ABSTRACT
Nup50 is nuclear pore complex component localized to the nuclear side of the pore and in the nucleoplasm. It has been characterized as an auxiliary factor in nuclear transport reactions. Our recent work indicates that it interacts with and stimulates RCC1, the sole guanine nucleotide exchange factor for the GTPase Ran. Here, we discuss how this interaction might contribute to Nup50 function in nuclear transport but also its other functions like control of gene expression, cell cycle and DNA damage repair.
Compartmentalization is crucial for cellular function. Arguably, the most prominent example constitutes, in eukaryotes, the separation of the nucleus from the remaining of the cell. This is achieved by the nuclear envelope, which establishes a diffusion barrier between the nucleus and the cytoplasm and is perforated by numerous nuclear pore complexes (NPCs) acting as transport gates. Understanding how NPCs assemble and integrate into the nuclear envelope is a challenging task [Citation1,Citation2]. Our recent work indicates that the NPC component Nup50, also referred as Npap60 [Citation3], plays an important regulatory role in this process by binding RCC1 (Regulator of Chromosome Condensation 1) and stimulating its guanine nucleotide exchange activity [Citation4]. Here, we discuss how the Nup50-RCC1 interaction might contribute to Nup50 long-discussed function in nuclear transport but also to other cellular events.
Nuclear pore complexes
NPCs are large protein complexes that regulate macromolecule traffic between the cytoplasm and the nucleus. They consist of approximately 30 different proteins called nucleoporins (for review, see [Citation5,Citation6]), each found in many copies in the NPC summing up to, in vertebrates, about 1000 individual proteins in each NPC [Citation6,Citation7]. Nucleoporins can be roughly categorized into two groups: Structural nucleoporins form the architectural backbone of the NPC, while largely unstructured nucleoporins with multiple phenylalanine-glycine (FG)-containing sequences are responsible for the permeability properties of the pore and act as docking sites for the transport reactions. Structurally, the NPC can be viewed as a huge and largely symmetric central part organized as a stack of three rings (). The inner ring, in vertebrates organized around Nup93, connects the pore membrane, i.e. the part of the nuclear envelope covered by the NPC, including transmembrane nucleoporins (Pom121, Gp210 and NDC1) with the central channel, mostly filled with FG-repeats nucleoporins of the Nup62-complex. Two outer rings, the cytoplasmic and nuclear rings, sandwich the inner ring and also contact the pore membrane. They are mostly composed of Y-complexes (also known as Nup107-160 complexes) which form, depending on the species, one or two ring assemblies within each of the outer rings [Citation8].
Figure 1. Nuclear pore complex scheme. Simplified scheme of the nuclear pore complex with the ring structures indicated: inner ring in light blue, cytoplasmic and nuclear rings in light orange, transmembrane nucleoporins in green. In addition, cytoplasmic filaments are in violet, nuclear basket in orange. FG-nucleoporins forming the central channel are in gray.
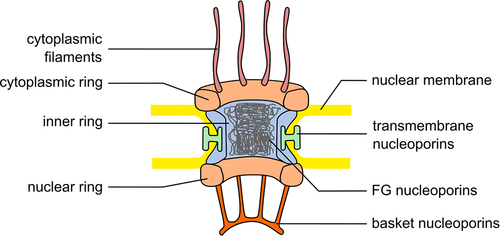
From this central part, asymmetric structures extend toward the cytoplasm and nucleoplasm: cytoplasmic filaments contain among other nucleoporins Nup358 [Citation9,Citation10] and interact with the nuclear trafficking machinery (see later). On the nuclear side, a basket structure is formed by TPR and ZC3HC1 [Citation11,Citation12] and anchored via Nup153 to the NPC [Citation13,Citation14]. Nup153 also recruits Nup50 to the nuclear side of NPCs [Citation14,Citation15].
