ABSTRACT
Retinopathy of prematurity (ROP), which is characterized by retinal neovascularization (RNV), is a major cause of neonatal blindness. The primary treatment for ROP is anti-vascular endothelial growth factor (VEGF) therapy, which is costly and can rapidly lead to desensitization. Celastrol, a bioactive compound extracted from Tripterygium wilfordii Hook F. (“Thunder of God Vine”), has been shown to exert anticancer and anti-inflammatory effects. However, whether celastrol has antiangiogenic activity and can suppress inflammation to inhibit ROP progression is unclear. This was investigated in the present study in vitro as well as in vivo using a mouse model of oxygen-induced retinopathy (OIR). Our results showed that celastrol treatment reduced neovascular and avascular areas in the retina and inhibited microglia activation and inflammation in OIR mice. Celastrol also inhibited proliferation, migration, and tube formation in cultured human retinal microvascular endothelial cells, and reversed the activation of the microRNA (miR)-17-5p/hypoxia-inducible factor (HIF)-1α/VEGF pathway in the retina of OIR mice. These results indicate that celastrol alleviates pathologic RNV in the retina by protecting neuroglia and suppressing inflammation via inhibition of miR-17-5p/HIF-1α/VEGF signaling, and thus has therapeutic potential for the prevention and treatment of ROP.
Abbreviations: BSA, bovine serum albumin; COX2, cyclooxygenase 2; ECM, endothelial cell medium; FBS, fetal bovine serum; HDAC, histone deacetylase; HIF-1, hypoxia-inducible factor 1; HRMEC, human retinal microvascular endothelial cell; Hsp70, heat shock protein; IB4, isolectin B4; ICAM-1, intercellular adhesion molecule 1; IL-1β/6, interleukin 1 beta/6; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein 1; miRNA, microRNA; MMP, matrix metalloproteinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-kappa B; OIR, oxygen-induced retinopathy; PBS, phosphate-buffered saline; PCNA, proliferating cell nuclear antigen; PI3K, phosphatidylinositol-3-kinase; qRT-PCR, quantitative real-time PCR; RNV, retinal neovascularization; ROP, retinopathy of prematurity; RTCA, real-time cell analyzer; RVO, retinal vaso-obliteration; TNF-α, tumor necrosis factor alpha; VCAM-1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor.
1. Introduction
Retinopathy of prematurity (ROP) is a major cause of neonatal blindness [Citation1]. Hyperoxic treatment in preterm infants impedes normal angiogenesis and consequently, incompletely formed blood vessels cannot meet the need for metabolic growth following the return to a relatively hypoxic environment. Vascular hyperplasia involving multiple factors occurs at the interface between vascular and avascular areas of the retina to supply adequate perfusion under hypoxia. Immature vasculature also undergoes pathologic changes such as vascular leakage, inflammation [Citation2], and impairment of the blood-retinal barrier [Citation3,Citation4]. Moreover, newborn blood vessels can grow from the inner layer of the retina into the vitreous body, causing irreversible damage or loss of vision [Citation5]. The oxygen-induced retinopathy (OIR) mouse model has been widely used to study ROP [Citation6].
Chinese medicinal plants offer broad prospects for drug development. However, their clinical application requires optimization of purification methods and a detailed understanding of the mechanisms of action of the bioactive components. Celastrol, a compound extracted from Tripterygium wilfordii Hook F. (“Thunder of God Vine”) used in traditional Chinese medicine, has been shown to alleviate oxidative stress [Citation7], suppress proinflammatory cytokine production [Citation8], and restore intestinal homeostasis [Citation9] by inhibiting the activation of several key signaling cascades involved in chronic inflammatory disorders such as the nuclear factor-kappa B (NF-κB) [Citation10] and phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathways [Citation11]. Celastrol has been shown to block the transcriptional activity of hypoxia-inducible factor (HIF)-1α and downstream target genes such as VEGF by decreasing hypoxia-induced accumulation of HIF-1α in vitro [Citation12,Citation13], and to inhibit angiogenesis under hypoxia via VEGF-induced Akt/mTOR/p70S6K signaling [Citation14,Citation15]. Additionally, celastrol protected human retinal pigment epithelial cells against hydrogen peroxide-induced apoptosis by promoting resistance to oxidation and suppressing the innate immune response through regulation of NF-κB and heat shock protein (Hsp)70 [Citation16]. However, whether the antiangiogenic and anti-inflammatory activities of celastrol can prevent ROP progression is unclear.
Micro (mi)RNA, a family of non-coding RNAs (20–25 nucleotides), has been known to play a pivotal role in ranges of physiological and pathological process via post-transcriptional regulation of downstream genes [Citation17]. Recently, the hypoxia-associated miRNAs targeting HIF-1α and VEGF mRNAs have been identified as the putative diagnostic markers and therapeutic agents in choroidal and retinal angiogenic diseases [Citation18]. The previous study systematically evaluated the miRNA profile in the retina and choroid during OIR, thereby uncovering the potential therapeutic values of specific miRNAs in the pathological progression of OIR [Citation19]. Besides, miR17 was reported to exert anti-angiogenic effects on neovascularization process [Citation20]. It remains unknown whether miR17 was involved in the biological role of celastrol in ROP progression.
To address this question, the present study investigated the role and mechanism of action of celastrol in hypoxia-induced human retinal microvascular endothelial cell (HRMEC) and in OIR mice. The results show that celastrol inhibits hypoxia-induced angiogenesis via modulation of a signaling axis composed of miRNA miR-17, VEGF, and HIF-1α.
