ABSTRACT
Gouty arthritis (GA) is caused by monosodium urate (MSU) crystal accumulation in the joints. MSU-mediated inflammation is an important inducing factor in gouty arthritis (GA). Recent studies have demonstrated that microRNAs can influence GA progression. Herein, the role and mechanism of miRNA-142-3p in GA were explored. To establish the in vitro and in vivo GA models, MSU was used to induce inflammatory response in human monocyte cell line THP-1 and male C57BL/6 mice. Protein levels, gene expression and proinflammatory cytokine secretion were respectively tested by Western blotting, RT-qPCR, and enzyme-linked immunosorbent assay (ELISA). Pathological changes in sagittal sections of ankle tissues were exhibited by hematoxylin-eosin (HE) staining. Binding relationship between miRNA-142-3p and zinc finger E-box binding homeobox 2 (ZEB2) was predicted and confirmed by bioinformatics analysis and luciferase reporter assay. In this study, MSU induced inflammatory response and upregulated miRNA-142-3p in THP-1 cells. Functionally, miRNA-142-3p knockdown inhibited inflammatory response in MSU-stimulated THP-1 cells and alleviated pathological symptoms of GA mice. Mechanically, miRNA-142-3p targeted ZEB2 in THP-1 cells. ZEB2 expression was elevated in MSU-administrated THP-1 cells and GA mice. ZEB2 downregulation reserved the inhibitory effect of miRNA-142-3p deficiency on inflammatory response in MSU-treated THP-1 cells. In addition, miRNA-142-3p activated NF-κB signaling by binding with ZEB2 in THP-1 cells upon MSU stimulation. Overall, miRNA-142-3p facilitates inflammatory response by targeting ZEB2 and activating NF-κB signaling in GA.
KEYWORDS:
Introduction
Gouty arthritis (GA) is the most prevalent form of inflammatory arthritis resulted from monosodium urate (MSU) deposition and hyperuricemia in the joints [Citation1]. GA is more likely to occur in men than in women, by a ratio of 10:1 [Citation2]. In recent years, the prevalence of GA has reached 11–13% and the incidence has reached 0.4% in people over 80 years old [Citation3]. The pathogenesis of GA is often accompanied with many comorbidities, such as cardiovascular diseases, diabetes, obesity, hypertension, and chronic kidney diseases. GA can be divided into chronic and acute forms of inflammation [Citation4]. Acute GA is featured with intense pain in the affected joints, which usually lasts 3–14 days [Citation5]. Monocytes/macrophages (a kind of inflammatory cells) can regulate MSU-induced inflammatory response in GA [Citation6]. Monocytes and macrophages have been reported to release more pro-inflammatory cytokines in synovial fluid of patients with acute GA [Citation7]. Additionally, MSU treatment has been demonstrated to activate caspase-1 and facilitate the productions of pro-inflammatory factors in monocytes/macrophages in an in vitro model of GA [Citation8]. At present, the first line drugs used for GA standard treatments include corticosteroids, colchicine and non-steroidal anti-inflammatory drugs [Citation9]. However, the side effects and low efficiency of these drugs hinder further clinical applications [Citation9]. Therefore, seeking more effective therapeutic approaches for GA treatment is important.
MicroRNAs (miRNAs) are single-stranded noncoding RNAs that modulate the expression of target genes by mediating mRNA cleavage, mRNA destabilization and translation repression [Citation10]. MiRNAs have been identified to regulate cellular processes (e.g. apoptosis, migration and differentiation) in various diseases [Citation11]. Increasing reports have substantiated the involvement of miRNAs in GA. For example, miR-302b significantly attenuates MSU-induced IL-1β secretion by targeting interleukin 1 receptor associated kinase 4 (IRAK4) and EPH receptor A2 (EphA2) to inhibit NF-κB and caspase-1 signaling [Citation12]. MiR-22-3p and miR-223-3p suppress gouty inflammation triggered by MSU stimulation by negatively regulating NLR family pyrin domain containing 3 (NLRP3) [Citation13]. MiR-155 decreases inositol polyphosphate-5-phosphatase D (SHIP-1) expression and promotes MSU-induced proinflammatory cytokine production [Citation14]. Numerous studies have substantiated that miR-142-3p serves as a key regulator of many inflammation-associated diseases, such as multiple sclerosis [Citation15], chronic rhinosinusitis with nasal polyposis [Citation16], intervertebral disc degeneration [Citation17] and rheumatoid arthritis [Citation18]. Although miR-142-3p expression has been demonstrated to be markedly elevated in the plasma of patients with asymptomatic hyperuricemia and primary gout in contrast with normouricemic controls [Citation19], the specific mechanism and role of miR-142-3p in GA remain unclear.
In this study, the in vivo and in vitro models of GA were established using MSU. The biological role and possible mechanism of miR-142-3p in the in vivo and in vitro GA models were investigated. We hypothesized that miR-142-3p might regulate inflammation in GA by regulating downstream genes. This study may contribute to the investigation of novel therapeutic targets for GA treatment.
Materials and methods
Bioinformatic analysis
Potential target genes (ZEB2, TASOR2, RICTOR and RHOBTB3) of miR-142-3p were identified by miRDB (http://www.mirdb.org/) (screening condition: target score > 98). The binding site between miR-142-3p and ZEB2 3ʹuntranslated region (3ʹUTR) was predicted by Targetscan (http://www.targetscan.org/).