Nuclear trafficking
NPCs are the gateways within the nuclear envelope. While smaller molecules can passively diffuse through this gate, larger molecules are imported or exported by interacting with nuclear transport receptors (NTRs, also called importins and exportins, depending on the transport direction, for review, see [Citation16]). Although there exists no rigorous cutoff for diffusion [Citation17,Citation18], most proteins and RNA-protein complexes rely on NTRs for NPC passage. For proteins imported into the nucleus, NTRs of the importin family bind their respective cargos via nuclear localization signals (NLSs) in the cytoplasm and mediate cargo translocation through the permeability barrier of NPCs (). On the nuclear side, RanGTP binds to importins and triggers cargo release. For a number of cargos with so-called classical NLSs, a complex of two NTRs, importin α and β, is required. Importin α binds the NLS and is, in turn, recognized by importin β, allowing for NPC passage. In this case, on the nuclear side RanGTP binds both importin α and β and induces cargo release. The NTR-RanGTP complexes are then recycled in the cytoplasm. While most NTRs complexed with RanGTP are directly exported, importin α-RanGTP is exported through CAS binding. In the cytoplasm, RanGAP (Ran GTPase-activating protein) together with RanBP1 (Ran Binding Protein 1) stimulates Ran GTPase activity causing GTP hydrolysis, which in turn induces the dissociation of Ran from NTR and/or the importin α-CAS complex. This reaction can also take place directly on cytoplasmic filaments of NPCs because SUMO-modified RanGAP forms a complex with Nup358, also known as RanBP2 (Ran Binding Protein 2), and the SUMO conjugating enzyme Ubc9 [Citation19,Citation20]. In both ways, NTR-RanGTP complexes are dissociated, and NTRs can be reused for another import cycle [Citation21].
Figure 2. Nuclear import and export cycles. An import complex consisting of an NLS-bearing cargo (violet) and importin (green) is formed in the cytoplasm. After NPC passage, RanGTP (blue), formed in the nucleus though the action of RCC1 (white), binds importin, resulting in nuclear cargo release. The importin-RanGTP complex returns to the cytoplasm through the NPC where the Ran GTPase-activating protein (RanGAP1, gray) stimulates GTP hydrolysis, releasing the importin for another import cycle. An export complex consisting of an NES-bearing cargo (pink), exportin (beige) and RanGTP is formed in the nucleus. After NPC passage RanGAP1 stimulates GTP hydrolysis, dissociating the export complex. The import of RanGDP and re-import of the exportin are, for the sake of simplicity, not shown.
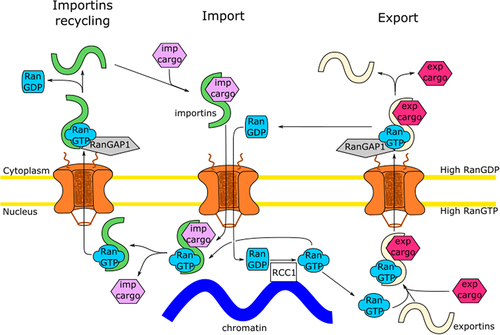
For protein export from the nucleus, RanGTP, an exportin, such as the Chromosomal Maintenance 1 (CRM1), and an NES (nuclear export signal) containing cargo form a trimeric complex in the nucleus (). This NES-bearing cargo–RanGTP–CRM1 complex is exported to the cytoplasm. Here, the interaction with RanGAP induces hydrolysis of Ran’s GTP to GDP, dissociating the export complex and releasing its components into the cytoplasm. Then, CRM1 translocates back into the nucleus to be available for another export cycle. RanGDP is separately imported through NTF2. In the nucleus, the RCC1, the guanine nucleotide exchange factor for Ran (RanGEF) catalyzes the exchange of GDP for GTP on Ran.
Passage of NTRs through NPCs occurs in both directions and the same would be, in principle, the case for importin-cargo and exportin-cargo complexes. Directionality is achieved by the asymmetric distribution of RanGTP. The only RanGEF, RCC1, is a chromatin binding protein, and hence Ran GDP to GTP exchange is in restricted to the nucleus, resulting a high nuclear RanGTP concentration. Likewise, Ran’s GTP hydrolysis is restricted to the cytoplasm as RanGAP is bound to cytoplasmic filaments or soluble in the cytoplasm. Consequently, in the cytoplasm Ran is predominantly present in its GDP-bound form.