2. Materials and methods
2.1. Mouse model of OIR
C57Bl/6 J mice were purchased from Vital River Biological Co. (Beijing, China). Animal care and experiments were carried out in accordance with the Association for Research in Vision and Ophthalmology guidelines [Citation21,Citation22] and were approved by the Experimental Animal Care and Use Committee of Nanjing Medical University. Newborn mice along with their mothers were exposed to 75% oxygen for 5 days from postnatal day (P)7 to P12, and then returned from P12 to P17 to normal conditions (21% oxygen). At P17, the mice were euthanized after anesthesia, and the eyes were removed for use in experiments.
2.2. Intravitreal injections of celastrol and miR-17 antagomir
Celastrol was diluted in phosphate-buffered saline (PBS) to 0, 10, 50, 100, and 1000 µM (Sigma-Aldrich, St. Louis, MO, USA) and then intravitreally injected into P12 mice (0.5 µl/eye). In another experiment, 2 nmol/µl miR-17 antagomir (RiboBio, Guangzhou, China) was intravitreally injected (0.5 µl/eye) 3 h before celastrol. Negative control antagomir was used as a control. A 33-G needle and Hamilton syringe were used for injections.
2.3. Immunofluorescent Staining
Mice were euthanized at P17 and the retina was isolated from the eye cups and prepared as flat-mount retinas. Whole-mount fixed retinas were washed in PBS containing 0.1% Tween-20 which was used for rinsing, then blocked for 1 hour in 0.2% bovine serum albumin and 0.3% Triton X-100, then incubated in blocked buffer with fluorescein-labeled isolectin B4 (1:150 dilution; Vector Laboratories, Burlingame, CA) overnight at 4°C. Retinas were then washed in 0.3% Triton X-100 three times, then mounted on slides with Prolong Gold Antifade Reagent.
Paraffin sections of mouse eyes were obtained from Servicebio (Wuhan, China) for immunohistochemistry. Antigen retrieval was performed by heating at 98°C for 8 h in citrate antigen retrieval solution (pH 6.0). After blocking with 1% bovine serum albumin (BSA) for 1 h at room temperature, the sections were incubated overnight at 4°C with primary antibodies for fluorophore-conjugated isolectin B4 (IB4; 1:150 dilution), glial fibrillary acidic protein (1:200; Proteintech, 16,825-1-AP, Wuhan, P.R.C), vascular endothelial growth factor (VEGF; 1:200 dilution, Abcam, ab52917), hypoxia-inducible factor 1 (HIF-1 alpha; 1:150 dilution, Abcam, ab1) or ionized calcium-binding adapter molecule (Iba)1 (1:200 dilution; Proteintech, 10,904-1-AP, Wuhan, P.R.C). Some samples were then incubated with fluorescein isothiocyanate (FITC)- and Cy3-conjugated secondary antibodies or directly protected with mounting medium (TA-030-FM, Mountant Permafluor; Lab Vision Corporation, Fremont, CA) the following day. Whole-mount retina samples or paraffin sections were photographed under a confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany).
2.4. Quantitation of retinal neovascularization (RNV) in OIR mice
Retinal vaso-obliteration (RVO) and RNV were quantitatively analyzed as previously described [Citation21]. Briefly, in images of whole-mount retinas and immunolabeled retinal tissue sections from OIR, OIR+celastral, and control mice, RVO and pathologic RNV were quantified by comparing the number of pixels within a given area with the total number of pixels in the retina using Image-Pro Plus software (Media Cybernetics, Silver Springs, MD, USA).
Images of retinal whole-mount endothelial cells, stained with IB4, were obtained using Zeiss AxioCam MRm, Zeiss AxioObserver.Z1 microscope and AxioVision 4.6.3.0 software. Select the magic wand tool. Then, set the tolerance to a level that will pick up new vessels while excluding normal vessels. Click the add to selection key and make sure the boxes next to “contiguous” and “Anti-alias” are checked. The process of imaging the cells with the microscope are as follows: select the wand icon and set the tolerance to a level, such that new vessels will be detected while ignoring the normal vessels. Once set click the add to selection button, being sure that both the ‘contiguous ‘ and “Anti-alias” boxes are checked. Now, using the wand tool, select regions containing new vessels – these areas will fluoresce with a greater intensity than the normal vessels – and color them in red [Citation21].
2.5. Human retinal microvascular endothelial cell (HRMEC) culture and treatment
HRMECs (Angio-Proteomie; Boston, MA; CAP-0010) were cultured in endothelial cell medium (ECM) (ScienCell, Carlsbad, CA, USA) according to the manufacturer’s instructions. Cells from passages 5–7 were used for experiments. The cells were transfected with micrOFF miRNA inhibitor (100 nM) or the negative control (RiboBio) using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. For hypoxia treatment, cells were placed in a homemade incubator [Citation23] that was flushed with a mixture of 1% O2, 5% CO2, and 94% N2 both with or without treatment using 0.1, 0.25, and 0.5 µM celastrol (Sigma-Aldrich).
2.6. Wound healing assay
Confluent HRMECs were scratched with a 100-µl pipet tip and detached cells were removed by washing with PBS. The cells were cultured with VEGF (10 ng/ml) and different concentrations of celastrol and/or miR-17 inhibitor (RiboBio, Guangzhou, China) in ECM containing 0.5% fetal bovine serum (FBS). Images of the cells were acquired after incubation for 24 and 48 h. Wound width was measured and quantified using Image-Pro Plus software.
2.7. Real-time cell analyzer (RTCA) proliferation and migration assays
RTCA proliferation and migration assays measure the effect of a treatment in real time [Citation24]. For RTCA proliferation experiments, 2 × 103 cells were seeded in an E-plate 16 (ACEA Biosciences, San Diego, CA, USA). After 8 h, the ECM was replaced with serum-free medium with or without 10 ng/ml VEGF, and different concentrations of celastrol. For RTCA migration experiments, cells were seeded in a CIM-16 plate (xCELLigence Roche, Penzberg, Germany) in serum-free medium with different concentrations of celastrol and 10 ng/ml VEGF added to the lower chamber as a chemoattractant.