Cell culture and MSU exposure
Human monocytic cell line THP-1 was purchased from American type culture collection (ATCC; Manassas, VA, USA). THP-1 cells were cultured in RPMI-1640 medium (ThermoFisher Scientific, USA) containing 10% fetal bovine serum (FBS; Gibco, USA) and 1% penicillin (100 U/mL)/streptomycin (100 μg/mL) (Gibco) at 37°C with 5% CO2. THP-1 cells (1.5 × 106 cells/well) were seeded in 6-well plates. To enhance the phagocytic properties of THP-1 cells and trigger proinflammatory cytokine release [Citation8], THP-1 cells were pre-treated with 0.5 μM phorbol 12-myristate 13-acetate (PMA; Sigma, USA) for 3 h [Citation20]. Next, MSU crystals (Invitrogen, Carlsbad, CA, USA) at the concentration of 250 μg/mL were used to treat THP-1 cells for 24 h to induce inflammatory response [Citation20]. THP-1 cells without MSU treatment served as the control (CTRL).
Cell transfection
Short hairpin RNA targeting ZEB2 (sh-ZEB2) was used to downregulate ZEB2 with sh-NC as a negative control. The miR-142-3p inhibitor was used to knockdown miR-142-3p with NC inhibitor as negative control. All plasmids were purchased from GenePharma (Shanghai, China). When the cell confluence reached 80%, a total of 100 nM sh-ZEB2, 100 nM sh-NC, 50 nM miR-142-3p inhibitor or 50 nM NC inhibitor were transfected into THP-1 cells at 37°C using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocols. The transfection efficiency was examined using RT-qPCR after 48 h.
Enzyme-linked immunosorbent assay (ELISA)
Inflammatory response was assessed by determining pro-inflammatory cytokine concentration using ELISA. Serum was separated from murine blood by centrifugation at 4°C at 3500 rpm for 15 min. According to the manufacturer’s instructions, the levels of pro-inflammatory cytokines in THP-1 cells and murine serum were detected using ELISA kits (R&D Systems, Minneapolis USA).
Animals and groups
The experimental unit was set as a single animal. Statistically, there should be more than 6 available data in each group. Thus, the number of mice in each group was set as nine with the consideration of other uncontrollable factors. A total of 36 healthy C57BL/6 mice (male, ten-week-old) used in this study were purchased from Shanghai Laboratory Animal Company (Shanghai, China). No other criteria were set for including and excluding animals during the experiment. All mice were fed ad libitum a standard diet and water and maintained separately at 22–24°C under a 12:12 h light/dark cycle. All mice were maintained in the same environment and then separately treated to minimize potential confounders. According to random number table, these mice were randomly divided into four groups (n = 9/group): sham (control), model, model+NC and model+miR-142-3p inhibitor. For establishment of GA mice model, MSU crystals (2 mg) suspended in 10 μL PBS were intraarticularly injected into both ankle joints of the mice from the model, model+NC and model+miR-142-3p inhibitor groups. Mice in the sham group received 10 μL PBS by intraarticular injection. Lentivirus-mediated miR-142-3p inhibitor (LV-miR-142-3p inhibitor) and its negative control (LV-miR-NC) were obtained from Genechem (Shanghai, China). After treatment with MSU crystals for 24 h, LV-miR-142-3p inhibitor (1 × 109 plaque-forming units) were injected into the ankles of mice in the model+miR-142-3p inhibitor group to silence miR-142-3p in vivo. Mice in the model+NC group received the equivalent amount of LV-miR-NC by contralateral ankle injection. After 19 d, all rats were anesthetized by intraperitoneal injection of 50 mg/kg sodium pentobarbital and the ankle tissues and blood samples were collected [Citation12]. Pathological changes in sagittal sections of ankle tissues in four groups were shown by hematoxylin-eosin staining. The release of proinflammatory cytokines (TNF-α, IL-6 and IL-1β) in the murine serum was examined using ELISA.
Group allocation, the conduct of the experiment, and outcome assessment as well as data analysis were independently performed by a designer, a conductor, and an evaluator. No adverse events were reported in the study. The experimental protocol was authorized by the Animal Ethics Committee of Zhoushan Hospital of Zhejiang Province (2019A-049B2, Zhejiang, China).
Hematoxylin-eosin (HE) staining
Ankle tissues were collected from mice with GA. Then, the tissues were fixed with 10% formalin, embedded in paraffin, cut into sagittal sections, and stained with a Hematoxylin and Eosin Staining Kit (Beyotime). A microscope (Olympus, Japan) was used to capture images for histological examination [Citation1].
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from THP-1 cells and murine ankle tissues by TRIzol reagent (Invitrogen, 1 mL) according to the manufacturer’s recommendations and then eluted with RNase-free water. The purity and concentrations of RNA samples were detected by a NanoDrop 2000/2000 c machine (Thermo Fisher Scientific, Waltham, MA, USA). Next, RNA (500 ng) was reverse transcribed to complementary DNA using reverse transcription cDNA synthesis kit (Vazyme, China) in a 20 μL reaction mixture following the manufacturer’s protocols. The reverse transcription reaction conditions were 37°C with 15 min and inactivation conditions were 85°C with 5 min. The concentration and purity of cDNA were measured by the NanoDrop 2000/2000 c machine (Thermo Fisher Scientific). Then, SYBR Green PCR kit (Takara) was used for qPCR on the 7900HT Fast Real-Time PCR System (Applied Biosystems). The qPCR was performed at 95°C for 5 min, followed by 40 cycles of two-step PCR: 95°C for 10s and 60°C for 30s, and extension at 72°C for 1 min. The expression levels of miR-142-3p and mRNAs were calculated by the 2−ΔΔCt method and were normalized to U6 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), respectively [Citation21]. The sequences of primers used are presented in .
