoptosis rate and Bax expression. These outcomes demonstrated that the effects of circ_0073748 knockdown were partially abrogated by miR-132-3p depletion and miR-132-3p downregulation was contributing to caerulein-induced pancreatic ductal cell injury.
MiR-132-3p targeted TRAF3 in HPDE6-C7 cells
TRAF3 3ʹUTR was predicted to be complementary to the “seed sequence” of miR-132-3p, and these sites mutation led to the unresponsiveness of luciferase reporter vector to miR-132-3p. Enrichment of miR-132-3p and TRAF3 were simultaneously found in Ago2-mediated precipitation. Both of TRAF3 mRNA and protein expressions were enhanced in bloods of patients with AP (P < 0.0001 and P < 0.001;), Besides, TRAF3 was upregulated by caerulein in a dose-dependent manner). TRAF3 mRNA level was inversely and linearly associated with miR-132-3p level in these patients. Moreover, miR-132-3p mimic and inhibitor could severally downregulated or upregulated the expression of TRAF3 protein in 10 nmol/L caerulein-induced HPDE6-C7 cells. These results suggested that TRAF3 was negatively regulated by miR-132-3p via target binding.
Reinforcing TRAF3 abated the restraining effect of miR-132-3p on caerulein-induced pancreatic ductal cell injury
TRAF3 overexpression vector was used for ectopic expression of TRAF3 in HPDE6-C7 cells (P < 0.0001;), and this vector could also upregulate TRAF3 protein level in miR-132-3p-silenced cells under 10 nmol/L caerulein induction. With miR-132-3p mimic transfection, cell viability and EdU positive cell rate of caerulein-inducedHPDE6-C7 cells were synchronously elevated. Contrarily, IL-6 and TNF-α under caerulein induction were lower secreted in response to miR-132-3p overexpression; apoptosis of caerulein-induced HPDE6-C7 cells was less induced in the presence of miR-132-3p mimic, as indicated by the decreased apoptosis rate and Bax expression, as well as the promoted Bcl-2 level). In addition, introducing miR-132-3p mimic lessened MDA level and advanced SOD activity in caerulein-induced HPDE6-C7 cells. Of note, these inhibiting/encouraging effects of miR-132-3p overexpression were overall abolished by reinforcing TRAF3 via vector transfection. These outcomes demonstrated that upregulating miR-132-3p could prevent HPDE6-C7 cells against caerulein-induced injuries by inhibiting TRAF3. Furthermore, circ_0073748 knockdown could also downregulate TRAF3 protein level under caerulein treatment, and this downregulation was almost countermanded by administrating anti-miR-132-3p. These data suggest that silencing circ_0073748 hampered caerulein-induced pancreatic ductal cell injury by inhibiting TRAF3 as well.
Circ_0073748 interference suppressed caerulein-activated canonical NF-κB signal via regulating miR-132-3p and TRAF3
Expressions of p-P65/P65 and p-IκB/IκB were highly induced in response to 10 nmol/L caerulein treatment (P < 0.0001 and P < 0.0001;), hinting that caerulein induced the activation of canonical NF-κB signaling pathway. Interfering circ_0073748 via its siRNA could suppress p-P65/P65 and p-IκB/IκB expressions, which was partially abolished by further transfecting anti-miR-132-3p or TRAF3 overexpression vector. These results demonstrated that silencing circ_0073748 suppressed canonical NF-κB signaling pathway in caerulein-induced HPDE6-C7 cells via upregulating miR-132-3p and downregulatingTRAF3.
Discussion
Existing document showed that three blood circRNAs including hsa_circRNA_101015, hsa_circRNA_101211 and hsa_circRNA_103470 could diagnose AP and estimate the severity [Citation11]. Thus, special circRNAs could serve as noninvasive biomarkers in the diagnosis of AP. Here, we focused on circ_0073748, and intended to determine its role and action-of-mechanism in caerulein-induced AP model in pancreatic ductal cells.