2.8. Aortic ring angiogenesis assay
Cross sections of the aorta of Sprague-Dawley rats (National Institutes of Health [NIH], Bethesda, MD, USA) were cut to a length of 1 mm. The aortic rings were embedded in a Matrigel matrix in a 96-well plate (Corning, NY, USA) and cultured in M119 medium supplemented with 2% FBS, VEGF (10 ng/ml), and 0.25 or 0.5 µM celastrol. The growth medium was changed every 2 days. After 6 days, microvessel sprouting was examined and photographed at 100× magnification under a light microscope (Olympus, Tokyo, Japan). The degree of sprouting was graded from 0 (least positive) to 5 (most positive) in a double-blinded manner.
2.9. Tube formation assay for HRMECs
HRMECs were cultured overnight in ECM containing 1% FBS without growth factors. The following day, 1 × 104 HRMECs resuspended in 100 µl ECM with VEGF (10 ng/ml) and different concentrations of celastrol and/or miR-17 inhibitor were seeded on a growth factor-reduced Matrigel matrix in a 96-well plate. After 4 and 8 h, capillary-like structures were observed by microscopy and analyzed using the Angiogenesis Analyzer tool for NIH ImageJ software (http://rsb.info.nih. gov/ij) between more than 3 images.
2.10. Apoptosis assay
After treatment with different concentrations of celastrol for 24 h, HRMECs were harvested and double-stained with Annexin V-fluorescein isothiocyanate/propidium iodide for 15 min. The percentage of apoptotic cells was determined by flow cytometry analysis (cat. no. FMSAV647-100, FCMACS Biotech Co. Ltd.,
Nanjing, China).
2.11. TUNEL staining
In situ cell apoptosis was detected in frozen retinal sections with TUNEL assay (Beyotime Biotechnology, Nanjing, China) following the manufacturer’s instructions. Briefly, the 4% PFA-fixed sections were permeated with 0.2% Triton X-100 for 10 min. Then, they were incubated with the TUNEL reaction mixture in a dark humidified atmosphere for 2 hours at room temperature. After that, the nuclei were stained with DAPI for 5 min in the dark. The stained slides were then photographed under a confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany).
2.12. Western blotting
Total protein from cells and tissues was extracted using radioimmunoprecipitation assay lysis buffer (Beyotime, Shanghai, China) supplemented with protease inhibitors (Roche) and phenylmethylsulfonyl fluoride (Beyotime). Equal amounts (30 µg) of protein per sample were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. After blocking with 5% BSA, the membrane was incubated overnight at 4°C with primary antibodies, including vascular endothelial growth factor (VEGF; 1:1000 dilution, Abcam, ab52917), hypoxia-inducible factor 1 (HIF-1 alpha; 1:1000 dilution, Abcam, ab1), proliferating cell nuclear antigen (PCNA; 1:2000 dilution, Proteintech, 10,205-2-AP), SAPK/JNK (1:1000 dilution, Cell Signaling Technology,#9252), Phospho-SAPK/JNK p-JNK (1:1000 dilution, Cell Signaling Technology, #4668), Erk1/2 (1:1000 dilution, Cell Signaling Technology,#4695), p-Erk1/2 (1:1000 dilution, Cell Signaling Technology,#8544), p38 (1:1000 dilution, Cell Signaling Technology,#14451), p-p38 (1:1000 dilution, Cell Signaling Technology,#4631), P300 (1:1000 dilution, Cell Signaling Technology, #86377), CBP (1:1000 dilution, Cell Signaling Technology, #7389), HDAC1 (1:1000 dilution, Cell Signaling Technology, #34589) and HDAC3 (1:1000 dilution, Cell Signaling Technology, #3949) against β-actin (1:2000 dilution, Proteintech, 60,008-1-Ig) and Lamin B (1:2000 dilution, Proteintech, 12,987-1-AP). After incubation with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature, immunoreactivity was detected with enhanced chemiluminescence reagent (Thermo Fisher Scientific, Waltham, MA, USA) and protein bands were analyzed and quantified using ImageJ software.
2.13. mRNA isolation and quantitative real-time (qRT-)PCR
TRIzol reagent (Invitrogen) was used to isolate total RNA from cells and tissue. After determining RNA concentration, cDNA was obtained by reverse transcription using PrimeScript RT reagent kit (Takara Bio, Otsu, Japan) according to the manufacturer’s protocol. The expression levels of miR-17-5p were measured using quantitative real-time PCR (qRT-PCR) performed with the Bulge-LoopTM miRNA Reverse Transcription Kit (RiboBio), the Bulge- LoopTM miRNA qRT-PCR Starter Kit (RiboBio), and miR-17 primers (RiboBio) and were normalized using small nuclear RNA (U6 snRNA, RiboBio) as the internal control. qRT-PCR was performed using SYBR green reagent and primers specific to human target gene sequences (Supplemental Table 1) on an ABI Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA, USA). Target gene expression levels relative to the level of the internal control β-actin were calculated with the 2−ΔΔCt method.
2.14. Ultraviolet (UV) detection of celastrol concentration
We diluted mother liquor of celastrol to 0,2,4,8,16,32 μg/mL with methanol and prepared the standard curve (absorbance vs. concentration of celastrol) by spectrophotometer under 424 nm UV light. Then, the retinas were isolated from mice receiving intravitreal injection of different concentrations of celastrol, and dissolved in 20 µl methanol per eye. After that, the samples were detected under 424 nm UV light; then the OD values were then quantified using the previously generated standard curve.