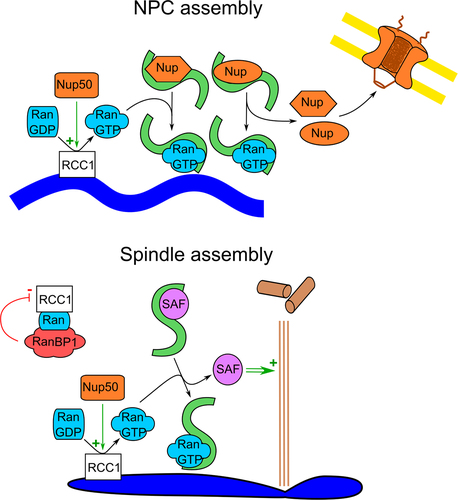
Thus, the RanGTP gradient across the nuclear envelope drives nuclear transport reactions in interphase. In addition, an asymmetric distribution of RanGTP and RanGDP persists also during mitosis when the nuclear envelope disappears and has important functions [Citation22]: RCC1 localizes to chromatin and generates here a high local RanGTP concentration. This local RanGTP enrichment around chromatin is crucial for the assembly of mitotic spindles because RanGTP releases central spindle assembly factors from their inhibitory binding to NTRs [Citation23–25]. Similarly, at the end of mitosis, RCC1 activity and high RanGTP concentrations around the chromatin direct NPC assembly into the reforming nuclear envelope on the chromatin surface [Citation26–29].
Nup50 in nuclear trafficking
Nup50 localizes to the nuclear side of NPCs and interacts with a number of nuclear transport factors, including importin α, importin β, transportin and RanBP7, RanGTP and probably CRM1 [Citation30–32] (see also ). Nup50 might thus act as a supporting factor in nuclear transport. It has been proposed that Nup50, by binding importin α and β, acts as shuttling factor between the cytoplasm and the nucleoplasm facilitating the NPC passage of the cargo-importin α/β complex [Citation31]. This is based on the findings that importin α can simultaneously bind NLS-containing cargos and the N-terminus of Nup50, and that Nup50 is not stably associated with the NPC. However, the latter is also true for many other nucleoporins that have a rather short half-time at NPCs [Citation33,Citation34]. In vitro assays show that under limiting importin α and importin β concentration, Nup50 enhances nuclear import of an artificial NLS import cargo [Citation31].
Figure 3. Domain organization of vertebrate Nup50. Scheme showing importin α and RCC1 binding domain (red) as well as the NPC localization domain (green) of Nup50. Also indicated are the importin β binding domain (light orange) and Ran binding domain (light yellow). Amino acid numbers indicating the domain borders refer to the Xenopus protein.
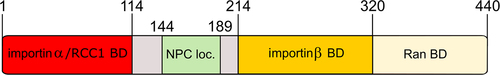
Alternatively, it has been suggested that Nup50 N-terminal domain rather competes with the imported cargo for importin α binding, enhancing cargo release in the nucleoplasm [Citation35–37]. This is supported by the crystal structure of importin α bound to the N-terminus of Nup50 [Citation37]: It identified two Nup50 binding sites on importin α, of which one overlaps with the cargo-binding site. Interaction of Nup50 with both sites is required to replace the NLS-cargo from importin α. Thus, whereas the first idea sees Nup50 as a dynamic factor moving between cytoplasm and nucleoplasm, this model places Nup50 function in the nucleus and resembles the proposed role of the Nup50 orthologue Nup2 in budding yeast [Citation38–41]. Notably, in humans, Nup50 is expressed as two versions, a longer and a shorter isoform [Citation35]. Although the longer isoform enhances nuclear import by promoting dissociation of the cargo-importin α/β complex, the shorter stabilizes this complex because it does not contain the part of Nup50 sequence, which competes with the NLS-cargo for importin α binding, and was thus suggested impeding efficient nuclear import.
Lastly, Nup50 may also be involved in nuclear export through its binding with CRM1, which is, however, controversial [Citation30,Citation31]. If true, Nup50 might serve as a binding site on the nuclear side of the NPC for exporting cargo–CRM1–RanGTP complexes [Citation30]. This latter view is supported by earlier experiments showing that microinjection of Nup50 antibodies blocks CRM1 mediated nuclear export but not nuclear import [Citation30]. However, coating the nuclear NPC side with antibodies might sterically impede nuclear export without Nup50 being directly involved.
Nup50 is required for nuclear pore complex assembly
In a recent publication [Citation4], we have shown that Nup50 also plays an unexpected role in NPC assembly. Depletion of the protein from Xenopus egg extract, where NPC assembly can be faithfully reconstituted, creates nuclei with a closed nuclear envelope devoid of NPCs. Consistently, in human U2OS cells, Nup50 downregulation severely reduces NPC numbers. The N-terminal region of Nup50 seems to be crucial for the protein function in NPC assembly (). It binds RCC1 and stimulates its GEF activity toward Ran. Thus, increased RCC1 activity via local increase of RanGTP concentrations might stimulate mitotic NPC assembly (), which is the assembly mode prevalent in the egg extraction system. Consistent with this hypothesis, the excess of RCC1 can, in this cell-free system, overcome Nup50 depletion.
