ABSTRACT
Arecoline, the most abundant alkaloid of the areca nut, induces toxicity to neurons. Hydrogen sulfide (H2S) is an endogenous gas with neuroprotective effects. We recently found that arecoline reduced endogenous H2S content in PC12 cells. In addition, exogenously administration of H2S alleviated the neurotoxicity of arecoline on PC12 cells. Increasing evidence has demonstrated the neuroprotective role of improvement of autophagic flux. Therefore, the aim of the present work is to explore whether improvement of autophagic flux mediates the protection of H2S against arecoline-caused neurotoxicity. Transmission electron microscope (TEM) for observation of ultrastructural morphology. Western blotting was used to detect protein expression of the related markers. Functional analysis contained LDH release assay, Hoechst 33,258 nuclear staining and flow cytometry were used to detect cytotoxicity and apoptosis. In the present work, we found that arecoline disrupted autophagy flux in PC12 cells as evidenced by accumulation of autophagic vacuoles, increase in LC3II/LC3I, and upregulation of p62 expression in PC12 cells. Notably, we found that sodium hydrosulfide (NaHS), the donor of H2S improved arecoline-blocked autophagy flux in PC12 cells. Furthermore, we found that blocking autophagic flux by chloroquine (CQ), the inhibitor of autophagy flux, antagonized the inhibitory role of NaHS in arecoline-induced cytotoxicity apoptosis and endoplasmic reticulum (ER) stress. In conclusion, H2S improves arecoline-caused disruption of autophagic flux to exert its protection against the neurotoxicity of arecoline.
Introduction
About 600 million persons are chewing areca nut in the word[Citation1]. Arecoline, the predominant alkaloid of the areca nut, readily crosses the blood–brain barrier and possesses parasympathomimetic properties with stimulation of muscarinic and nicotinic receptors, influencing on the central and the autonomic nervous systems. Remarkably, arecoline also shows potential effects in patients with schizophrenia [Citation2], Alzheimer dementia (AD) [Citation3] as well as cerebral ischemia [Citation4]. Recently, the neuron toxicity of arecoline has been reported. Shih et al. found that arecoline increases reactive oxygen species and activates NADPH oxidase, attenuates antioxidant defense in neurons by decreasing glutathione level and superoxide dismutase activity. Additionally, arecoline enhances apoptosis by upregulating proapoptotic proteins including cytochrome c, Bax, caspase-9 and caspase-3, and downregulating the antiapoptotic protein Bcl-2 in neurons [Citation5]. We recently also confirmed the neurotoxic effects of arecoline on PC12 cells [Citation6]. Therefore, it is of great clinical significance to reduce neurotoxicity of arecoline for prevention and treatment of arecoline-associated neurological diseases.
Hydrogen sulfide (H2S) has been defined as the third endogenous gaseous molecule in company with nitric oxide (NO) and carbon monoxide (CO) [Citation7,Citation8]. Several lines of literatures have demonstrated that H2S plays neuroprotective roles in anti-oxidation [Citation9], anti-apoptosis [Citation10,Citation11], and anti-ER stress [Citation12,Citation13]. Interestingly, our recent study has documented the protective function of H2S against the neurotoxicity caused by arecoline [Citation6]. However, the mechanism underlying the protection of H2S against arecoline-induced neurotoxicity remains unknown.
Autophagy is a highly conserved intracellular lysosome-dependent process for eliminating and recycling the long-lived proteins and damaged organelles. The rate of the cytoplasmic cargo degradation through autophagy is referred to as autophagic flux, which is the dynamic process of autophagy and a measure of autophagic degradation activity [Citation14]. During the process of autophagy, autophagosomes formation, and functional autophagic flux is required to clearance of autophagic cargo [Citation15]. It is well reported that high autophagic flux is a critical characteristic of neurons [Citation16]. Increasing evidence has demonstrated that impaired autophagic flux in the central nervous system contributes to the aggregates of abnormal proteins, axonal degeneration as well as neuronal death, and enhancing autophagic flux may exert neuroprotection [Citation16–18]. In the present study, we explored whether autophagic flux is involved in H2S-elicited neuroprotective function against the neurotoxicity of arecoline.
In the present study, we demonstrated that arecoline disrupted autophagic flux and sodium hydrosulfide (NaHS), the donor of H2S restored autophagic flux in arecoline-treated PC12 cells. Furthermore, chloroquine (CQ), the inhibitor of autophagic flux, reversed the protection of NaHS against toxicity, apoptosis, and endoplasmic reticulum (ER) stress in arecoline-induced PC12 cells. Our findings for the first time demonstrate that H2S enhances arecoline-impaired autophagic flux, which contributes to the protective effects of H2S against the neurotoxicity of arecoline.
Materials and methods
Reagents
Arecoline, sodium hydrosulfide (NaHS), and chloroquine (CQ) were procured from Sigma (St. Louis, MO, USA). The lactic dehydrogenase (LDH) release assay kit is purchased from USCN Life Science Inc. (Wuhan, China). FITC Annexin V Apoptosis Detection Kit is supplied by BD Biosciences (San Jose, CA, USA). Hoechst 33,258 is provided by Beyotime Biotechnology (Shanghai, China).