Table 1. Sequences of primers used for reverse transcription-quantitative PCR
Western blotting
Total protein was isolated from THP-1 cells and mouse ankle tissues utilizing RIPA lysis buffer (Beyotime). A BCA assay kit (Beyotime) was used to quantify the obtained proteins. Based on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), proteins were transferred onto polyvinylidene fluoride (PVDF) membranes, and then incubated with primary antibodies including anti-ZEB2 (ab191364; 1:500), anti-p65 (ab32536; 1:1000), anti-p-p65 (ab76302; 1:1000), anti-p-IκBα (ab133462; 1:10,000), anti-IκBα (ab76429; 1:500), anti-β-actin (ab8226, 1:1000) and anti-GAPDH (ab8245; 1:500) at 4°C overnight and subsequently incubated with secondary antibody (ab6721; 1:2000) at room temperature for 2 h. All antibodies were purchased from Abcam (Cambridge, MA, USA). Protein bands were visualized by BeyoECL Plus (Beyotime) and analyzed using sodium Image Lab 3.0 (Invitrogen) [Citation22].
Luciferase reporter assay
The ZEB2 3ʹUTR fragment containing binding site of miR-142-3p was inserted into the pmirGLO vector (Promega, Madison, WI, USA) to form wild-type luciferase reporter ZEB2-Wt. ZEB2 3ʹUTR-Mut was formed by mutating the predicted binding site using a Phusion Site-Directed Mutagenesis Kit (Thermo Fisher Scientific). ZEB2 3ʹUTR Wt/Mut was transfected into THP-1 cells with miR-142-3p inhibitor/NC inhibitor using Lipofectamine 2000 (Invitrogen). After 48 h of transfection, the luciferase activity (Firefly/Renilla) of each group was assessed using Luciferase Reporter Assay System (Promega) [Citation23]. The activity of firefly luciferase was normalized to that of Renilla luciferase.
Statistical analysis
All the experimental data were obtained from three independent measurements and were analyzed using GraphPad Prism software 6.0 (La Jolla, CA, USA) and SPSS 19.0 software (IBM, Armonk, NY, USA). All data are presented as the mean ± standard error (SE). Normality of the data was tested using the Kolmogorov-Smirnov test. An independent-sample t-test was used for two-group comparisons, and one-way ANOVA followed by Bonferroni’s post hoc test was used for multiple-group comparisons. Correlation between miR-142-3p expression and ZEB2 expression in ankle tissues of GA mice was analyzed by Pearson correlation analysis. For each experimental group, no animals, experimental units, or data points were not included in the analysis. For each analysis, n = 9 in each experimental group. A value of p < 0.05 was considered statistically significant.
Results
MSU treatment induces inflammatory response and upregulates miR-142-3p in THP-1 cells
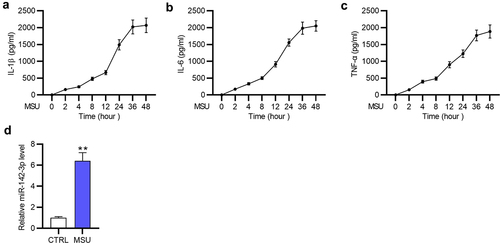
GA in vitro model was established using 250 μg/mL MSU to stimulate inflammatory response in THP-1 cells. Since inflammatory factor secretion is the indicator of gouty inflammatory response, the levels of TNF-α, IL-6 and IL-1β in THP-1 cells after MSU treatment were measured by ELISA at indicated time points. The results revealed that the release of these pro-inflammatory factors was elevated in a time-dependent manner in MSU-stimulated THP-1 cells (), which suggested that MSU treatment triggered inflammatory response in THP-1 cells and an in vitro GA model was established successfully. As demonstrated by RT-qPCR, miR-142-3p was markedly (**p < 0.01) highly expressed in MSU-treated THP-1 cells compared with that in the control (CTRL) group ()). Therefore, miR-142-3p may participate in MSU-medicated inflammatory response in GA.
Figure 1. MSU treatment induces inflammatory response and elevates miR-142-3p in THP-1 cells. (a-c) Accumulation of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) in THP-1 cells after MSU treatment were detected by ELISA at indicated time points. (d) MiR-142-3p level in THP-1 cells with or without MSU administration was examined using RT-qPCR. **p < 0.01.
MiR-142-3p knockdown inhibits inflammatory response in MSU-treated THP-1 cells
Next, the role of miR-142-3p in regulating inflammatory response in THP-1 cells was explored. MiR-142-3p expression was significantly (***p < 0.001) reduced by miR-142-3p inhibitor in MSU-stimulated THP-1 cells ()). Proinflammatory cytokine levels in MSU-treated THP-1 cells were evaluated as shown by ELISA and RT-qPCR (). ELISA revealed that MSU-induced increase in IL-1β, IL-6, and TNF-α concentration was partially (**p < 0.01) reversed by miR-142-3p depletion (). Consistently, the increase in mRNA levels of TNF-α, IL-6 and IL-1β induced by MSU administration was significantly (**p < 0.01) counteracted by miR-142-3p silencing in THP-1 cells (). Overall, MiR-142-3p knockdown suppresses inflammation of MSU-treated THP-1 cells.
Figure 2. MiR-142-3p knockdown inhibits inflammatory response in MSU-treated THP-1 cells. (a) MiR-142-3p level in MSU-stimulated THP-1 cells transfected with miR-142-3p inhibitor or NC inhibitor was examined by RT-qPCR. (b-d) The release of proinflammatory cytokines in THP-1 cells with or without MSU stimulation after transfection with miR-142-3p inhibitor or NC inhibitor was evaluated by ELISA. (e-g) The mRNA levels of proinflammatory cytokines were detected in THP-1 cells with or without MSU stimulation after transfection with miR-142-3p inhibitor or NC inhibitor via RT-qPCR. **p < 0.01, ***p < 0.001.