First of all, expression of circ_0073748 was validated in this cohort of patients with AP, and its level was increased in the plasma with AP. Moreover, circ_0073748 expression was dominantly discovered in the cytoplasmic fraction and with resistance to RNase R digestion, which were consistent with the notion that exon-derived circRNAs always showed loop conformation and cytoplasmic localization [Citation21]. Besides, circRNAs harboring these properties could act as miRNA sponges in ceRNA regulation model [Citation21]. Next, we searched and identified that circ_0073748 could target and sponge miR-132-3p in pancreatic ductal HPDE6-C7 cells. Functionally, interfering circ_0073748 improved cell proliferation of caerulein-induced HPDE6-C7 cells, and suppressed pro-inflammatory response, oxidative stress and apoptosis. These effects could conclude a protective role of circ_0073748 downregulation in caerulein-induced pancreatic ductal cell injury, suggesting that inhibiting circ_0073748 might display medicinal benefit in AP.
In this study, miR-132-3p was lowly expressed in the plasma of AP patients and caerulein-induced pancreatic ductal cells. However, the link between miR-132-3p expression and pancreatitis was controversially reported. For example, Kumstel et al. [Citation19] indicated that miR-132-3p was upregulated in blood plasma of mice with caerulein-induced pancreatitis; on the contrary, Dixit et al. [Citation18] considered a downregulation of miR-132-3p in caerulein-induced mouse pancreatic acinar cells. This study might be the first evidence of miR-132-3p expression in AP patients, and it was downregulated in patients’ plasma with AP. Of note, this finding suggested miR-132-3p as a potential noninvasive biomarker for AP. Functionally, reinforcing miR-132-3p improved cell proliferation of caerulein-induced pancreatic ductal HPDE6-C7 cells, and suppressed pro-inflammatory response, oxidative stress and apoptosis. Therefore, miR-132-3p upregulation could prevent caerulein-induced pancreatic ductal cell injury, indicating a potential therapeutic target for the treatment of AP. Mechanically, miR-132-3p and miR-212-3p were equipped with the identical seed sequence, hinting that the both could target the same genes [Citation22]. Yet, miR-212-3p seemed to be unstudied in pancreatitis. But, miR-212-3p/miR-132-3p were deregulated in pancreatic islets involved in mediating beta cell dysfunction [Citation23,Citation24], and in PDAC implicated in tumorigenesis and development [Citation25].
As previously demonstrated, TRAF3 could be a common functional target gene for different miRNAs in rat pancreatic acinar cells for AP [Citation26–28], and we further confirmed TRAF3 was targeted by miR-132-3p in human pancreatic ductal cells. In this study, TRAF3 expression was increased in patient’s blood with AP, and in human pancreatic ductal HPDE6-C7 cells with caerulein treatment. TRAF3 downregulation was allied with interfering circ_0073748 and restoring miR-132-3p in their inhibiting roles in caerulein-induced HPDE6-C7 cell injury and canonical NF-κB signaling pathway. Furthermore, direct evidence had also been also provided that TRAF3 inhibition mediated treatment effect on caerulein-induced rat pancreatic acinar cells by suppressing inflammatory response and autophagy [Citation28]. Moreover, oxidative stress was well known as an early phenomenon in pancreatic tissue in the course of caerulein-induced AP [Citation29]. Interfering circ_0073748 and restoring miR-132-3p both decreased caerulein-induced high levels of MDA, IL-6 and TNF-α in human pancreatic ductal cells and upregulated SOD activity. TRAF3 was required for the activation of canonical NF-κB pathway [Citation8], whereas it was one negative regulator of the non-canonical NF-κB pathway [Citation30]. Moreover, CD40 was essential in TRAF3 upregulation, NF-κB activation, and NF-κB-dependent pro-inflammatory genes expression in mice [Citation31]; in turn, TRAF3 could participate in the formation of CD40 ligand-induced transcriptional complex with P65 [Citation32]. Accidently, TRAF3-MAPK P38 signal activation was previously demonstrated to be underlying the acute pancreatitis response induced by caerulein [Citation9,Citation33].