Cell culture and grouping
Differentiated PC12 cells were kindly supplied by the Experimental Animal Center of Sun Yat-Sen University (Guangzhou, China). PC12 cells were cultured in Dulbecco’s modified Eagle’s Medium (DMEM; GibicoBRL, Ground Island, USA) containing 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin-streptomycin and grown in a humidified incubator maintained at 37°C and 5% CO2. The cell culture media was changed every other day. The cells were, respectively, treated with 0.5, 1, and 2 mM of arecoline, 100, 200 and 400 μM of sodium hydrosulfide (NaHS), 10 μM of chloroquine (CQ), arecoline (1 mM) + CQ (10 μM), arecoline (1 mM) + NaHS (400 μM), arecoline (1 mM) + NaHS (400 μM) + CQ (10 μM) based on the different experimental purposes.
Lactate dehydrogenase (LDH) release assay for analysis of cytotoxicity
PC12 cells were seeded in 6-well (1 x 106 cells per well). When the cell density reached up to 70–80%, cells were incubated with the different drugs for 24 h. After centrifugation at 1000 g for 20 min, cell culture supernatants were collected. Briefly, 50 μL of supernatants were added to the 96-well pre-coated with an antibody specific to LDH and incubated at 37°C for 2 h. The liquid of each well was removed without wash. 100 μL of prepared detection reagent A was added to each well and incubated for 1 h at 37°C. After washing 3 times, 100 μL of prepared detection reagent B was added into each well and incubated for 30 min at 37°C. After washing 5 times, 90-μL substrate solution was added to all wells and incubated for 10–20 min at 37°C in the dark. The liquid will turn blue by adding substrate solution. Subsequently, 50 μL of stop solution was added to each well and the liquid will appear yellow. Immediately, the absorbance of each well was measured using the microplate reader at a wavelength of 450 nm.
Annexin V-FITC/PI staining for assessment of apoptosis
PC12 cells were seeded in 6-well (1 x 106 cells per well). After being subjected to the experimental treatments, the culture supernatants were collected, and the cells were digested with trypsin followed by harvested into the tubes containing the corresponding cell supernatant. The tubes were then centrifugated at 1000 g for 5 min and cells were collected at a density of 105 cells/tube. Subsequently, cells were washed with PBS three times and re-suspended in binding buffer. Following that step, 5 µl of Annexin- V-FITC labeling solution and 5 µl of PI solution were added to the mixture and incubated in the dark at room temperature for 20 min and then analyzed with a flow cytometer (BD Biosciences, San Jose, CA, USA) [Citation19].
Hoechst 33,258 nuclear staining for evaluation of apoptosis
PC12 cells were plated in 24-well at a density of 1 × 105 cells per well. After the cell concentration reached up to 70–80%, cells were treated with the indicated drugs for 24 h. Cells were fixed with 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) at 4°C for 10 min. After washing three times with PBS, the cells were stained with Hoechst 33,258 for 10 min. After three rinses with PBS, the PC12 cells were observed under a florescence microscope (Bx50-FLA; Olympus, Tokyo, Japan). Viable cells displayed a uniform blue fluorescence throughout the nucleus and normal nuclear size. However, apoptotic cells showed condensed, distorted or fractured nuclei [Citation20].
Transmission electron microscope (TEM) for observation of ultrastructural morphology
After different treatment, PC12 cells were placed in 2.5% glutaraldehyde fixing solution and dehydrated through an ascending ethanol series. Subsequently, the cells were placed in a dilute solution of plastic embedding medium epoxy resin mixture. Sections 70-nm thick were embedded for orientation purposes and then stained with uranyl acetate and lead citrate. Changes in ultrastructure were examined using a JEM-1200EX transmission electron microscope (JEOL Ltd., Tokyo, Japan) [Citation21].
Western blotting analysis for expressions of LC3, p62, GRP78, and cleaved caspase-12
Following drug treatment, PC12 cells were harvested and lyzed with cell lysis solution at 4°C for 30 min. The lysates were centrifugated at 12,000 g for 10 min at 4°C. Subsequently, the supernatants were collected. Total proteins were quantified using a BCA Protein Assay Kit (Solarbio, Beijing, China). After addition of loading buffer, the cytosolic extracts were denatured for 5 min at 100°C. Equal amounts of the samples were run on 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then blotted onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% fat-free milk in fresh blocking buffer [0.1% Tween-20 in Tris-buffered saline (TBS-T) at room temperature for 2 h, and subsequently incubated with primary antibodies such as anti-LC3 antibody (4108S, 1:1000 dilution), anti-p62 antibody (39749S, 1:1000 dilution), anti-GRP78 (3183S, 1:1000 dilution), and anti-caspase-12 antibody (ab62484, 1:2000 dilution) in freshly prepared TBS-T with 5% free fat milk with gentle agitation at 4°C overnight. β-actin (20,536-1-AP, 1:2000 dilution) was served as loading control. Following washing for 10 min with TBS-T three times, the membranes were incubated with HRP-conjugated secondary antibodies (mouse and rabbit; SA00001-1 and SA00001-2, 1:5000 dilution) at room temperature for 2 h. The membranes were again washed with TBS-T, and visualized with the enhanced chemiluminescence kit (Millipore, Billerica, MA, USA). Gray value analysis of protein bands was used by Image J software, which was relative to the control group. β-actin was regarded as the loading control [Citation22].