MiR-142-3p silencing attenuates MSU-induced inflammatory response in vivo
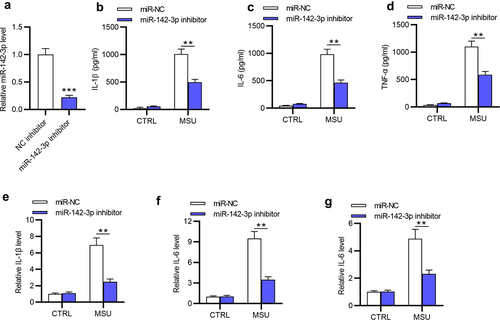
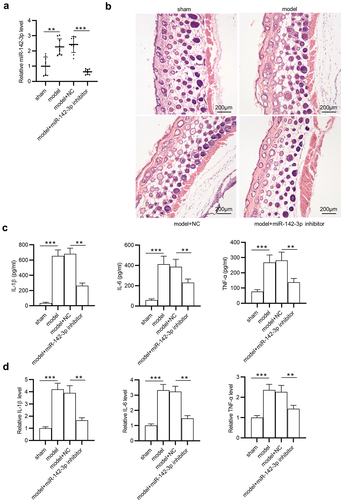
Next, in vivo GA model was established by intraarticularly injecting MSU into ankle joints of C57BL/6 mice. Then, LV-miR-142-3p inhibitor and LV-miR-NC were injected into murine ankle tissues. MiR-142-3p expression in mouse ankle tissues was examined by RT-qPCR, showing that miR-142-3p was highly (**p < 0.01) expressed in the model group in contrast with the sham group ()). After injection with LV-miR-142-3p inhibitor, miR-142-3p expression was significantly (***p < 0.001) decreased in the model group compared with that in model+NC group ()). In addition, histological examination demonstrated that the recruitment of inflammatory cells was enhanced in the model group and model+NC group, and miR-142-3p deficiency suppressed the MSU-induced enhancement ()). Compared with those in the sham group, IL-1β, IL-6 and TNF-α concentration and mRNA levels in the murine serum and ankle tissues were significantly (***p < 0.001) elevated in the model group (). miR-142-3p deficiency greatly (**p < 0.01) reduced MSU-mediated high levels of TNF-α, IL-6 and IL-1β in the model mice compared with the model+NC group (). Overall, miR-142-3p silencing mitigates the MSU-triggered inflammatory response in vivo.
Figure 3. MiR-142-3p silencing attenuates the inflammatory response induced by MSU in vivo. (a) Measurement of miR-142-3p level in ankle tissues of different groups (sham, model, model+LV-NC inhibitor, model+LV-miR-142-3p inhibitor) via RT-qPCR. (b) Pathological changes in sagittal sections of ankle tissues in above four groups were exhibited via HE staining (scar bar: 200 μm). (c) Proinflammatory cytokine release in the murine serum in above four groups was evaluated by ELISA. (d) The mRNA levels of proinflammatory cytokines in the murine ankle tissues in the four groups were evaluated by RT-qPCR. **p < 0.01, ***p < 0.001.
MiR-142-3p targets ZEB2
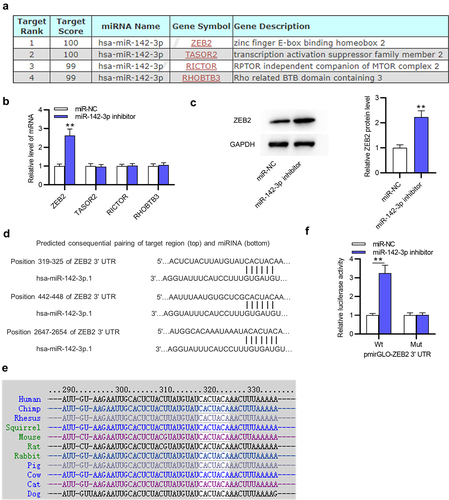
To figure out the underlying mechanism of miR-142-3p in GA, the target gene of miR-142-3p was investigated. The miRDB website was used to predict potential mRNAs containing binding sites for miR-142-3p, and the first four genes (ZEB2, TASOR2, RICTOR and RHOBTB3) with target score > 98 were identified ()). Among these candidates, only ZEB2 was greatly (**p < 0.01) upregulated by miR-142-3p inhibitor in THP-1 cells ()). In addition, ZEB2 protein level was significantly (**p < 0.01) elevated in THP-1 cells after depleting miR-142-3p ()). Subsequently, three binding sites between miR-142-3p and ZEB2 3ʹUTR were predicted by Targetscan, and position 319–325 of ZEB2 3ʹUTR are highly conserved among multiple species (). Luciferase reporter assay further confirmed the interaction between miR-142-3p and ZEB2 3ʹUTR, which showed that miR-142-3p downregulation markedly (**p < 0.01) increased the luciferase activity of ZEB2 3ʹUTR-Wt rather than that of ZEB2 3ʹUTR-Mut ()). In conclusion, miR-142-3p targets ZEB2 in THP-1 cells.
Figure 4. MiR-142-3p targets ZEB2. (a) The miRDB website was employed to predict potential target genes (ZEB2, TASOR2, RICTOR and RHOBTB3) of miR-142-3p. (b) The expression of these potential target genes was detected in THP-1 cells after transfection with miR-142-3p inhibitor or NC inhibitor using RT-qPCR. (c) ZEB2 protein level in THP-1 cells transfected with NC inhibitor or miR-184-3p inhibitor was tested by Western blotting. (d-e) Three binding sites between miR-142-3p and ZEB2 3ʹUTR were predicted by Targetscan and position 319–325 of ZEB2 3ʹUTR are highly conserved among multiple species. (f) Luciferase reporter assay was performed to examine the interaction between miR-142-3p and ZEB2 in THP-1 cells. **p < 0.01.