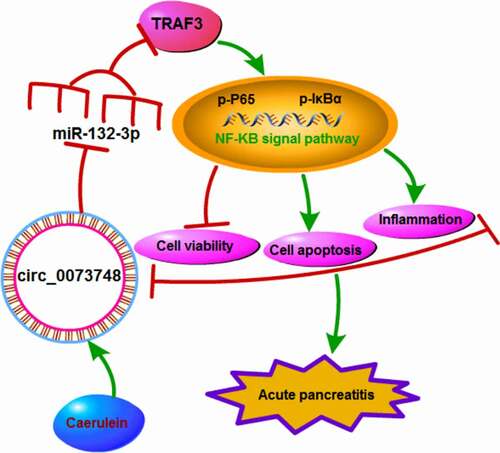
All in all, caerulein could induce circ_0073748 upregulation, and thus promoted miR-132-3p-targeted TRAF3 expression, eventually mediating NF-κB signal activation, cell proliferation inhibition, and responses of apoptosis, inflammation and oxidative stress in pancreatic ductal cells in AP (). Furthermore, interfering circ_0073748 might alleviate caerulein-induced pancreatic ductal cell injury, and circ_0073748/miR-132-3p/TRAF3 ceRNA pathway might be one underlying mechanism and therapeutic target of AP.
Figure 1. Circ_0073748 was an abnormally upregulated circRNA in patients and cells with AP. (a, b) qPCR detected relative circ_0073748 expression in (a) blood plasma of AP patients (n = 23) and normal controls (n = 19), and (b) pancreatic ductal HPDE6-C7 cells induced by caerulein. (c, d) qPCR detected relative RNA levels of (c) circ_0073748, U6 and GAPDH in HPDE6-C7 cells-derived cytoplasmic and nuclear fractions, and (d) circ_0073748 and LMNB1 in HPDE6-C7 cells-derived total RNAs treated with mock and RNase R. *P < 0.05 and ****P < 0.0001.
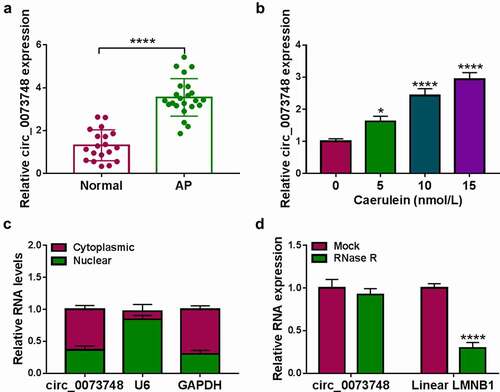
Figure 2. Silencing circ_0073748 alleviated caerulein-induced pancreatic ductal cell injury. HPDE6-C7 cells were transfected with si-NC and si-circ_0073748 prior to caerulein (10 nmol/L) induction. (a) qPCR detected relative circ_0073748 expression. (b) MTS assay measured cell viability (%). (c) ELISA examined concentrations of IL-6 and TNF-α. (d) EdU assay determined EdU positive cells (%), normalized to Hoechst 33342 positive cells. (e) FITC/PI assay and FCM determined apoptosis rate (%) in FITC+/PI± quadrants. (f) Western blotting detected relative protein expression of Bcl-2 and Bax, corrected by GAPDH. (g, h) Assay kits examined relative MDA level and SOD activity. **P < 0.01, ***P < 0.001 and ****P < 0.0001.