Statistical analysis
Measured data conforming to the normality of distribution were test using K-S test. The data were shown as mean ± standard error of means (S.E.M.). Differences between groups were analyzed by one-way analysis of variance (ANOVA) by using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) and followed by LSD post hoc comparison tests. Statistical significance was set at P < 0.05.
Results
Arecoline blocks the autophagic flux in PC12 cells
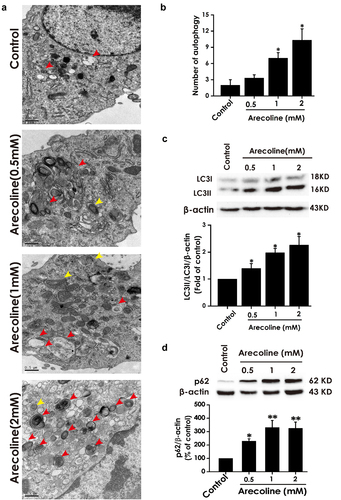
We first explored the effect of arecoline on the autophagic flux in PC12 cells. As illustrated in , comparing to the control group, treatment of PC12 cells with arecoline (1 mM or 2 mM) for 24 h, the numbers of autophagic vacuoles including autophagosomes and autolysosomes were markedly increased. LC3 (LC3 I and LC3II) as well as p62 protein were the two key molecules spotlighted in autophagic events. We also found that treatment with arecoline (0.5, 1, and 2 mM) obviously upregulated LC3II/LC3I () and p62 protein () in PC12 cells. These data suggested arecoline impairs the autophagic flux of PC12 cells.
Figure 1. Effect of arecoline on the level of autophagy in PC12 cells. PC12 cells were exposed to arecoline (0.5, 1, and 2 mM) for 24 h. (a) Images show ultrastructural analysis by transmission electron microscope (TEM), which indicate the typical morphological changes of autophagy. Red arrows show autophagosomes and yellow arrows indicate autolysosomes. (b) Statistical analysis of the number of autophagy including autophagosomes and autolysosomes in A. LC3 protein (as indicated by LC3II and LC3I) (c) and p62 protein (d) expressions were measured by Western blot using anti-LC3 antibody and anti-p62 antibody, respectively. In all blots, β-actin was used as a loading control. Data are representative images of three independent experiments and values are the mean ± S.E.M., *P < 0.05, **P < 0.01, versus control group.
CQ treatment does not reverse autophagic flux in arecoline-exposed PC12 cells
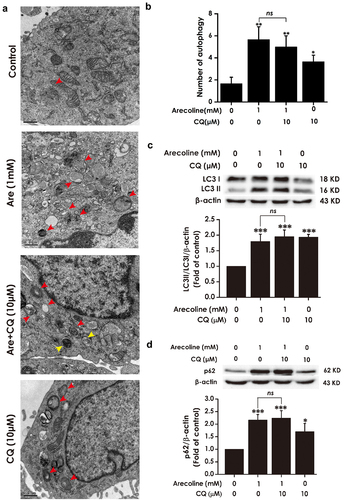
In the following test, we investigated the effect of the autophagic flux inhibitor CQ on the autophagic flux in arecoline-induced PC12 cells. As shown in , the number of autophagic vacuoles, the ratio of LC3II/LC3I, and the level of p62 protein in arecoline (1 mM) co-treated with CQ (10 μM) in PC12 cells remain unchanged compared to arecoline (1 mM)-induced group. CQ alone resulted in accumulation of autophagic vacuoles and increases in LC3II/LC3I as well as p62 level relative to the control group. Taken together, these findings conformed that arecoline blocked the autophagic flux in PC12 cells.
Figure 2. Effect of CQ on arecoline-disrupted autophagic flux of PC12 cells. PC12 cells were preincubated with CQ (10 μM) for 30 min and then co-treated with arecoline (1 mM) for 24 h. (a) Images show ultrastructural analysis by transmission electron microscope (TEM), which indicate the typical morphological changes of autophagy. Red arrows show autophagosomes and yellow arrows indicate autolysosomes. (b) Statistical analysis of the number of autophagy including autophagosomes and autolysosomes in A. LC3 protein (as indicated by LC3II and LC3I) (c) and p62 protein (d) expressions were measured by Western blot using anti-LC3 antibody and anti-p62 antibody, respectively. Data are representative images of three independent experiments and values are the mean ± S.E.M., *P < 0.05, ***P < 0.001, versus control group. ns, means no significant difference.