Figure 4. RCC1 function in mitosis. The establishment of RanGTP gradient by the chromatin (dark blue) bound RCC1 (white) is critical for NPC assembly (upper panel). RanGTP (blue) binding to importin (green) will induce the release of key nucleoporins, allowing NPC formation. The establishment of RanGTP gradient by the chromosome (dark blue) bound RCC1 is also critical for spindle assembly (lower panel). RanGTP (blue) binding to importin will release spindle assembly factors (SAF, pink), allowing spindle assembly (brown). Nup50 (orange) might stimulate RCC1 activity in both cases. By forming a complex with Ran and RCC1, RanBP1 (red) inhibits RCC1 activity in early mitosis.
Surprisingly, for its function in NPC assembly, Nup50 does not need to be localized to NPCs. A short-conserved motif of 46 amino acids is required and sufficient for Nup50 NPC targeting, mediating an interaction of the two nucleoporins Nup153 and ELYS/MEL28 (). Mutations interrupting these interactions or fragments lacking the respective motifs do not interfere with Nup50 function in NPC assembly. This might also explain the older data indicating that mouse Nup50, although essential, is not required for NPC formation [Citation32]. Rodents possess two Nup50 orthologues, a canonical NPC bound form, and a relative, which due to amino acid exchanges in the NPC targeting motif, does not localize to NPCs. In contrast to the long and short versions of human Nup50, these orthologues arise from two different gene loci and resemble the long human isoform. In the Nup50 knockout mice, the second nuclear Nup50 protein might sustain sufficient RCC1 activity for NPC assembly. Consistent with this idea, only the downregulation of both Nup50 orthologues in mouse NIH3T3 cells severely reduces NPC numbers [Citation4].
It remains open whether Nup50 also stimulates RCC1 activity during other phases of the cell cycle and in early mitosis, for example, to stimulate spindle assembly (). During mitosis, the GEF activity of RCC1 is spatially controlled by interaction with different partners [Citation25,Citation42]: Chromatin-bound RCC1 promotes RanGDP to GTP exchange, while RCC1 activity in a soluble complex with Ran and RanBP1 is inhibited. It will be interesting to see how Nup50 influences the balance of active to inactive RCC1 during open mitosis.
A fresh perspective on Nup50 function in nuclear traffic
The newly defined role of Nup50 as stimulator of the RCC1 GEF activity can not only explain Nup50 function in NPC assembly but also sheds new light on Nup50 function in nuclear transport. As Nup50 is localized to the nuclear side of the NPC, its stimulation of RCC1 activity could locally increase the RanGTP concentration, allowing for efficient cargo-NTR-complex dissociation (). In this scenario, it remains open whether the Ran binding domain, located in the C-terminus of Nup50 would further contribute to this effect. In this way, the stimulatory effect of Nup50 on nuclear import would not be limited to the classical import pathway via importin α and β [Citation31,Citation35,Citation37,Citation43] but could extend to the non-classical pathway (i.e. other NTRs) dependent on RanGTP. Because some nuclear export routes, such as CRM1, CAS, exportins 4–7 and exportin-t also require RanGTP (for review see Ref. [Citation44]), Nup50 might generally promote Ran-dependent nuclear transport routes. In the case of the CRM1 this might be independent of whether Nup50 directly interacts with CRM1 [Citation30] or not [Citation31].
Figure 5. Possible implication of Nup50 in nuclear trafficking. By acting as a scaffold at the nuclear basket, Nup50 (orange) could help the dissociation of the import cargo (violet) on the nuclear side. Additionally, by enhancing the RCC1 GEF activity, Nup50 could help to sustain the RanGTP nuclear gradient. This could boost both import cargo dissociation and export cargo formation (pink). The possible interaction between Nup50 and the exportin CRM1 might also enhance nuclear export.