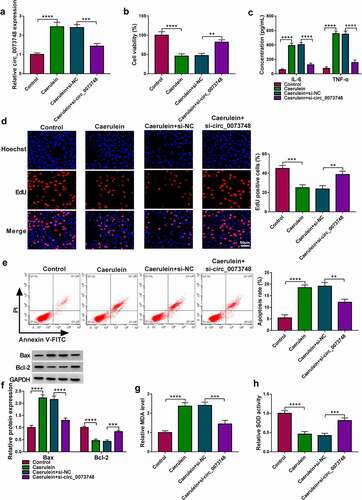
Figure 3. MiR-132-3p was downregulated in AP and targeted by circ_0073748. (a) Computational intact binding sites of miR-132-3p in WT-circ_0073748 and the mutations in MUT-circ_0073748 were shown. (b) qPCR detected relative miR-132-3p expression in HPDE6-C7 cells after transfections with miR-132-3p and miR-NC. (c) Dual-luciferase reporter system determined relative luciferase activity of reporter vectors expressing WT-circ_0073748 or MUT-circ_0073748 in HPDE6-C7 cells transfected with miR-132-3p mimic and miR-NC mimic. (d) qPCR tested relative RNA levels of circ_0073748 and miR-132-3p in Ago2 RIP with normalization to IgG RIP. (e, g) qPCR detected relative miR-132-3p expression in (e) blood plasma of AP patients (n = 23) and normal controls (n = 19), and (g) HPDE6-C7 cells induced by caerulein or not. (f) Correlation coefficient (r) between circ_0073748 and miR-132-3p expression in AP blood plasma was confirmed by Spearman’s rank. (h, i) qPCR detected relative circ_0073748 and miR-132-3p expressions in 10 nmol/L caerulein-induced HPDE6-C7 cells transfected with si-NC, si-circ_0073748, pCD5-ciR vector, and circ_0073748 vector. **P < 0.01, ***P < 0.001 and ****P < 0.0001.
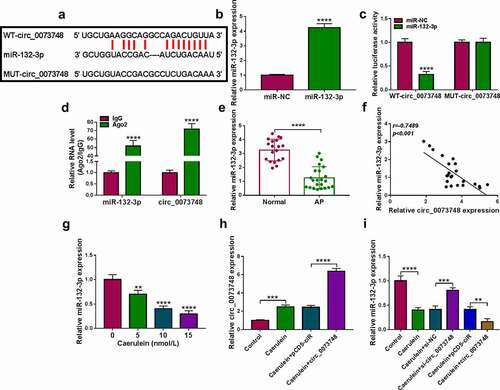
Figure 4. Exhausting miR-132-3p contributed to caerulein-induced pancreatic ductal cell injury with circ_0073748 silencing. (a, b) qPCR detected relative miR-132-3p expression in HPDE6-C7 cells after transfections of anti-miR-132-3p and anti-miR-NC, and in 10 nmol/L caerulein-induced HPDE6-C7 cells transfected with si-NC, si-circ_0073748, si-circ_0073748 coupled with anti-miR-132-3p, si-circ_0073748 coupled with anti-miR-NC. (c) MTS assay measured cell viability (%). (d) ELISA examined concentrations of IL-6 and TNF-α. (e) EdU assay determined EdU positive cells (%). (e) FITC/PI assay determined apoptosis rate (%) in FITC+/PI± quadrants. (g) Western blotting detected relative protein expression of Bcl-2 and Bax, corrected by GAPDH. (h, i) Assay kits examined relative MDA level and SOD activity. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
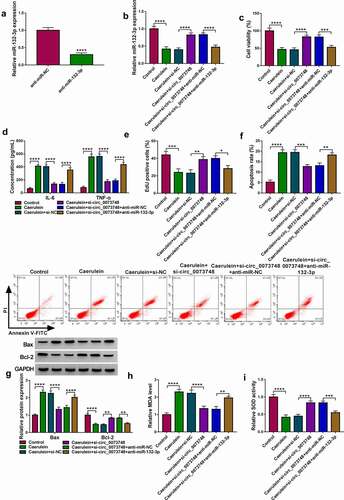
Figure 5. MiR-132-3p targeted TRAF3 in human pancreatic ductal cells. (a) Simple graph showed miR-132-3p-binding sites in WT-TRAF3 3ʹUTR and the mutations in MUT-TRAF3 3ʹUTR. (b) Dual-luciferase reporter system determined relative luciferase activity of reporter vectors expressing WT-TRAF3 3ʹUTR or MUT-TRAF3 3ʹUTR in HPDE6-C7 cells transfected with miR-132-3p and miR-NC. (c) qPCR tested relative RNA levels of TRAF3 and miR-132-3p in Ago2 RIP with normalization to IgG RIP. (d-f) qPCR and Western blotting detected relativeTRAF3 mRNA and protein expressions in (d, e) bloods of AP patients and normal controls, and (f) HPDE6-C7 cells induced by caerulein or not. (g) Correlation coefficient (r) between TRAF3 mRNA and miR-132-3p expression in AP bloods was confirmed by Spearman’s rank. (h, i) qPCR and Western blotting respectively detected relative miR-132-3p and TRAF3 protein expressions in 10 nmol/L caerulein-induced HPDE6-C7 cells transfected with miR-NC, miR-132-3p, anti-miR-132-3p, and anti-miR-NC. **P < 0.01, ***P < 0.001 and ****P < 0.0001.