H2S enhances the autophagic flux of PC12 cells
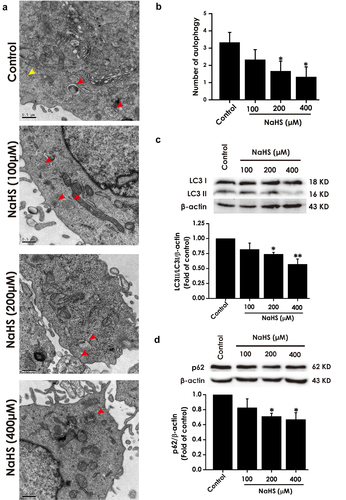
Next, we investigated the effect of H2S on the autophagic flux in PC12 cells. As illustrated in , 200 and 400 μM of sodium hydrosulfide (NaHS), the donor of H2S, reduced the number of autophagic vacuoles, decreased the ratio of LC3II/LC3I, and downregulated the expression of p62 in PC12 cells when compared to the control group, indicating that H2S promoted the autophagic flux in PC12 cells.
Figure 3. Effect of H2S on autophagic flux of PC12 cells. PC12 cells were treated with NaHS (100 μM, 200 μM and 400 μM) for 24 h. (a) Images show ultrastructural analysis by transmission electron microscope (TEM), which indicate the typical morphological changes of autophagy. Red arrows show autophagosomes and yellow arrows indicate autolysosomes. (b) Statistical analysis of the number of autophagy including autophagosomes and autolysosomes in A. LC3 protein (as indicated by LC3II and LC3I) (c) and p62 protein (d) expressions were measured by Western blot using anti-LC3 antibody and anti-p62 antibody, respectively. Data are representative images of three independent experiments and values are the mean ± S.E.M., *P < 0.05, **P < 0.01, versus control group.
H2S rescues arecoline-disrupted autophagic flux in PC12 cells
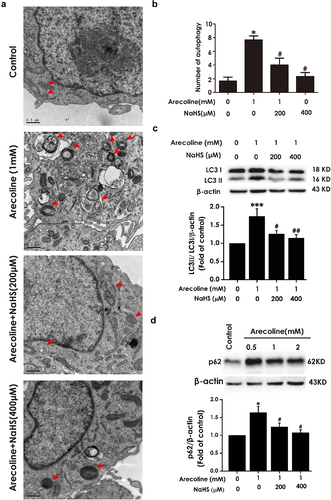
We also explored the effect of H2S on arecoline-blocked autophagic flux in PC12 cells. As shown in , the numder of autophagic vacuoles the rate of LC3II/LC3I and the levelof p62 expression were reduced in arecoline co-treated with NaHS in PC12 cells relative to arecoline-treated group, indicating that H2S improves the autophagic flux in arecoline-treated PC12 cells.
Figure 4. Effect of H2S on arecoline-induced disruption of autophagic flux in PC12 cells. After pretreatment with NaHS (200 and 400 μM) for 30 min, PC12 cells were co-treated with arecoline (1 mM) for 24 h. (a) Images show ultrastructural analysis by transmission electron microscope (TEM), which indicates the typical morphological changes of autophagy. Red arrows show autophagosomes and yellow arrows indicate autolysosomes. (b) Statistical analysis of the number of autophagy including autophagosomes and autolysosomes in A. LC3 protein (as indicated by LC3II and LC3I) (c) and p62 protein (d) expressions were measured by Western blot using anti-LC3 antibody and anti-p62 antibody, respectively. Data are representative images of three independent experiments and values are the mean ± S.E.M., ***P < 0.001, versus control group; #P < 0.05, ##P < 0.01, versus arecoline-treated alone group.
Inhibition of autophagic flux attenuates H2S-improved the autophagic flux in arecoline-exposed PC12 cells
Next, we investigated whether CQ abrogates the improvement of H2S on autophagic flux in arecoline-caused PC12 cells. As illustrated in , comparing to the arecoline + NaHS group, the number of autophagic vacuoles, LC3II/LC3I ratio, and the level of p62 protein were elevated in arecoline + NaHS + CQ group, indicating that CQ prevents H2S to improve the autophagic flux in arecoline-exposed PC12 cells.
Figure 5. Effect of CQ on attention of H2S on arecoline-induced disruption of autophagic flux in PC12 cells. PC12 cells were pretreated with CQ (10 μM) for 30 min before incubation with NaHS (400 μM) for 30 min prior to 24 h exposure of arecoline (1 mM). (a) Images show ultrastructural analysis by transmission electron microscope (TEM), which indicates the typical morphological changes of autophagy. Red arrows show autophagosomes and yellow arrows indicate autolysosomes. (b) Statistical analysis of the number of autophagy including autophagosomes and autolysosomes in A. LC3 protein (as indicated by LC3II and LC3I). (c) and p62 protein (d) expressions were measured by Western blot using anti-LC3 antibody and anti-p62 antibody, respectively. Data are representative images of three independent experiments and values are the mean ± S.E.M., *P < 0.05, **P < 0.01, versus control group; #P < 0.05, versus arecoline-treated alone group; &P < 0.05, versus co-treated with NaHS and arecoline group.