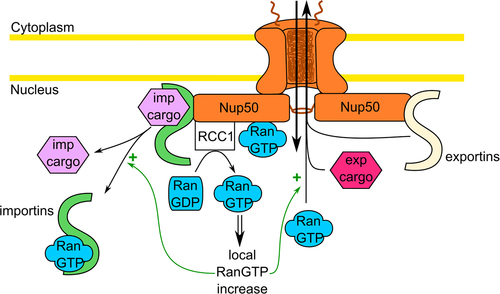
It is unclear whether RCC1 and importin α, both of them bind to the N-terminal part of Nup50 [Citation4,Citation31], can interact with the nucleoporin simultaneously. If so, Nup50 could be envisioned as an interaction hub bringing all relevant partners together. Alternatively, the stimulation of RCC1 activity and importin α/β-cargo dissociation might constitute two separate functions of Nup50, which nevertheless both increase the nuclear import efficiency.
NPC localization of Nup50 might be important for its auxiliary role in nuclear transport reactions e.g. by locally increasing the RanGTP concentration at the nuclear exit sites of NPCs. However, for its function in the mitotic NPC assembly, Nup50 does not necessarily need to be localized to NPCs [Citation4]. Nup50 has one of the shortest residence time of all the nucleoporins at the NPC [Citation34]. Consistently, Nup50 is a dynamic nucleoporin found both at NPCs and within the nucleoplasm [Citation30,Citation45,Citation46]. The nuclear pool of Nup50 could have a role in nuclear trafficking e.g. by titrating importin α away from the NPC to facilitate importin α-cargo dissociation, or increasing nucleoplasmic RanGTP concentration by stimulating RCC1.
Nup50 in transcriptional regulation
In the nucleoplasm, Nup50 is more associated with euchromatin than with heterochromatin [Citation45]. Nup50 mobility in the nucleoplasm requires an ongoing RNA polymerase II transcription and is thus increased in transcriptional highly active compared to quiescent cells. Interestingly, Nup50 knockdown inhibits the differentiation of myoblasts into myotubes, indicating that Nup50 might have an active role in gene transcription or regulation [Citation45]. Consistently, Nup50 interacts with transcribed genes (mainly developmental ones) and stimulates their expression in Drosophila [Citation47]. Recently, the function of Nup50 but also of Nup153 and TPR in total RNA expression and export was compared using an inducible auxin-degron systems [Citation48]. While the TPR downregulation has the strongest impact on RNA expression, RNA changes induced by Nup50 downregulation are mild and the RNAs affected mostly do not overlap with the ones influenced by TPR or Nup153 downregulation. It is unlikely that the Nup50 effects of RNA expression are due to RNA export defects and, indeed, a follow-up study showed that there is little to no involvement of Nup50 in mRNA export [Citation49].
Nup50 in cell cycle control
Nup50 has been originally identified as an interactor of the CDK2 inhibitor p27Kip1 [Citation32]. p27Kip1 is involved in cell cycle control, particularly in but not limited to the G1/S transition. Mutations of p27Kip1 or decrease in its cellular levels have been reported in different cancer types (for review, see Ref. [Citation50]). p27Kip1 level is controlled through its degradation after phosphorylation and ubiquitination [Citation51] and cellular compartmentalization, as it possesses both an NLS and an NES [Citation52–54]. In mice, Nup50 knockout disturbs p27Kip1 expression and causes a neural tube abnormality resulting in embryonic death [Citation32]. Disruption of p27Kip1 interaction with Nup50 leads to its cytosolic accumulation and defects in cell proliferation [Citation55]. Nuclear localization of this mutant p27Kip1 can be rescued by cyclin E and CDK2 co-expression, but the mutant accumulates in the nucleus in a phosphorylated form where its degradation is impaired, indicating that the interaction with Nup50 is somehow necessary for the degradation of nuclear p27Kip1 [Citation55]. Interestingly, p27Kip1 expression might be linked to RCC1: RCC1 knockdown in lung adenocarcinoma cells increases p27Kip1 expression, both at mRNA and protein levels, and impedes cell proliferation [Citation56]. It is thus also possible that Nup50 influences p27Kip1 activity via its interaction with and activation of RCC1.