2.15. Statistical analysis
The data is expressed as mean ± SEM. One-way analysis of variance followed by Bonferroni’s post hoc test was used to evaluate the significance of mean differences between more than 2 groups. Values were considered statistically significant for P < 0.05. Statistical analyses were performed using Prism v7.0 software (GraphPad, La Jolla, CA, USA).
3. Results
3.1. Celastrol inhibits VEGF-induced HRMEC proliferation and migration
To investigate the inhibitory effects of celastrol on VEGF-induced angiogenesis in vitro, we first examined the induction of apoptosis by celastrol. Apoptosis was increased ~2.0-fold by treatment with celastrol at a concentration of 1 µM (*P < 0.05), while no effect was observed after 24 h at concentrations of 0.01, 0.1, 0.25, and 0.5 µM (), p>0.05). The wound healing assay and the RTCA showed a dose-dependent inhibition of VEGF-induced HRMEC migration in the presence of 0.25 or 0.5 µM celastrol, with a more potent effect at the higher concentration ()) (Fig. S1A). A similar inhibitory effect was observed on cell proliferation. In the RTCA assay, 0.5 µM celastrol decreased VEGF-induced proliferation (~2.0-fold), which was confirmed by the reduction in proliferating cell nuclear antigen (PCNA) expression observed by immunofluorescence analysis ()).
Figure 1. Celastrol inhibits VEGF-induced HRMEC proliferation and migration. (a-b) Flow cytometry analysis of apoptosis in HRMECs treated with indicated concentrations of celastrol. (c–e)The effects of celastrol (0.01, 0.1, 0.25, 0.5 μM) on 10 ng/ml VEGF-induced migration and angiogenic potential in HRMECs were evaluated with the wound healing assay (c) and tube formation (d) and aortic ring (e) assays. (f-g) Effects of celastrol on VEGF-induced proliferation and migration of HRMECs evaluated with the RTCA proliferation (f) and migration (g) assays. (h-i) Analysis of cell proliferation by immunofluorescence detection of PCNA (red) in HRMECs treated with celastrol (0.01, 0.1, 0.25, 0.5 μM). Nuclei were stained with DAPI (blue). Scale bar, 100 μm. Data are shown as mean ± SEM (n = 6 per group). NS, not significant. *P < 0.05, VEGF group vs control group; #, $,£P < 0.05, 0.1 μM, 0.25 μM, 0.5 μM celastrol vs VEGF group.
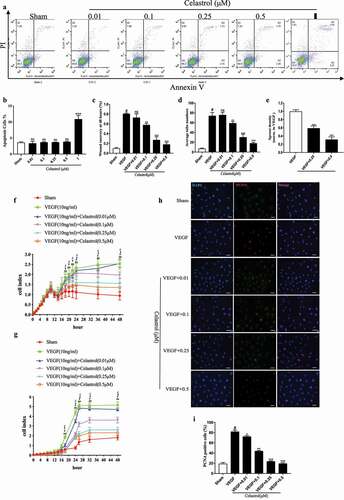
We evaluated the effect of celastrol on angiogenesis with the tube formation assay. HRMECs were treated with different concentrations of celastrol and placed in growth factor-reduced Matrigel. Treatment with 0.25 or 0.5 µM celastrol blocked VEGF-induced tube formation, suggesting that angiogenesis was inhibited ()) (Fig.S1B). We also cultured aortic rings with or without celastrol and evaluated vessel sprouting. In the presence of VEGF, microvessels formed a network around the aortic rings; however, this was suppressed in a dose-dependent manner by application of celastrol, with the most obvious effect (~3.0-fold) at a concentration of 0.5 µM ()) (Fig.S1C). Taken together, these results indicate that celastrol prevents VEGF-induced angiogenesis in vitro by inhibiting endothelial cell migration, proliferation, and tube formation.
3.2. Celastrol suppresses hypoxia-induced expression of VEGF and HIF-1α in vitro
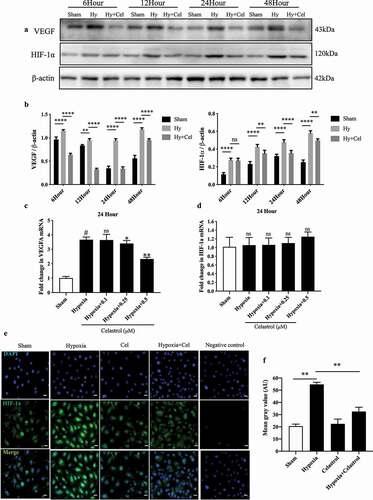
We examined the effect of celastrol on the expression of VEGF and HIF-1α induced by culture under hypoxia for 24 h by western blotting and qRT-PCR. Celastrol (0.5 µM) suppressed the ~2.0-fold and ~5.0-fold expression of VEGF and HIF-1α proteins at the 24-h time point, respectively; however, while the same treatment decreased the mRNA level of VEGF (~1.5-fold), it had no effect on that of HIF-1α. Immunofluorescence analysis revealed that celastrol could reduce hypoxia-induced activation and expression of HIF-1α in HRMECs ()). In addition, results of western blotting shown in FigureS2C indicated that nuclear import of HIF-1α was also blocked by celastrol.