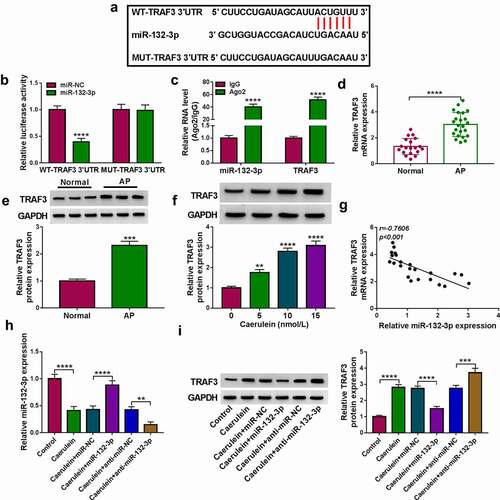
Figure 6. Reinforcing TRAF3 abated the restraining effect of miR-132-3p on caerulein-induced pancreatic ductal cell injury. (a, b) Western blotting detected relative TRAF3 protein expression in (a) HPDE6-C7 cells transfected TRAF3 vector and pcDNA vector, and in (b) 10 nmol/L caerulein-induced HPDE6-C7 cells transfected with miR-NC, miR-132-3p, miR-132-3p along with TRAF3 vector, and miR-132-3p along with pcDNA vector. (c) MTS assay measured cell viability (%). (d) ELISA examined concentrations of IL-6 and TNF-α. (e) EdU assay determined EdU positive cells (%). (E) FITC/PI assay determined apoptosis rate (%) in FITC+/PI± quadrants. (g) Western blotting detected relative protein expression of Bcl-2 and Bax, corrected by GAPDH. (h, i) Assay kits examined relative MDA level and SOD activity. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
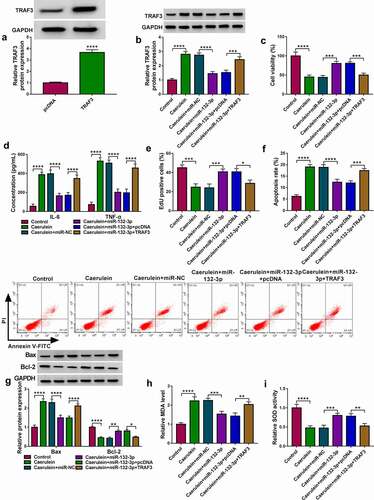
Figure 7. MiR-132-3p mediated the modulation of circ_0073748 on TRAF3 expression. (a) qPCR and (b) Western blotting detected relative TRAF3 mRNA and protein expressions in 10 nmol/L caerulein-induced HPDE6-C7 cells transfected with si-NC alone, si-circ_0073748 alone and allied with anti-miR-NC or anti-miR-132-3p. ****P < 0.0001.
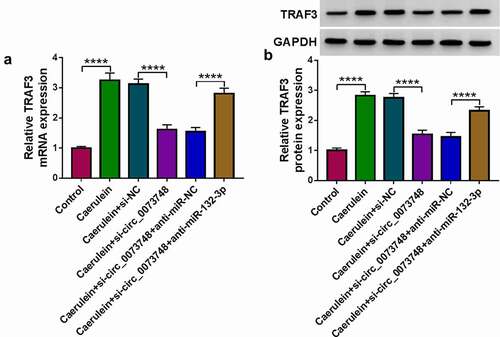
Figure 8. Circ_0073748 interference suppressed caerulein-induced NF-κB signal activation via regulating miR-132-3p and TRAF3. Western blotting detected relative protein expressions of p-P65, P65, p-IκB, IκB, and GAPDH in (a) 10 nmol/L caerulein-induced HPDE6-C7 cells and (b) these cells pre-transfected with si-NC alone, si-circ_0073748 alone, and si-circ_0073748 allied with anti-miR-NC, anti-miR-132-3p, pcDNA vector and TRAF3 vector. ****P < 0.0001.