ZEB2 is upregulated in GA
Subsequently, ZEB2 expression in GA cell model and mouse model was examined. As ) indicated, ZEB2 mRNA and protein levels were significantly (**p < 0.01, ***p < 0.001) decreased in MSU-stimulated THP-1 cells compared with those in the CTRL group. GA mice exhibited significantly (**p < 0.01) decreased ZEB2 expression at the mRNA and protein levels in the ankle tissues compared to mice in the sham group (). In addition, Pearson correlation analysis showed that miR-142-3p expression was negatively (R2 = 0.155, p < 0.05) correlated with ZEB2 expression in ankle tissues of GA mice ()). All these findings suggested ZEB2 plays a potential role in GA.
Figure 5. ZEB2 is upregulated in GA. (a-c) ZEB2 mRNA and protein levels in THP-1 cells with or without MSU treatment were investigated using RT-qPCR and Western blotting. (d-f) ZEB2 mRNA and protein levels in the sham and model groups were investigated using RT-qPCR and Western blotting. (g) Correlation between miR-142-3p expression and ZEB2 expression in ankle tissues of GA mice was analyzed by Pearson correlation analysis. **p < 0.01, ***p < 0.001.
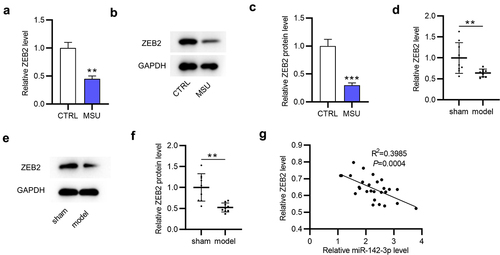
ZEB2 knockdown reverses the suppressive impact of miR-142-3p silencing on inflammatory response in MSU-treated THP-1 cells
To investigate whether the miR-142-3p/ZEB2 axis participates in MSU-induced inflammation, rescue experiments were conducted. After transfection with sh-ZEB2, ZEB2 mRNA and protein levels were significantly (***p < 0.001) downregulated in MSU-stimulated THP-1 cells (). ZEB2 deficiency significantly (**p < 0.01) attenuated the inhibitory effect (**p < 0.01, ***p < 0.001) of miR-142-3p knockdown on proinflammatory factor production in MSU-treated THP-1 cells, as suggested by ELISA (). Consistently, the outcome of RT-qPCR revealed that miR-142-3p silencing markedly (**p < 0.01, ***p < 0.001) decreased the mRNA expression of inflammatory cytokines in MSU-stimulated THP-1 cells, which was significantly (**p < 0.01) neutralized by ZEB2 downregulation (). Collectively, ZEB2 knockdown reverses the suppressive effect of miR-142-3p silencing on inflammatory response in MSU-treated THP-1 cells.
Figure 6. ZEB2 deficiency reverses the suppressive effect of miR-142-3p deficiency on inflammatory response in MSU-administrated THP-1 cells. (a-c) ZEB2 mRNA and protein levels in MSU-treated THP-1 cells transfected with sh-ZEB2 or sh-NC were investigated via RT-qPCR and Western blotting. (d-f) Proinflammatory cytokine release in MSU-treated THP-1 cells transfected with miR-NC, miR-142-3p inhibitor or miR-142-3p inhibitor+sh-ZEB2 was evaluated by ELISA. (g-i) Proinflammatory cytokine mRNA levels in MSU-treated THP-1 cells transfected with miR-NC, miR-142-3p inhibitor or miR-142-3p inhibitor+sh-ZEB2 were detected via RT-qPCR. **p < 0.01, ***p < 0.001.
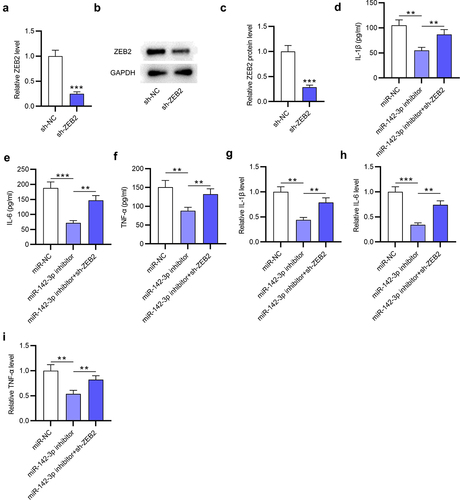
MiR-142-3p activates the NF-κB signaling via ZEB2
The NF-κB signaling plays a key role in regulating the inflammation cytokines [Citation24], and ZEB2 was demonstrated to regulate the NF-κB signaling in some diseases [Citation10]. To explore the effect of the miR-142-3p/ZEB2 axis on the NF-κB signaling in THP-1 cells, protein levels of factors (p-p65, p65, IκBα and p-IκBα) involved in NF-κB pathway were detected by Western blotting. As ) indicated, p-p65 and p-IκBα protein levels were significantly (**p < 0.01) increased in MSU-treated THP-1 cells compared to the CTRL group, suggesting that MSU stimulation in THP-1 cells activated the NF-κB signaling. Meanwhile, the decrease in p-p65 and p-IκBα protein levels that was caused by miR-142-3p knockdown was significantly (*p < 0.05, **p < 0.01) counteracted by ZEB2 deficiency in MSU-administrated THP-1 cells (). The results demonstrated that the inactivation of the NF-κB signaling mediated by miR-142-3p silencing was attenuated by ZEB2 deficiency. All these results indicated that miR-142-3p activates the NF-κB signaling by negatively regulating ZEB2 expression in MSU-stimulated THP-1 cells.