Nup50 function in DNA repair
DNA maintenance and repair are cardinal cellular functions, and Nup50 could be involved in it. This is illustrated by the finding that, together with Nup153, Nup50 promotes the recruitment of 53BP1 in place of BRCA1 to damaged DNA [Citation57]. 53BP1 is involved in the error-prone non-homologous end-joining repair, while BRCA1 acts as a high-fidelity homologous recombination-mediated DNA repair pathway. RCC1 interaction could be a point of entry for Nup50: RCC1 overexpression inhibits DNA-damage induced senescence in telomerase-immortalized normal epithelial RPE1 cells and decreases the number of 53BP1 foci per cells, which, together with mitosis recovery, is a hallmark of a faster DNA repair [Citation58]. Interestingly, this mechanism appears to be dependent on Ran and nuclear transport as Ran knockdown or inhibition of nuclear export with the CRM1 inhibitor KPT-330 delays DNA repair termination [Citation58]. The importance of Ran and RCC1 in DNA repair is also illustrated in a cellular model of the Hutchinson–Gilford progeria syndrome: Here disruption of the nuclear lamina disturbs heterochromatin organization, which hampers RCC1 activity and RanGTP gradient formation, which in turn impedes DNA repair [Citation59]. Given the interaction of Nup50 with both Ran and RCC1 as well as its DNA binding capability, it is tempting to speculate that Nup50 is also more directly involved in DNA repair.
Conclusion
Thus, Nup50 is involved as an interaction hub and stimulatory factor for the Ran system in nuclear transport reactions and NPC assembly. It remains open whether its additional functions in cell cycle regulation, DNA repair and transcriptional regulation are similarly related to Ran via stimulation of RCC1.
In budding yeast, the Nup50 orthologue Nup2 is similarly localized to the nuclear side of the NPC via the Nup153 orthologue Nup60 [Citation60,Citation61]. Nup2 also interacts with the yeast importin α orthologue Srp1 and has been thus suggested to similarly act as an auxiliary factor for nuclear import reactions [Citation39,Citation41]. However, an interaction between Nup2 and the yeast RCC1, Prp20, could not be detected [Citation39]. It thus remains open whether Nup50–RCC1 interaction is a metazoan or even a vertebrate specific adaptation opening doors to new functions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no datasets were generated or analyzed.
Additional information
Funding
References
- Dultz E, Wojtynek M, Medalia O, et al. The nuclear pore complex: birth, life, and death of a cellular Behemoth. Cells. 2022;11(9):1456.
- Kutay U, Juhlen R, Antonin W. Mitotic disassembly and reassembly of nuclear pore complexes. Trends Cell Biol. 2021;31(12):1019–1033.
- Fan F, Liu CP, Korobova O, et al. cDNA cloning and characterization of Npap60: a novel rat nuclear pore-associated protein with an unusual subcellular localization during male germ cell differentiation. Genomics. 1997;40(3):444–453.
- Holzer G, De Magistris P, Gramminger C, et al. The nucleoporin Nup50 activates the Ran guanine nucleotide exchange factor RCC1 to promote NPC assembly at the end of mitosis. EMBO J. 2021;40(23):e108788.
- Schwartz TU. The structure inventory of the nuclear pore complex. J Mol Biol. 2016;428(10):1986–2000.
- Lin DH, Hoelz A. The structure of the nuclear pore complex (An update). Annu Rev Biochem. 2019;88(1):725–783.
- Beck M, Hurt E. The nuclear pore complex: understanding its function through structural insight. Nat Rev Mol Cell Biol. 2017;18(2):73–89.
- Ori A, Banterle N, Iskar M, et al. Cell type-specific nuclear pores: a case in point for context-dependent stoichiometry of molecular machines. Mol Syst Biol. 2013;9(1):648.
- Wu J, Matunis MJ, Kraemer D, et al. Nup358, a cytoplasmically exposed nucleoporin with peptide repeats, Ran-GTP binding sites, zinc fingers, a cyclophilin A homologous domain, and a leucine-rich region. J Biol Chem. 1995;270(23):14209–14213.
- Yokoyama N, Hayashi N, Seki T, et al. A giant nucleopore protein that binds Ran/TC4. Nature. 1995;376(6536):184–188.
- Gunkel P, Iino H, Krull S, et al. ZC3HC1 is a novel inherent component of the nuclear basket, resident in a state of reciprocal dependence with TPR. Cells. 2021;10(8):1937.
- Gunkel P, Cordes VC. ZC3HC1 is a structural element of the nuclear basket effecting interlinkage of TPR polypeptides. bioRxiv. 2022;2021. DOI:10.1101/2021.12.30.474576.
- Walther TC, Fornerod M, Pickersgill H, et al. The nucleoporin Nup153 is required for nuclear pore basket formation, nuclear pore complex anchoring and import of a subset of nuclear proteins. EMBO J. 2001;20(20):5703–5714.