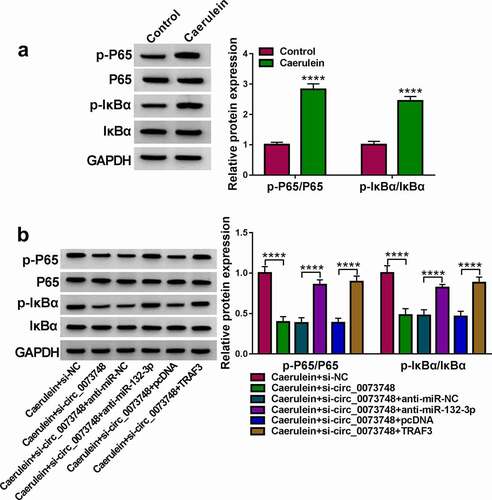
Figure 9. Caerulein induced pancreatic ductal cell injury in AP presumably through activating circ_0073748/miR-132-3p/TRAF3 axis and NF-κB signaling pathways. Caerulein induced circ_0073748 upregulation and promoted miR-132-3p-targeted TRAF3 expression, eventually mediating NF-κB signal activation and pancreatic ductal cell injury in AP.
Supplemental Material
Download Zip (11.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Jiang X, Zheng YW, Bao S, et al. Drug discovery and formulation development for acute pancreatitis. Drug Deliv. 2020;27:1562–1580.
- Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180–1193.
- Steer ML, Meldolesi J. The cell biology of experimental pancreatitis. N Engl J Med. 1987;316:144–150.
- Yu JH, Kim H. Oxidative stress and inflammatory signaling in cerulein pancreatitis. World J Gastroenterol. 2014;20:17324–17329.
- Liu T, Zhang L, Joo D, et al. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2. DOI:https://doi.org/10.1038/sigtrans.2017.23
- Jakkampudi A, Jangala R, Reddy BR, et al. NF-kappaB in acute pancreatitis: mechanisms and therapeutic potential. Pancreatology. 2016;16:477–488.
- Shi JH, Sun SC. Tumor necrosis factor receptor-associated factor regulation of nuclear factor kappaB and mitogen-activated protein kinase pathways. Front Immunol. 2018;9:1849.
- Fochi S, Bergamo E, Serena M, et al. TRAF3 is required for NF-kappaB pathway activation mediated by HTLV tax proteins. Front Microbiol. 2019;10:1302.
- Jia R, Ma J, Xiang S, et al. Caerulin-induced pro-inflammatory response in macrophages requires TRAF3-p38 signaling activation. Biochem Biophys Res Commun. 2017;494:358–364.
- Yu S, Li Y, Liao Z, et al. Plasma extracellular vesicle long RNA profiling identifies a diagnostic signature for the detection of pancreatic ductal adenocarcinoma. Gut. 2020;69:540–550.
- Liu C, Zhu X, Niu X, et al. Elevated hsa_circRNA_101015, hsa_circRNA_101211, and hsa_circRNA_103470 in the human blood: novel biomarkers to early diagnose acute pancreatitis. Biomed Res Int. 2020;2020:2419163.
- Yang Y, Ren J, Huang Q, et al. CircRNA expression profiles and the potential role of CircZFP644 in mice with severe acute pancreatitis via sponging miR-21-3p. Front Genet. 2020;11:206.
- Wang B, Wu J, Huang Q, et al. Comprehensive analysis of differentially expressed lncRNA, circRNA and mRNA and their ceRNA networks in mice with severe acute pancreatitis. Front Genet. 2021;12:625846.