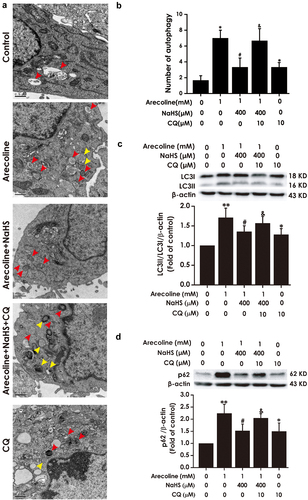
Inhibition of autophagic flux reserves the protective effect of H2S against cytotoxicity and apoptosis in arecoline-induced PC12 cells
The above-mentioned results hint that basal autophagic flux may mediate the protection of H2S against arecoline-induced neurotoxicity. Thus, we investigated whether the inhibition of autophagic flux by CQ antagonized the protective function of H2S against arecoline-induced neurotoxicity in PC12 cells to further determine that autophagic flux plays a mediatory role in H2S-elicited protection against the neurotoxicity of arecoline.
First, we detected the content of LDH in cell supernatants using Lactate dehydrogenase (LDH) release assay kit to explore the effect of pretreatment with CQ on the protection of H2S against the toxicity in arecoline-treated PC12 cells. Compared to the arecoline + NaHS group, LDH release was increased in arecoline + NaHS + CQ group (), which indicates that blocking autophagic flux could antagonize H2S-ameliorated cytotoxicity induced by arecoline in PC12 cells.
Figure 6. Effect of CQ on H2S-induced inhibition on arecoline-elicited apoptosis in PC12 cells. PC12 cells were pretreatment with CQ (10 μM) for 30 min before preincubated with NaHS (400 μM) for 30 min prior to 24-h exposure of arecoline (1 mM). The release of lactic dehydrogenase (LDH) was detected by LDH release assay (a). Quantitative analysis of the percentage of apoptotic cells by flow cytometry after Annexin V-FITC/PI staining (b). The nuclear morphology of cell stained with Hoechst 33,258 was observed by fluorescence microscopy (200×). Cells with brightly fluorescent and fragmented nuclei were apoptotic cells (c). Data are representative images of three independent experiments and values are the mean ± S.E.M., **P < 0.01, versus control group; #P < 0.05, versus arecoline-treated alone group; &P < 0.05, &&P < 0.01, versus co-treated with NaHS and arecoline group.
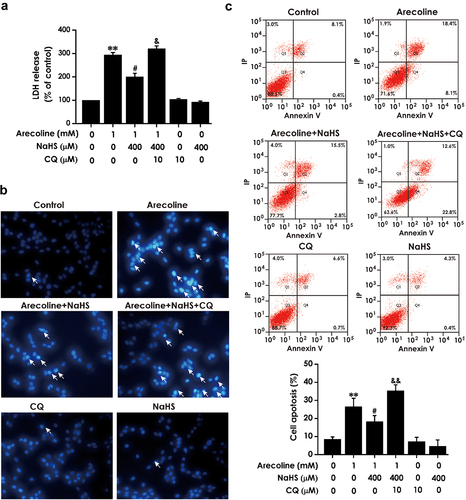
We further explored the effect of pretreatment with CQ on the protective effects of H2S- against the apoptosis induced by arecoline in PC12 cells. comparing to the arecoline + NaHS group, the cell apoptosis rate () and the fluorescence signal of Hoechst 33,258, which exhibits the typical apoptosis characteristics with nuclei fragmentation and chromatin condensation, were enhanced in arecoline + NaHS + CQ group (). These data reveal that suppressing autophagic flux could abolish the protection of H2S on arecoline-triggered cytotoxicity and apoptosis.
Inhibition of autophagic flux reserves the protective effect of H2S against arecoline-induced ER stress in PC12 cells
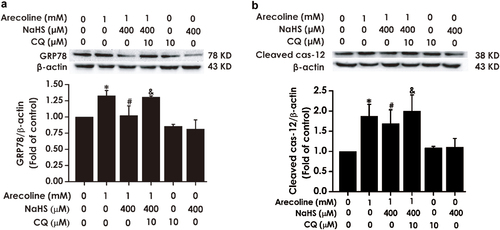
Finally, we explored the effect of pretreatment with CQ on the inhibitory effect of H2S on ER stress in arecoline-exposed PC12 cells. We measured the expressions of glucose-regulated protein 78 (GRP78) and Cleaved cysteinyl aspartate-specific proteinase-12 (Cleaved caspase-12), which are the molecular markers of ER stress [Citation13,Citation24]. As shown in , the expression levels of GRP78 and Cleaved caspase-12in in arecoline + NaHS + CQ group were upregulated in in arecoline + NaHS + CQ group relative to the arecoline + NaHS group, suggesting that disruption of autophagic flux antagonizes the inhibitory action of H2S on arecoline-induced ER stress.