- Hase ME, Cordes VC. Direct Interaction with Nup153 mediates binding of TPR to the periphery of the nuclear pore complex. Mol Biol Cell. 2003;14(5):1923–1940.
- Makise M, Mackay DR, Elgort S, et al. The Nup153-Nup50 protein interface and its role in nuclear import. J Biol Chem. 2012;287(46):38515–38522.
- Paci G, Caria J, Lemke EA. Cargo transport through the nuclear pore complex at a glance. J Cell Sci. 2021;134(2). DOI:10.1242/jcs.247874.
- Mohr D, Frey S, Fischer T, et al. Characterisation of the passive permeability barrier of nuclear pore complexes. EMBO J. 2009;28(17):2541–2553.
- Timney BL, Raveh B, Mironska R, et al. Simple rules for passive diffusion through the nuclear pore complex. J Cell Biol. 2016;215(1):57.
- Mahajan R, Delphin C, Guan T, et al. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 1997;88(1):97–107.
- Bernier-Villamor V, Sampson DA, Matunis MJ, et al. Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell. 2002;108(3):345–356.
- Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007;8(3):195–208.
- Forbes DJ, Travesa A, Nord MS, et al. Nuclear transport factors: global regulation of mitosis. Curr Opin Cell Biol. 2015;35:78–90.
- Carazo-Salas RE, Guarguaglini G, Gruss OJ, et al. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature. 1999;400(6740):178–181.
- Moore W, Zhang C, Clarke PR. Targeting of RCC1 to chromosomes is required for proper mitotic spindle assembly in human cells. Curr Biol. 2002;12(16):1442–1447.
- Yau KC, Arnaoutov A, Aksenova V, et al. RanBP1 controls the Ran pathway in mammalian cells through regulation of mitotic RCC1 dynamics. Cell Cycle. 2020;19(15):1899–1916.
- Zhang C, Clarke PL. Chromatin-independent nuclear envelope assembly induced by Ran GTPase in Xenopus egg extracts. Science. 2000;288(5470):1429–1432.
- Hetzer M, Bilbao-Cortes D, Walther TC, et al. GTP hydrolysis by Ran is required for nuclear envelope assembly. Mol Cell. 2000;5(6):1013–1024.
- Walther TC, Askjaer P, Gentzel M, et al. RanGTP mediates nuclear pore complex assembly. Nature. 2003;424(6949):689–694.
- Askjaer P, Galy V, Hannak E, et al. Ran GTPase cycle and importins α and β are essential for spindle formation and nuclear envelope assembly in living Caenorhabditis elegans embryos. Mol Biol Cell. 2002;13(12):4355–4370.
- Guan T, Kehlenbach RH, Schirmer EC, et al. Nup50, a nucleoplasmically oriented nucleoporin with a role in nuclear protein export. Mol Cell Biol. 2000;20(15):5619–5630.
- Lindsay ME, Plafker K, Smith AE, et al. Npap60/Nup50 is a tri-stable switch that stimulates importin-alpha:beta-mediated nuclear protein import. Cell. 2002;110(3):349–360.
- Smitherman M, Lee K, Swanger J, et al. Characterization and targeted disruption of murine Nup50, a p27 Kip1 -interacting component of the nuclear pore complex. Mol Cell Biol. 2000;20(15):5631–5642.
- D’Angelo MA, Raices M, Panowski SH, et al. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136(2):284–295.
- Rabut G, Doye V, Ellenberg J. Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat Cell Biol. 2004;6(11):1114–1121.
- Ogawa Y, Miyamoto Y, Asally M, et al. Two isoforms of Npap60 (Nup50) differentially regulate nuclear protein import. Mol Biol Cell. 2010;21(4):630–638.
- Pumroy RA, Nardozzi JD, Hart DJ, et al. Nucleoporin Nup50 stabilizes closed conformation of armadillo repeat 10 in importin alpha5. J Biol Chem. 2012;287(3):2022–2031.
- Matsuura Y, Stewart M. Nup50/Npap60 function in nuclear protein import complex disassembly and importin recycling. EMBO J. 2005;24(21):3681–3689.
- Hood JK, Casolari JM, Silver PA. Nup2p is located on the nuclear side of the nuclear pore complex and coordinates Srp1p/importin-alpha export. J Cell Sci. 2000;113(8):1471–1480.
- Solsbacher J, Maurer P, Vogel F, et al. Nup2p, a yeast nucleoporin, functions in bidirectional transport of importin alpha. Mol Cell Biol. 2000;20(22):8468–8479.