Figure 2. Celastrol inhibits hypoxia-induced VEGF and HIF-1α expression in vitro. (a, b) VEGF and HIF-1α protein levels determined by western blotting in HRMECs exposed to hypoxic conditions with or without celastrol (0.5 μM) treatment at 6, 12, 24, and 48 h; β-actin was used as a loading control. (c, d) VEGF (c) and HIF-1α (d) mRNA expression levels in HRMECs determined by qRT-PCR and normalized to β-actin level. (e, f) Representative immunofluorescence images (e) and quantification (f) of HIF-1α expression (red) in HRMECs. Nuclei were stained with DAPI (blue). Scale bar, 100 μm. Data are shown as mean ± SEM (n = 6 per group). NS, not significant. *P < 0.05, **P < 0.01, ***P < 0.001, #vs control group; *vs hypoxia group.
3.3. Celastrol alleviates angiogenesis in vivo by blocking HIF-1α-VEGF signaling
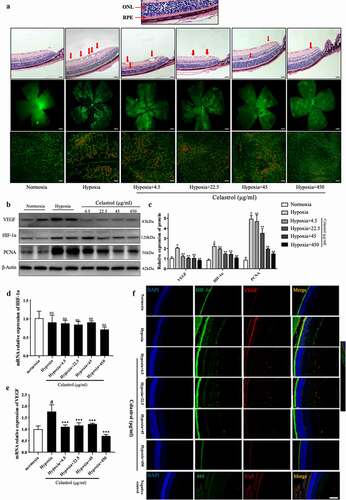
To confirm the effects of celastrol on neovascularization in the retina, we injected different concentrations of celastrol into the vitreous humor of P12 mouse pups and assessed vessel formation by isolectin (I)B4 staining of whole-mount retinal tissue specimens. First, as shown in FigureS4, no obvious retinal apoptosis could be observed by TUNEL staining in frozen sections of retinal tissues after exposed to different concentrations (4.5, 22.5, 45, 450 μg/mL) of celastrol for 5 days (d12-17). Then, neovascular and avascular areas were observed in the retina in the OIR group but not in the control group. However, these were markedly reduced in a dose-dependent manner following injection of celastrol (), FigureS3). Western blot analysis showed that HIF-1α, VEGF, and PCNA protein levels were decreased about 2.0–3.0-fold in the OIR group ()), and immunofluorescence analysis confirmed the dose-dependent inhibition of HIF-1α and VEGF expression by celastrol ()). Thus, the HIF-1α/VEGF pathway is activated in the retina of OIR mice, but this is reversed by celastrol treatment.
Figure 3. Celastrol blocks angiogenesis in vivo by inhibiting HIF-1α/VEGF pathway activation. (a) Representative images of hematoxylin-eosin (HE) staining in retinal tissue sections (top; scale bar, 200 μm), IB4 immunolabeling in whole-mount retinal tissue specimens (middle; scale bar, 500 μm), and high-magnification views (bottom, scale bar, 100 μm). (b, c) Western blot analysis of VEGF, HIF-1α, and PCNA protein levels in retinal tissues samples (left) and corresponding densitometric analysis (right); β-actin was used as the loading control. (d, e) HIF-1α (d) and VEGF (e) mRNA levels in retinal tissues determined by qRT-PCR and normalized to β-actin level. (f) Representative immunofluorescence images of HIF-1α (green) and VEGF (red) expression in retinal tissue sections. Nuclei were stained with DAPI (blue). Scale bar, 20 μm. Data are shown as mean ± SEM (n = 8 per group). NS, not significant. #vs control group; *P < 0.05, **P < 0.01, ***P < 0.001 vs hypoxia group.
3.4. Celastrol improves hypoxic-induced glial cell imbalance and reduces inflammatory cytokine expression in the retina
Pathologic retinal angiogenesis and retinal glial cell imbalance have been implicated in the progression of OIR. To determine whether the latter can be alleviated by celastrol, astrocytes and microglia were detected by immunolabeling with antibodies against glial fibrillary acidic protein and ionized calcium-binding adapter molecule. In the OIR group, the number of astrocytes was decreased while the microglia number was increased relative to control animals. These trends were reversed in a dose-dependent manner by celastrol treatment ()). The more detailed panels have been shown in Figure.S6-7.
Figure 4. Celastrol reverses hypoxia-induced glial cell imbalance and increased inflammatory cytokine levels in the retina. (A–H) IL-1β, IL-6, TNF-α, MCP-1, MMP-9, COX-2, ICAM-1, and VCAM-1 mRNA levels in retinal tissue were determined by qRT-PCR and normalized to β-actin level. (I) Representative immunofluorescence images of IB4 (green) and glial fibrillary acidic protein (GFAP; red) or ionized calcium-binding adapter molecule (Iba)1 (red) expression in retinal tissue sections. Nuclei were stained with DAPI (blue). Scale bar, 20 μm. Data are shown as mean ± SEM (n = 8 per group). NS, not significant. #vs control group; *P < 0.05, **P < 0.01, ***P < 0.001 vs hypoxia group.
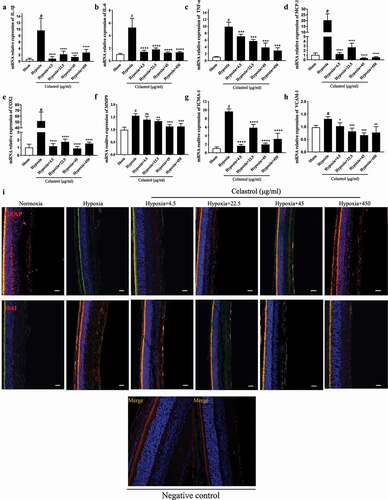
We also examine the effects of celastrol on inflammation in the retina of OIR mice at P17. The mRNA levels of the proinflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α were upregulated over 5-fold in the retina of OIR mice but downregulated following celastrol treatment. Similar trends were observed for markers of chronic inflammation (cyclooxygenase [COX]2 and monocyte chemoattractant protein [MCP]-1) and adhesion (matrix metalloproteinase [MMP], intercellular adhesion molecule [ICAM]-1, and vascular cell adhesion molecule [VCAM]-1) ()).