Figure 7. Effect of CQ on the inhibitory role of H2S in arecoline-induced ER stress in PC12 cells. PC12 cells were preincubated with CQ (10 μM) for 30 min before treatment with NaHS (400 μM) for 30 min prior to 24 h exposure of arecoline (1 mM). The expressions of GRP78 (a) and Cleaved caspase-12 (b) proteins were measured by Western blot. Western blot images exhibit representative results from three independent experiments. In all blots, β-actin was used as a loading control. Values are expressed as the mean ± S.E.M., *P < 0.05, versus control group; #P < 0.05, versus arecoline-treated alone group; $P < 0.05, versus co-treated with NaHS and arecoline group.
Discussion
We recently showed that H2S exerts protective function against the neurotoxicity induced by arecoline. Emerging observations have documented that basal level or appropriate induction of autophagic flux has neuroprotective effects [Citation14,Citation25]. The purpose of the present work is to explore the mediated role of autophagic flux in the protection of H2S against arecoline-caused neurotoxicity. The main findings of the current study were the following: (1) arecoline blocks autophagic flux of PC12 cells; (2) NaHS, the donor of H2S, promotes arecoline-blocked autophagic flux in PC12 cells; (3) Inhibiting autophagic flux alleviates the protective actions of H2S against arecoline-induced cytotoxicity, apoptosis, and ER stress in PC12 cells. This is the first report that H2S rescues arecoline-caused impairment of autophagic flux, which contributes to the protection of H2S against the neurotoxicity of arecoline.
Arecoline is a naturally occurring psychoactive alkaloid isolated from betel nut and is considered as the major active ingredient of betel nut. Numerous researches have demonstrated that arecoline exhibits cytotoxicity [Citation26], neurotoxicity [Citation5], genotoxicity [Citation4] and immunotoxicity [Citation27]. Our recent study found that arecoline significantly inhibited the viability and elevated the apoptosis and ER stress of PC12 cells. H2S, a novel gaseous mediator, is reported to evoke neuroprotective effects [Citation10,Citation11]. We have previously also found that arecoline decreased the content of H2S in the culture supernatant and the expression levels of cystathionine b-synthase and 3-mercaptopyruvate sulfurtransferase, two major enzymes for endogenous H2S production [Citation6]. Moreover, exogenously increasing H2S level relieved the neurotoxicity of arecoline on PC12 cells. However, the mechanism underlying this protection of H2S remains unclear.
Autophagy is a highly conserved degradation process, which involves a sequential set of events encompassing double-membrane formation, elongation, vesicle maturation and ultimately delivery of the damaged cytoplasmic constituents to the lysosome [Citation28,Citation29]. Autophagy plays a critical role in maintenance of cellular homeostasis and can protect cells from multiple insults such as misfolded and aggregated proteins and impaired organelles, which is particularly vital to neuronal development, aging, survival, and death [Citation30]. In this study, to explore whether autophagy is involved in protection of H2S against the neurotoxicity of arecoline, we first observed the effects of arecoline on the autophagy levels of PC12 cells. TEM observations demonstrated that the ultrastructure of PC12 cells were impaired after arecoline administration, manifested by aggregation of autophagic vacuoles containing autophagosomes and autolysosomes. The level of LC3-II is generally considered as a marker for monitoring the progression of autophagy. The results showed that the levels of LC-3II/LC3I were heightened in arecoline-induced PC12 cells. Notably, owing to the dynamic process of autophagy, the accumulation of LC3-II or autophagosomes might be resulted from either enhancement of autophagy or blockage of autophagic degradation [Citation31]. Autophagic flux refers to the entire process of cargo moving through the autophagic system [Citation32]. Autophagy adaptor p62 protein (also known as SQSTM1) binds to ubiquitinated substrates as well as LC3, and is degraded in lysosomes after autophagosomes fuse with lysosomes. SQSTM1/p62 is essential to reflect the status of autophagic flux, and its accumulation indicates impairment of autophagic flux [Citation32]. We further manifested that p62 protein level was enhanced in arecoline-treated PC12 cells. Monitoring the conversion of LC3-I to LC3-II in the presence of lysosomal proteinase inhibitors is another commonly used method for detecting autophagic flux [Citation23]. If drugs-induced increases in the ratio of LC3II/LC3I remain unchanged in the presence of lysosomal protease inhibitors, it is possibly that drugs block autophagic degradation [Citation33]. We further observed that PC12 cells co-treated with CQ and arecoline did not change ratio of LC3II/LC3I compared to arecoline-treated group. Taken together, our results indicated that arecoline can cause the inhibition of autophagic flux.