Figure 7. MiR-142-3p activates the NF-κB pathway by targeting ZEB2. (a-c) Western blotting was performed to examine the IκBα, p-IκBα, p65 and p-p65 protein levels in THP-1 cells of different groups (CTRL, MSU, miR-NC, miR-142-3p inhibitor, miR-142-3p inhibitor+sh-ZEB2). *p < 0.05, **p < 0.01, ***p < 0.001.
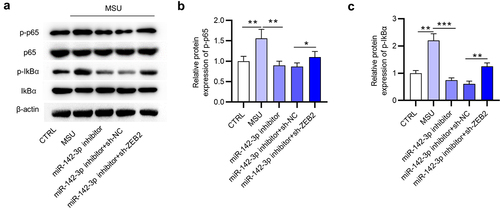
Schematic illustration for the potential mechanism of miR-142-3p mediating inflammatory response in GA in vitro
Collectively, our findings demonstrate the critical role of miR-142-3p in MSU-triggered inflammatory injury. According to the schematic illustration, we can know that MSU administration elevates miR-142-3p expression and miR-142-3p activates the NF-κB signaling by negatively regulating ZEB2, subsequently accelerating cellular inflammatory response in THP-1 cells (). Our findings may provide a new strategy to prevent excessive inflammation in GA.
Figure 8. Schematic illustration shows the mechanism of miR-142-3p mediating inflammatory response in GA in vitro. After MSU administration, miR-142-3p is upregulated in THP-1 cells. miR-142-3p directly targets ZEB2 to activate the NF-κB signaling, subsequently promoting cellular inflammatory response.
Discussion
GA is a common arthropathy characterized by recurrence of joint inflammation in response to MSU stimulation [Citation25]. MSU crystals are crystalline forms of uric acid deposition in joints and other tissues, which induce the pro-inflammatory factor release and subsequently lead to aseptic inflammation [Citation26]. Herein, MSU was applied to treat THP-1 cells and C57BL/6 mice to induce gouty inflammation, and thus to explore the role of miR-142-3p in regulating MSU-stimulated inflammatory response.
Multiple miRNAs have been verified to be implicated in GA pathogenesis, including miR-221-5p [Citation27], miR-146a [Citation28], miR-488 and miR-920 [Citation29]. Studies have revealed the regulation of miR-142-3p in different types of arthritis, such as osteoarthritis [Citation30], rheumatoid arthritis [Citation31] as well as GA [Citation19]. MiR-142-3p has been demonstrated to be elevated in the plasma of patients diagnosed with gout [Citation19]. Consistently, herein, miR-142-3p expression was elevated in MSU-treated GA mice and THP-1 cells, suggesting that miR-142-3p participates in GA progression. Accumulating evidence has confirmed that miR-142-3p plays a pivotal role in other inflammation-associated diseases. MiR-142-3p can act as anti-inflammatory gene in some pathological processes. For example, miR-142-3p upregulation alleviates inflammatory response of lipopolysaccharide (LPS)-treated human periodontal ligament cells by increasing pro-inflammatory factor secretion in periodontitis [Citation32]. MiR-142-3p overexpression improves cardiac function by coronary microembolization-induced inflammation in myocardiac injury [Citation33]. However, miR-142-3p can also exert pro-inflammatory effects under other conditions. MiR-142-3p inhibition protects human keratinocyte HaCaT cells against inflammation by targeting Sema3A in psoriasis [Citation34]. MiR-142-3p is upregulated in systemic lupus erythematosus (SLE) and overexpressing miR-142-3p in monocyte-derived dendritic cells increases SLE-related cytokines, including TNF-α, interleukin 6 (IL-6), C-X-C motif chemokine ligand 8 (CXCL8), C-C motif chemokine ligand 5 (CCL5) and C-C motif chemokine ligand 2 (CCL2) [Citation35]. Therefore, miR-142-3p has different regulatory effects on inflammation in different diseases. Herein, miR-142-3p knockdown inhibited inflammatory response in THP-1 cells and mice treated with MSU, which were evidenced by the decrease in proinflammatory cytokine production in cells and the inhibition of inflammatory cell infiltration in the ankle tissues Hence, we concluded that miR-142-3p facilitates MSU-induced inflammation in both in vitro and in vivo GA models.
Zinc finger E-box binding homeobox 2 (ZEB2) was identified as the target of miR-142-3p in this study. ZEB2, also known as SIP1, SIP-1, HSPC082, ZFHX1B or SMADIP1, was firstly found in 1999. ZEB2 belongs to the ZEBs protein family and interacts with Smad proteins [Citation36,Citation37]. Growing evidence has confirmed that ZEB2 can modulate hypoxia signals and proinflammatory cytokines and mediate the epithelial-mesenchymal transition (EMT) process [Citation38]. ZEB2 has been demonstrated to be a mediator of inflammation in some diseases. For example, ZEB2 elevation inhibits the proinflammatory cytokine production in LPS-mediated L-02 cells in acute liver injury [Citation39]. Conversely, upregulation of ZEB2 increases cellular senescence and inflammatory responses in pulmonary emphysema [Citation40]. Hence, ZEB2 can act as a suppressor or activator of inflammation. In our study, ZEB2 was downregulated in MSU-stimulated THP-1 cells and GA mice. Furthermore, ZEB2 expression was negatively correlated with miR-142-3p expression in ankle tissues of GA mice. Functionally, ZEB2 knockdown reversed the restrictive effects of miR-142-3p silencing on inflammation in MSU-administrated THP-1 cells, suggesting that ZEB2 repressed inflammation in GA. From all findings from our study and previous reports, we speculated that different cellular environment of different diseases may be responsible for the duple effects of ZEB2 on inflammatory response. More experiments should be conducted to verify this phenomenon in the future.