3.5. Celastrol promotes miR-17 expression and inhibits HIF-1α/VEGF signaling in HRMECs
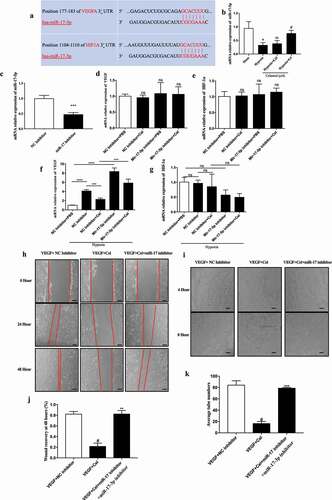
The mitogen-activated protein kinase (MAPK) pathway is involved in retinal angiogenesis [Citation25]. We found here that treatment with celastrol did not reverse the increase in p38 MAPK and extracellular signal-regulated kinase phosphorylation caused by hypoxia in HRMECs (Fig.S2A-B). HIF-1α has been shown to regulate the expression of miR-17 [Citation26]; we therefore examined whether celastrol inhibits angiogenesis in retinal cells via regulation of miR-17 expression. miR-17 was downregulated ~4.0-fold in HRMECs under hypoxia; which was reversed by treatment with celastrol (). Using TargetScan (http://www.targetscan.org/vert_72/), we determined that both HIF-1α and VEGF are potential target genes of miR-17 ()). Treatment of HRMECs with miR-17 inhibitor had no effect on the mRNA levels of VEGF and HIF-1α under normoxic conditions ()). However, under hypoxia, the levels of VEGF were increased about 4-fold following pretreatment with miR-17 inhibitor compared to cells treated with celastrol alone ()). The results of the wound healing and tube formation assays showed that miR-17 inhibitor reversed the suppression of VEGF-induced angiogenesis by celastrol ()). Thus, celastrol inhibits HIF-1α/VEGF signaling and angiogenesis by increasing the expression of miR-17.
Figure 5. Celastrol promotes miR-17 expression in HRMECs and suppresses HIF-1α/VEGF signaling. (a) Identification of HIF-1α and VEGF as target genes of miR-17 using TargetScan software. (b) miR-17-5p levels in HRMECs determined by qRT-PCR. (c) Effect of miR-17-5p inhibitor treatment in HRMECs. (d–g) mRNA levels of HIF-1α (e, g) and VEGF (d, f) under normoxia and hypoxia following treatment with miR-17-5p inhibitor. (h-k) Migratory and angiogenic potential of HRMECs evaluated with the wound healing (h, j) and tube formation (i, k) assays, respectively. Scale bar, 200 μm. Data are shown as mean ± SEM (n = 6 per group). NS, not significant. #vs control group; *P < 0.05, **P < 0.01, ***P < 0.001.
3.6. Inhibiting miR-17 expression reverses the effects of celastrol on angiogenesis and inflammatory cytokine release in vivo
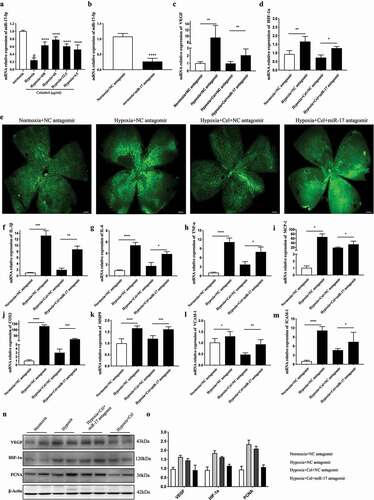
We next examined whether celastrol negatively regulates miR-17 expression in vivo. Intravitreal injection of miR-17 antagomir 3 h before celastrol treatment in a mice model of OIR reduced miR-17 level in the retina, confirming the inhibition of miR-17 ()). IB4 staining of whole-mount retinal tissue specimens showed that miR-17 antagomir blocked the inhibitory effect of celastrol on angiogenesis ()). In addition, HIF-1α, VEGF protein and mRNA levels were significantly increased ~1.5–2.0-fold in mice treated with miR-17 antagomir plus celastrol compared those treated with celastrol alone (). Similar trends were observed for inflammatory factors ()). These data suggest that celastrol suppresses hypoxia-induced angiogenesis and inflammation by increasing miR-17 expression.
Figure 6. Inhibition of miR-17 reverses the effect of celastrol on angiogenesis and inflammatory cytokine release in vivo. (a) miR-17-5p levels in retinal tissues determined by qRT-PCR. (b–d) Effects of miR-17-5p antagomir (b), and HIF-1α (d) and VEGF (c) mRNA levels in retinal tissue. (e) Representative images of IB4 expression (green) in whole-mount retinal tissue specimens. Scale bar, 500 μm. (f–m) IL-1β, IL-6, TNF-α, MCP-1, MMP-9, COX-2, ICAM-1, and VCAM-1 mRNA levels in retinal tissue determined by qRT-PCR and normalized to β-actin level. (n, o) Western blot analysis of VEGF, HIF-1α, and PCNA protein levels in retinal tissue (left) and corresponding densitometric analysis (right). β-Actin was used as the loading control. Data are shown as mean ± SEM (n = 6 per group). NS, not significant. #vs control group; *P < 0.05, **P < 0.01, ***P < 0.001.