Notably, our and other studies showed that H2S can promote autophagic flux and restore impairment of autophagic flux associated with cognitive damage in diabetic rats, neuronal senescence caused by high glucose and the neurotoxicity of acrylonitrile [Citation34–36]. Subsequently, we focused on the influence of H2S on impairment of autophagic flux induced by arecoline. Our data demonstrated that NaHS obviously increased autophagic flux and rescued autophagic flux disrupted by arecoline in PC12 cells. Emerging researches have indicated that autophagy, which plays a key role in cell survival and cell death, is closely associated to ER stress [Citation37]. Autophagy can prevent cells that are under ER from apoptosis by removing damaged organelles as well as misfolded/unfolded proteins. However, dysfunction of autophagy may result in several pathological conditions by inducing cell death [Citation38]. CQ, the lysosomotropic agent, can raise lysosomal pH and thereby preventing the digestive activity of hydrolases, contributing to inhibition of both the fusion of the autophagosome with the lysosome and the degradative activity of the autolysosomes [Citation39]. In the present study, we showed that inhibition of autophagic flux reserved improvement of H2S on autophagic flux in arecoline-exposed PC12 cells. We also show that inhibiting autophagy flux significantly ameliorated the effects of H2S on cytotoxicity, apoptosis, and ER stress caused by arecoline in PC12 cells. This is in line with previous findings suggesting that autophagy triggered by ER stress can relieve cell injury, and that inhibition of autophagy results in the misfolding or aggregation of proteins contributing to neuronal damage [Citation40]. Taken together, our data indicated that H2S is implicated in rescuing autophagy function to inhibit ER stress and thereby prevent neuronal apoptosis.
In summary, the present study demonstrates that H2S improves autophagic flux caused by arecoline, which mediates the protection of H2S against arecoline–induced neurotoxicity. Our data offer a helpful insight into the mechanism underlying H2S-exerted protection against the neurotoxicity of arecoline.
Data availability
The original data used to support the findings of this study are included in the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Oliveira NG, Ramos DL, Dinis-Oliveira RJ. Genetic toxicology and toxicokinetics of arecoline and related areca nut compounds: an updated review. Arch Toxicol. 2021;95:375–393.
- Bales A, Peterson MJ, Ojha S, et al. Associations between betel nut (Areca catechu) and symptoms of schizophrenia among patients in Nepal: a longitudinal study. Psychiatry Res. 2009;169:203–211.
- Sadashiva CT, Chandra JN, Kavitha CV, et al. Synthesis and pharmacological evaluation of novel N-alkyl/aryl substituted thiazolidinone arecoline analogues as muscarinic receptor 1 agonist in Alzheimer’s dementia models. Eur J Med Chem. 2009;44:4848–4854.
- Monmaur P, Allix M, Schoevaert-Brossault D, et al. Effects of transient cerebral ischemia on the hippocampal dentate theta (theta) profile in the acute rat: a study 4-5 months following recirculation. Brain Res. 1990;508:124–134.
- Shih YT, Chen PS, Wu CH, et al. Arecoline, a major alkaloid of the areca nut, causes neurotoxicity through enhancement of oxidative stress and suppression of the antioxidant protective system. Free Radic Biol Med. 2010;49:1471–1479.
- Jiang JM, Wang L, Gu HF, et al. Arecoline induces neurotoxicity to PC12 cells: involvement in ER stress and disturbance of endogenous H2S generation. Neurochem Res. 2016;41:2140–2148.
- Olah G, Modis K, Toro G, et al. Role of endogenous and exogenous nitric oxide, carbon monoxide and hydrogen sulfide in HCT116 colon cancer cell proliferation. Biochem Pharmacol. 2018;149:186–204.
- Moustafa A. Changes in nitric oxide, carbon monoxide, hydrogen sulfide and male reproductive hormones in response to chronic restraint stress in rats. Free Radic Biol Med. 2021;162:353–366.
- Tabassum R, Jeong NY. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases. Int J Med Sci. 2019;16:1386–1396.
- Ghanbari F, Khaksari M, Vaezi G, et al. Hydrogen sulfide protects hippocampal neurons against methamphetamine neurotoxicity via inhibition of apoptosis and neuroinflammation. J Mol Neurosci. 2019;67:133–141.
- Lv S, Wu N, Wang Q, et al. Endogenous hydrogen sulfide alleviates methotrexate-induced cognitive impairment by attenuating endoplasmic reticulum stress-induced apoptosis via CHOP and caspase-12. Fundam Clin Pharmacol. 2020;34:559–570.
- Tang YY, Wang AP, Wei HJ, et al. Role of silent information regulator 1 in the protective effect of hydrogen sulfide on homocysteine-induced cognitive dysfunction: involving reduction of hippocampal ER stress. Behav Brain Res. 2018;342:35–42.
- Wei HJ, Xu JH, Li MH, et al. Hydrogen sulfide inhibits homocysteine-induced endoplasmic reticulum stress and neuronal apoptosis in rat hippocampus via upregulation of the BDNF-TrkB pathway. Acta Pharmacol Sin. 2014;35:707–715.