ZEB2 was reported to attenuate inflammation via the NF-κB signaling in HK-2 cells [Citation38]. The activation of NF-κB signaling is the core mediator of the inflammatory response [Citation41] and is also closely related to MSU-triggered inflammation [Citation42]. Detecting expression levels of NF-κB signaling-associated genes are very important for the exploration of molecules or drugs in GA treatment [Citation43]. For example, curcumin effectively alleviates MSU-caused inflammation via repressing IκBα degradation and NF-κB signaling activation in THP-1 cells [Citation42]. Heat shock protein 60 (HSP60) exacerbates mitochondrial dysfunction and activates the TLR4-MyD88-NF-κB signaling in MSU-induced GA [Citation44]. Herein, MSU stimulation significantly increased p-p65 and p-IκBα protein levels in THP-1 cells, indicating that MSU activated NF-κB signaling in GA. MiR-142-3p knockdown suppressed the phosphorylation of p65 and IκBα in THP-1 cells with MSU-treatment, which was counteracted by ZEB2 depletion. Therefore, we concluded that miR-142-3p activates the NF-κB signaling by targeting ZEB2 in GA.
In conclusion, miR-142-3p promotes MSU-induced inflammatory response by targeting ZEB2 to activate the NF-κB signaling. Our findings highlighted the potential role of the miR-142-3p/ZEB2/NF-κB axis in anti-inflammation therapy for GA treatment. However, improvements should be made in this study. For example, the upstream mechanism of miR-142-3p in GA and whether the miR-142-3p/ZEB2 axis can modulate other signaling pathways in GA are worth to be further explored.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data available within the article or its supplementary materials.
Additional information
Funding
References
- He M, Hu C, and Chen M, et al. Effects of gentiopicroside on activation of NLRP3 inflammasome in acute gouty arthritis mice induced by MSU. J Nat Med. 2022;76(1):178–187 .
- Patil T, Soni A, Acharya S. A brief review on in vivo models for gouty arthritis. Metabol Open. 2021 Sep;11:100100.
- Singh JA, Gaffo A. Gout epidemiology and comorbidities. Semin Arthritis Rheum. 2020 Jun;50(3s):S11–s16.
- Senthelal S, Li J, Goyal A, et al. Arthritis. Treasure Island (FL): StatPearls Publishing. 2021. StatPearls. Copyright © 2021, StatPearls Publishing LLC.
- Hsu DZ, Chen SJ, Chu PY, et al. Therapeutic effects of sesame oil on monosodium urate crystal-induced acute inflammatory response in rats. Springerplus. 2013;2:659.
- Pascual E, Pedraz T. Gout. Curr Opin Rheumatol. 2004 May;16(3):282–286.
- Jeong JH, Hong S, Kwon OC, et al. CD14(+) cells with the phenotype of infiltrated monocytes consist of distinct populations characterized by anti-inflammatory as well as pro-inflammatory activity in gouty arthritis. Front Immunol. 2017;8:1260.
- Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006 Mar 9;440(7081):237–241.
- Ghosh P, Cho M, Rawat G, et al. Treatment of acute gouty arthritis in complex hospitalized patients with anakinra. Arthritis Care Res (Hoboken). 2013 Aug;65(8):1381–1384.
- Yang J, Wu M, Fang H, et al. Puerarin prevents acute liver injury via inhibiting inflammatory responses and ZEB2 expression. Front Pharmacol. 2021;12:727916.
- Mead B, Tomarev S. The role of miRNA in retinal ganglion cell health and disease. Neural Regen Res. 2022 Mar;17(3):516–522.
- Ma T, Liu X, Cen Z, et al. MicroRNA-302b negatively regulates IL-1β production in response to MSU crystals by targeting IRAK4 and EphA2. Arthritis Res Ther. 2018 Feb 26;20(1):34.
- Wang X, Chi J, Dong B, et al. MiR-223-3p and miR-22-3p inhibit monosodium urate-induced gouty inflammation by targeting NLRP3. Int J Rheum Dis. 2021 Apr;24(4):599–607.
- Jin HM, Kim TJ, Choi JH, et al. MicroRNA-155 as a proinflammatory regulator via SHIP-1 down-regulation in acute gouty arthritis. Arthritis Res Ther. 2014 Apr 7;16(2):R88.
- Mandolesi G, De Vito F, Musella A, et al. miR-142-3p is a key regulator of IL-1β-dependent synaptopathy in neuroinflammation. J Neurosci. 2017 Jan 18;37(3):546–561.
- Qing X, Zhang Y, Peng Y, et al. Mir-142-3p regulates inflammatory response by contributing to increased TNF-α in chronic rhinosinusitis with nasal polyposis. Ear Nose Throat J. 2021 Jan;100(1): Np50–np56.
- Zhu L, Shi Y, Liu L, et al. Mesenchymal stem cells-derived exosomes ameliorate nucleus pulposus cells apoptosis via delivering miR-142-3p: therapeutic potential for intervertebral disc degenerative diseases. Cell Cycle. 2020 Jul;19(14):1727–1739.
- Meng D, Li J, Li H, et al. Salvianolic acid B remits LPS-induced injury by up-regulating miR-142-3p in MH7A cells. Biomed Pharmacother. 2019;115:108876.
- Bohatá J, Horváthová V, Pavlíková M, et al. Circulating microRNA alternations in primary hyperuricemia and gout. Arthritis Res Ther. 2021 Jul 10;23(1):186.