4. Discussion
The results of our study demonstrate that celastrol, a bioactive compound that is used in traditional Chinese medicine, regulates hypoxia-induced retinal neovascularization by maintaining the function of microvascular endothelial cells. This is the first evidence that celastrol inhibits sprouting of retinal microvessels and pathologic angiogenesis in a hypoxic environment by negatively modulating the miR-17/HIF-1α/VEGF signaling axis.
As a major cause of neonatal blindness, ROP is characterized by increased vascular permeability, neovascularization, loss of microvasculature, and inflammation [Citation27]. The OIR mouse model is widely used to study ROP and other proliferative retinal vasculopathies [Citation22,Citation28]. Here we investigated whether celastrol, which has been shown to promote the maintenance of a healthy endothelial barrier during inflammation-induced angiogenesis by suppressing VEGF secretion and inhibiting endogenous peroxynitrite formation in endothelial cells [Citation29–31], has the same effect on pathologic angiogenesis under hypoxic conditions. Our results showed that celastrol treatment suppressed HRMEC proliferation, microvessel sprouting, and migration induced by VEGF or hypoxia. Pathologic angiogenesis and glial cell dysfunction in the retina are mutually dependent in OIR [Citation32]. A decrease in the number of retinal glial cells – including Müller cells and astrocytes located in the nerve fiber layer – can result in the abnormal development and function of retinal microvessels [Citation33], and the absence of proper guidance of retinal astrocytes during development has been shown to result in pathologic neovascularization in vivo [Citation34]. In the present study, we found that celastrol treatment protected retinal microvasculature and promoted vascular reconstruction in avascular areas of the retina in OIR mice by preserving the number of astrocytes.
Like astrocytes, microglia aggregate and attach to vascular tufts in the retina following activation under pathologic conditions such as ischemia-induced injury to stimulate angiogenesis [Citation35,Citation36]. The increase in microglia numbers in OIR can lead to excessive release of proinflammatory cytokines, leading to microvascular injury and pathologic angiogenesis [Citation37]. We observed that celastrol treatment attenuated the hypoxia-induced activation of retinal microglia by suppressing the levels of proinflammatory cytokines including IL-1, IL-6, TNF-α, and MCP-1 as well as associated enzymes such as COX2 and MMP9, suggesting a close relationship between the immune response and retinal angiogenesis. This is supported by the finding that celastrol exerts anti-inflammatory effects in rheumatoid and other immune diseases by reducing proinflammatory cytokine expression of via NF-κB or other pathways [Citation7,Citation10,Citation38,Citation39].
HIF-1α/VEGF signaling is the main mechanism by which the immune response acts in coordination with local or systemic stimuli to modulate microvascular permeability and angiogenesis in endothelial cells [Citation40]. HIF-1α is a transcription factor that is translocated to the nucleus under oxygen tension [Citation41,Citation42], and its expression was shown to be increased by proinflammatory factors in a normoxic environment [Citation43]. Several antineoplastic drugs attenuate tumor angiogenesis by decreasing VEGF levels through HIF-1α inhibition [Citation44]. Previous studies have shown that the HIF-1α/VEGF pathway plays a critical role in retinal angiogenesis and endothelial cell proliferation [Citation36]. As celastrol was reported to play an antiangiogenic role in various cancers and inhibit the transcriptional activity of HIF-1α [Citation45], we evaluated its effect on HIF-1α and VEGF expression in our study. Our results showed that celastrol treatment resulted in the downregulation of VEGF and HIF-1α and prevented the nuclear import of HIF-1α in HRMECs. Celastrol also suppressed pathologic angiogenesis triggered by hypoxia via inhibition of HIF-1α/VEGF signaling, providing insight into the molecular basis of the antiangiogenic activity of celastrol. Interestingly, in our vitro study, hypoxia could induce the increase of protein rather than mRNA levels of HIF-1α. In addition to our study, previous reports have also shown that HIF-1α protein accumulates rapidly during hypoxia without a significant increase in HIF-1α mRNA levels [Citation46,Citation47]. Some researchers demonstrated that hypoxia-induced increase of protein levels of HIF-1α depends on the increased stability of the proteins themselves rather than the increase of mRNA expression [Citation48]. Further, histone deacetylase (HDAC)1, which regulates HIF-1α activity, was inhibited by the effect of celastrol, suggesting that the negative regulation of VEGF by celastrol partly depends on HIF-1α stabilization through HDAC1 (Fig.S2C-E).
MicroRNAs (miRNAs), the phylogenetically conserved endogenous non-coding small single-stranded RNAs in multicellular organisms, play a critical role in regulating the output of many protein-coding genes by influencing mRNAs stability and translational efficiency [Citation49,Citation50]. Notably, several miRNAs, including the miR-17 family, are known to promote or suppress angiogenesis by integrating into introns of protein-coding genes [Citation51,Citation52].
Doebele et al., showed that the miR-17-92 cluster could downregulate sprouting in endothelial cells [Citation53]. Recently, miR17 has also been reported to inhibit neovascularization in vivo, suggesting its anti-angiogenic properties in the maintenance of vascular integrity [Citation20]. Downregulation of miR-17 family members was also shown to play a key role in early angiogenic transformation leading to altered expression of HIF-1α and VEGF [Citation54,Citation55]. Previous studies suggested that HIF-1α agonist-activated miR-17-5p overexpression promotes gastric cancer development and metastasis by repressing PDCD4 as well [Citation56], which provides a strongly link between miR-17 and HIF-1α. Also, another study has proved celastrol to be an inhibitor of HIF-1α activation in human hepatoma cells [Citation57]. Moreover, celastrol was found to downregulate the expression of miR-17, thereby resulting in the induction of autophagy in vitro [Citation26]. Therefore, the link between celastrol, HIF-1α and miR-17-5p is reliable. Thus, we aimed to further verify whether miR-17 is also involved in the anti-angiogenesis effects of celastrol on attenuating OIR. In our study, we showed that celastrol treatment enhanced the hypoxia-induced decrease in miR-17 level in HRMECs, while miR-17 inhibitor reversed the inhibitory effect of celastrol on VEGF expression. Moreover, miR-17 inhibition increased RNV in mice treated with celastrol by increasing VEGF and HIF-1α levels. These results indicate that celastrol blocks angiogenesis by decreasing HIF-1α/VEGF pathway activation through upregulation of miR-17.