- Wang J, Li X, Liu Y, et al. CircHIPK3 promotes pyroptosis in acinar cells through regulation of the miR-193a-5p/GSDMD axis. Front Med (Lausanne). 2020;7:88.
- Xiang H, Tao X, Xia S, et al. Targeting MicroRNA function in acute pancreatitis. Front Physiol. 2017;8:726.
- Yang Y, Huang Q, Luo C, et al. MicroRNAs in acute pancreatitis: from pathogenesis to novel diagnosis and therapy. J Cell Physiol. 2020;235:1948–1961.
- Haviv R, Oz E, Soreq H. The stress-responding miR-132-3p shows evolutionarily conserved pathway interactions. Cell Mol Neurobiol. 2018;38:141–153.
- Dixit AK, Sarver AE, Yuan Z, et al. Comprehensive analysis of microRNA signature of mouse pancreatic acini: overexpression of miR-21-3p in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2016;311:G974–G980.
- Kumstel S, Janssen-Peters H, Abdelrahman A, et al. MicroRNAs as systemic biomarkers to assess distress in animal models for gastrointestinal diseases. Sci Rep. 2020;10:16931.
- Li J, Chen J, Tang W. The consensus of integrative diagnosis and treatment of acute pancreatitis-2017. J Evid Based Med. 2019;12:76–88.
- Li X, Yang L, Chen LL. The biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71:428–442.
- Park JK, Henry JC, Jiang J, et al. miR-132 and miR-212 are increased in pancreatic cancer and target the retinoblastoma tumor suppressor. Biochem Biophys Res Commun. 2011;406:518–523.
- Dalgaard LT, Eliasson L. An ‘alpha-beta’ of pancreatic islet microribonucleotides. Int J Biochem Cell Biol. 2017;88:208–219.
- Esguerra JLS, Nagao M, Ofori JK, et al. MicroRNAs in islet hormone secretion. Diabetes Obes Metab. 2018;20(Suppl 2):11–19.
- Zhang H, Liu A, Feng X, et al. MiR-132 promotes the proliferation, invasion and migration of human pancreatic carcinoma by inhibition of the tumor suppressor gene PTEN. Prog Biophys Mol Biol. 2019;148:65–72.
- Zhu Y, Liu S, Wang F. MicroRNA MiR-27a-5p alleviates the cerulein-induced cell apoptosis and inflammatory injury of AR42J cells by targeting Traf3 in acute pancreatitis. Inflammation. 2020;43:1988–1998.
- Liu S, Zou H, Wang Y, et al. miR-155-5p is negatively associated with acute pancreatitis and inversely regulates pancreatic acinar cell progression by targeting rela and Traf3. Cell Physiol Biochem. 2018;51:1584–1599.
- Sun H, Tian J, Li J. MiR-92b-3p ameliorates inflammation and autophagy by targeting TRAF3 and suppressing MKK3-p38 pathway in caerulein-induced AR42J cells. Int Immunopharmacol. 2020;88:106691.
- Dabrowski A, Konturek SJ, Konturek JW, et al. Role of oxidative stress in the pathogenesis of caerulein-induced acute pancreatitis. Eur J Pharmacol. 1999;377:1–11.
- Sun SC. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17:545–558.
- Song Z, Jin R, Yu S, et al. CD40 is essential in the upregulation of TRAF proteins and NF-kappaB-dependent proinflammatory gene expression after arterial injury. PLoS One. 2011;6:e23239.
- El Hokayem J, Brittain G, Nawaz Z, et al. Tumor Necrosis Factor Receptor Associated Factors (TRAFs) 2 and 3 form a transcriptional complex with phosho-RNA polymerase II and p65 in CD40 ligand activated Neuro2a cells. Mol Neurobiol. 2017;54:1301–1313.
- Jia R, Ma J, Meng W, et al. Dihydromyricetin inhibits caerulin-induced TRAF3-p38 signaling activation and acute pancreatitis response. Biochem Biophys Res Commun. 2018;503:1696–1702.