- Gilchrist D, Mykytka B, Rexach M. Accelerating the rate of disassembly of karyopherin.cargo complexes. J Biol Chem. 2002;277(20):18161–18172.
- Matsuura Y, Lange A, Harreman MT, et al. Structural basis for Nup2p function in cargo release and karyopherin recycling in nuclear import. Embo J. 2003;22(20):5358–5369.
- Zhang MS, Arnaoutov A, Dasso M. RanBP1 governs spindle assembly by defining mitotic Ran-GTP production. Dev Cell. 2014;31(4):393–404.
- Moore MS. Npap60: a new player in nuclear protein import. Trends Cell Biol. 2003;13(2):61–64.
- Güttler T, Görlich D. Ran-dependent nuclear export mediators: a structural perspective. EMBO J. 2011;30(17):3457–3474.
- Buchwalter AL, Liang Y, Hetzer MW. Nup50 is required for cell differentiation and exhibits transcription-dependent dynamics. Mol Biol Cell. 2014;25(16):2472–2484.
- Duheron V, Chatel G, Sauder U, et al. Structural characterization of altered nucleoporin Nup153 expression in human cells by thin-section electron microscopy. Nucleus. 2014;5(6):601–612.
- Kalverda B, Pickersgill H, Shloma VV, et al. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell. 2010;140(3):360–371.
- Aksenova V, Smith A, Lee H, et al. Nucleoporin TPR is an integral component of the TREX-2 mRNA export pathway. Nat Commun. 2020;11(1):4577.
- Li Y, Aksenova V, Tingey M, et al. Distinct roles of nuclear basket proteins in directing the passage of mRNA through the nuclear pore. Proc Natl Acad Sci USA. 2021;118(37:e2015621118. DOI:10.1073/pnas.2015621118.
- Bencivenga D, Caldarelli I, Stampone E, et al. p27(Kip1) and human cancers: a reappraisal of a still enigmatic protein. Cancer Lett. 2017;403:354–365.
- Tsvetkov LM, Yeh K-H, Lee S-J, et al. p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–S662.
- Zeng Y, Hirano K, Hirano M, et al. Minimal requirements for the nuclear localization of p27Kip1, a cyclin-dependent kinase inhibitor. Biochem Biophys Res Commun. 2000;274(1):37–42.
- Connor MK, Kotchetkov R, Cariou S, et al. CRM1/Ran-mediated nuclear export of p27 Kip1 involves a nuclear export signal and links p27 export and proteolysis. Mol Biol Cell. 2003;14(1):201–213.
- Borriello A, Cucciolla V, Oliva A, et al. p27Kip1 metabolism: a fascinating labyrinth. Cell Cycle. 2007;6(9):1053–1061.
- Muller D, Thieke K, Burgin A, et al. Cyclin E-mediated elimination of p27 requires its interaction with the nuclear pore-associated protein mNPAP60. EMBO J. 2000;19(10):2168–2180.
- Zeng X, Zhong M, Yang Y, et al. Down-regulation of RCC1 sensitizes immunotherapy by up-regulating PD-L1 via p27 kip1 /CDK4 axis in non-small cell lung cancer. J Cell Mol Med. 2021;25(8):4136–4147.
- Mackay DR, Howa AC, Werner TL, et al. Nup153 and Nup50 promote recruitment of 53BP1 to DNA repair foci by antagonizing BRCA1-dependent events. J Cell Sci. 2017;130(19):3347–3359.
- Cekan P, Hasegawa K, Pan Y, et al. RCC1-dependent activation of Ran accelerates cell cycle and DNA repair, inhibiting DNA damage-induced cell senescence. Mol Biol Cell. 2016;27(8):1346–1357.
- Dworak N, Makosa D, Chatterjee M, et al. A nuclear lamina-chromatin-Ran GTPase axis modulates nuclear import and DNA damage signaling. Aging Cell. 2019;18(1):e12851.
- Cibulka J, Bisaccia F, Radisavljević K, et al. Assembly principle of a membrane-anchored nuclear pore basket scaffold. Sci Adv. 2022;8(6):eabl6863.
- Dilworth DJ, Suprapto A, Padovan JC, et al. Nup2p dynamically associates with the distal regions of the yeast nuclear pore complex. J Cell Biol. 2001;153(7):1465–1478.