- Lumkwana D, du Toit A, Kinnear C, et al. Autophagic flux control in neurodegeneration: progress and precision targeting-Where do we stand? Prog Neurobiol. 2017;153:64–85.
- Chen H, Chen W, Yao Y, et al. Upregulation of CFTR protects against palmitate-induced endothelial dysfunction by enhancing autophagic flux. Oxid Med Cell Longev. 2020;2020:8345246.
- Valencia M, Kim SR, Jang Y, et al. Neuronal autophagy: characteristic features and roles in neuronal pathophysiology. Biomol Ther (Seoul). 2021;29:605–614.
- Wu J, Lipinski MM. Autophagy in neurotrauma: good, bad, or dysregulated. Cells. 2019;8(7):693.
- Fowler AJ, Moussa CE. Activating autophagy as a therapeutic strategy for parkinson’s disease. CNS Drugs. 2018;32:1–11.
- O’Brien MC, Bolton WE. Comparison of cell viability probes compatible with fixation and permeabilization for combined surface and intracellular staining in flow cytometry. Cytometry. 1995;19:243–255.
- Zhen Y, Pan W, Hu F, et al. Exogenous hydrogen sulfide exerts proliferation/anti-apoptosis/angiogenesis/migration effects via amplifying the activation of NF-kappaB pathway in PLC/PRF/5 hepatoma cells. Int J Oncol. 2015;46:2194–2204.
- Dai Y, Zhao X, Chen P, et al. Neuropeptide FF promotes recovery of corneal nerve injury associated with hyperglycemia. Invest Ophthalmol Vis Sci. 2015;56:7754–7765.
- Xie XJ, Ma LG, Xi K, et al. Effects of microRNA-223 on morphine analgesic tolerance by targeting NLRP3 in a rat model of neuropathic pain. Mol Pain. 2017;13:1744806917706582.
- Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544.
- Li X, Zhang KY, Zhang P, et al. Hydrogen sulfide inhibits formaldehyde-induced endoplasmic reticulum stress in PC12 cells by upregulation of SIRT-1. PloS one. 2014;9:e89856.
- Hirano K, Fujimaki M, Sasazawa Y, et al. Neuroprotective effects of memantine via enhancement of autophagy. Biochem Biophys Res Commun. 2019;518:161–170.
- Tseng SK, Chang MC, Su CY, et al. Arecoline induced cell cycle arrest, apoptosis, and cytotoxicity to human endothelial cells. Clin Oral Investig. 2012;16:1267–1273.
- Chang LY, Lai YL, Yu TH, et al. Effects of areca nut extract on lipopolysaccharides-enhanced adhesion and migration of human mononuclear leukocytes. J Periodontol. 2014;85:859–867.
- Farre-Alins V, Narros-Fernandez P, Palomino-Antolin A, et al. Melatonin reduces NLRP3 inflammasome activation by increasing alpha7 nAChR-mediated autophagic flux. Antioxidants (Basel). 2020;9. doi:https://doi.org/10.3390/antiox9121299.
- Ichimiya T, Yamakawa T, Hirano T, et al. Autophagy and autophagy-related diseases: a review. Int J Mol Sci. 2020;21(23):8974.
- Zhang Z, Miah M, Culbreth M, et al. Autophagy in neurodegenerative diseases and metal neurotoxicity. Neurochem Res. 2016;41:409–422.
- Gonzalez-Rodriguez A, Mayoral R, Agra N, et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014;5:e1179.
- Yin Y, Sun G, Li E, et al. ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res Rev. 2017;34:3–14.
- Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545.
- Wu L, Chen Y, Wang CY, et al. Hydrogen sulfide inhibits high glucose-induced neuronal senescence by improving autophagic flux via up-regulation of SIRT1. Front Mol Neurosci. 2019;12:194.
- Kang X, Li C, Xie Y, et al. Hippocampal ornithine decarboxylase/spermidine pathway mediates H2S-alleviated cognitive impairment in diabetic rats: involving enhancment of hippocampal autophagic flux. J Adv Res. 2021;27:31–40.
- Yang B, Bai Y, Yin C, et al. Activation of autophagic flux and the Nrf2/ARE signaling pathway by hydrogen sulfide protects against acrylonitrile-induced neurotoxicity in primary rat astrocytes. Arch Toxicol. 2018;92:2093–2108.
- Bhardwaj M, Leli NM, Koumenis C, et al. Regulation of autophagy by canonical and non-canonical ER stress responses. Semin Cancer Biol. 2020;66:116–128.
- Sakamaki JI, Wilkinson S, Hahn M, et al. Bromodomain protein BRD4 is a transcriptional repressor of autophagy and lysosomal function. Mol Cell. 2017;66:517–32 e9.
- Mauthe M, Orhon I, Rocchi C, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14:1435–1455.
- Song S, Tan J, Miao Y, et al. Crosstalk of autophagy and apoptosis: involvement of the dual role of autophagy under ER stress. J Cell Physiol. 2017;232:2977–2984.