- Meng Q, Meng W, Bian H, et al. Total glucosides of paeony protects THP-1 macrophages against monosodium urate-induced inflammation via MALAT1/miR-876-5p/NLRP3 signaling cascade in gouty arthritis. Biomed Pharmacother. 2021;138:111413.
- Erster O, Mendelson E, and Levy V, et al. Rapid and high-throughput reverse transcriptase quantitative PCR (RT-qPCR) assay for identification and differentiation between SARS-CoV-2 variants B.1.1.7 and B.1.351. Microbiol Spectr. 2021;9((2): ;e0050621.
- Mishra M, Tiwari S, Gunaseelan A, et al. Improving the sensitivity of traditional Western blotting via streptavidin containing poly-horseradish peroxidase (PolyHRP). Electrophoresis. 2019 Jul;40(12–13):1731–1739.
- Brzuzan P, Mazur-Marzec H, Florczyk M, et al. Luciferase reporter assay for small-molecule inhibitors of MIR92b-3p function: screening cyanopeptolins produced by Nostoc from the Baltic Sea. Toxicol In Vitro. 2020;68:104951.
- Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013 Aug 2;12:86.
- Chen H, Zhou GX, He XJ, et al. Efficacy of fire needle on acute gouty arthritis induced by monosodium urate in rat. J Tradit Chin Med. 2021 Aug;41(4):564–570.
- Wang Y, Zhu W, Lu D, et al. Tetrahydropalmatine attenuates MSU crystal-induced gouty arthritis by inhibiting ROS-mediated NLRP3 inflammasome activation. Int Immunopharmacol. 2021 Sep 3;100:108107.
- Li G, Zhang H, Ma H, et al. MiR-221-5p is involved in the regulation of inflammatory responses in acute gouty arthritis by targeting IL-1β. Int J Rheum Dis. 2021 Mar;24(3):335–340.
- Chen X, Gao Q, Zhou L, et al. MiR-146a alleviates inflammation of acute gouty arthritis rats through TLR4/MyD88 signal transduction pathway. Eur Rev Med Pharmacol Sci. 2019 Nov;23(21):9230–9237.
- Zhou W, Wang Y, Wu R, et al. MicroRNA-488 and −920 regulate the production of proinflammatory cytokines in acute gouty arthritis. Arthritis Res Ther. 2017 Sep 15;19(1):203.
- Gao Y, Zhao H, Li Y. LncRNA MCM3AP-AS1 regulates miR-142-3p/HMGB1 to promote LPS-induced chondrocyte apoptosis. BMC Musculoskelet Disord. 2019 Dec 13;20(1):605.
- Renman E, Brink M, and Ärlestig L, et al. Dysregulated microRNA expression in rheumatoid arthritis families-a comparison between rheumatoid arthritis patients, their first-degree relatives, and healthy controls. Clin Rheumatol. 2021 Jun ;40(6 2387–2394).
- Hu HY, Wang JQ. [MiR-142-3p inhibitslipopolysaccharide-induced inflammatory response in human periodontal ligament cells through targeting IRAK1]. Shanghai Kou Qiang Yi Xue. 2016 Dec;25(6):682–687.
- Su Q, Lv X, Ye Z, et al. The mechanism of miR-142-3p in coronary microembolization-induced myocardiac injury via regulating target gene IRAK-1. Cell Death Dis. 2019 Jan 25;10(2):61.
- Zhang D, Wang Y, Xia Y, et al. Repression of miR-142-3p alleviates psoriasis-like inflammation by repressing proliferation and promoting apoptosis of keratinocytes via targeting Sema3A. Mol Cell Probes. 2020;52:101573.
- Wang Y, Liang J, Qin H, et al. Elevated expression of miR-142-3p is related to the pro-inflammatory function of monocyte-derived dendritic cells in SLE. Arthritis Res Ther. 2016 Nov 16;18(1):263.
- Verschueren K, Remacle JE, Collart C, et al. SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5’-CACCT sequences in candidate target genes. J Biol Chem. 1999 Jul 16;274(29):20489–20498.
- Rasouly HM, Kumar S, Chan S, et al. Loss of Zeb2 in mesenchyme-derived nephrons causes primary glomerulocystic disease. Kidney Int. 2016 Dec;90(6):1262–1273.
- Ding Q, Wang Y, Zhang AL, et al. ZEB2 attenuates LPS-induced inflammation by the NF-κB pathway in HK-2 cells. Inflammation. 2018 Mar;41(2):722–731.
- Yang J, Xu L, Wu M, et al. Paeonol derivative-6 attenuates inflammation by activating ZEB2 in acute liver injury. Int Immunopharmacol. 2021;91:107235.
- Shen Z, Xuan W, Wang H, et al. miR-200b regulates cellular senescence and inflammatory responses by targeting ZEB2 in pulmonary emphysema. Artif Cells Nanomed Biotechnol. 2020 Dec;48(1):656–663.
- Ghosh S, and Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002 Apr;109 Suppl: S81–S96 .
- Chen B, Li H, Ou G, et al. Curcumin attenuates MSU crystal-induced inflammation by inhibiting the degradation of IκBα and blocking mitochondrial damage. Arthritis Res Ther. 2019 Aug 27;21(1):193.
- Wang Z, Zhao Y, Phipps-Green A, et al. Differential DNA methylation of networked signaling, transcriptional, innate and adaptive immunity, and osteoclastogenesis genes and pathways in gout. Arthritis Rheumatol. 2020 May;72(5):802–814.
- Huang Q, Gao W, Mu H, et al. HSP60 regulates monosodium urate crystal-induced inflammation by activating the TLR4-NF-κB-MyD88 signaling pathway and disrupting mitochondrial function. Oxid Med Cell Longev. 2020;2020:8706898.