The activation of endothelial cells (ECs) plays a pivotal role in angiogenesis triggered by oxygen deficiency by mediating the transcription of multiple angiogenic genes, including VEGF [Citation58]. Endothelial cells have a high expression of miR-17-5p [Citation59]. Silencing miR-17-5p in vascular smooth muscle under hypoxic conditions has an anti-apoptotic effect [Citation60]. The previous study also showed that miR-17-5p loss-of function could protect heart function after AMI through decreasing the rate of apoptosis on ECs and repairing vascular injury [Citation61]. In addition, celastrol has been reported to attenuate EC damage through regulating apoptosis, oxidative stress, and immune response via several signal pathways under different pathological conditions [Citation62–64]. Considering the close association between celastrol and miR-17 discussed above, it is reasonable for us to suppose that the protective role of miR-17 in improving the vascular repair via attenuating the apoptosis in ECs could be another protective mechanism accounting for therapeutic effects of celastrol on OIR. However, this remains to be further confirmed.
Notably, in an effort to further accelerate the potential of celastrol to be utilized in future clinical practice, researchers have been investigating on the various therapeutic mechanisms and targets of celastrol that underlie its broad spectrum of pharmacological properties [Citation65]. However, the toxicological drawbacks of celastrol may limit its clinical use [Citation66]. The dosage used in our study was quite small, and no obvious retinal apoptosis of celastrol could be observed in vivo and in vitro. Our results show that, despite using a low dosage to the point of having minimal detection (Fig.S5A-B), there was a very strong anti-angiogenesis effect. Therefore we have reason to believe that the potency of celastrol in the retina under hypoxic conditions is significant. Further clinical trials may be conducted to provide a clear insight into the safety of celastrol, as well as to determine its optimum dosage in the treatment of OIR in the future.
In summary, the results of our study demonstrate for the first time that celastrol treatment can preserve glial cell function, reduce inflammation, and prevent neovascularization in the retina in OIR via inhibition of the miR-17/HIF-1α/VEGF signaling axis. These findings highlight the therapeutic potential of celastrol for the treatment of ROP ().
Ethics approval and consent to participate
All procedures were approved by the Experimental Animal Care and Use Committee of Nanjing Medical University and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85–23, revised 1996). The ethical approval number is IACUC-1710006.
Biosecurity statement
All standard biosecurity and institutional safety procedures have been adhered to in all experiments described in this article.
Author contributions
YC, CY and PZ designed the work and prepared the manuscript. KZ and YJ performed the experiments, conducted data analyses, and wrote the manuscript. JZ and XW analyzed and interpreted the data. All authors discussed the results, and read and approved the final version of the manuscript for publication.
Supplemental Material
Download Zip (24.4 MB)Acknowledgments
This study was supported by the Youth Project of Shanghai Municipal Commission of Health (grant number 20194Y0238), the research fund project of Yangpu hospital of Tongji University (grant number Se1201821), the morning light project for young talents of Yangpu hospital of Tongji University (grant number Ye1201903).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384101.2022.2087277
Additional information
Funding
References
- Good WV, Hardy RJ, Dobson V, et al., Early Treatment for Retinopathy of Prematurity Cooperative, G. The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116:15–23.
- Rivera JC, Dabouz R, Noueihed B, et al. Ischemic retinopathies: oxidative stress and inflammation. Oxid Med Cell Longev. 2017;2017:3940241.
- Grisanti S, Tatar O. The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog Retin Eye Res. 2008;27(4):372–390.
- Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48.
- Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367(26):2515–2526.
- Zhou Y, Yoshida S, Nakao S, et al. M2 macrophages enhance pathological neovascularization in the mouse model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2015;56(8):4767–4777.
- Shaker ME, Ashamallah SA, Houssen ME. Celastrol ameliorates murine colitis via modulating oxidative stress, inflammatory cytokines and intestinal homeostasis. Chem Biol Interact. 2014;210:26–33.
- Venkatesha SH, Yu H, Rajaiah R, et al. Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J Biol Chem. 2011;286(17):15138–15146.
- Nanjundaiah SM, Venkatesha SH, Yu H, et al. Celastrus and its bioactive celastrol protect against bone damage in autoimmune arthritis by modulating osteoimmune cross-talk. J Biol Chem. 2012;287(26):22216–22226.
- Lee JH, Koo TH, Yoon H, et al. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem Pharmacol. 2006;72(10):1311–1321.
- Polivka J Jr., Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142(2):164–175.
- Huang L, Zhang Z, Zhang S, et al. Inhibitory action of celastrol on hypoxia-mediated angiogenesis and metastasis via the HIF-1alpha pathway. Int J Mol Med. 2011;27(3):407–415.
- Hudson CC, Liu M, Chiang GG, et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22(20):7004–7014.
- Pang X, Yi Z, Zhang J, et al. Celastrol suppresses angiogenesis-mediated tumor growth through inhibition of AKT/mammalian target of rapamycin pathway. Cancer Res. 2010;70(5):1951–1959.
- Zhou YX, Huang YL. Antiangiogenic effect of celastrol on the growth of human glioma: an in vitro and in vivo study. Chin Med J (Engl). 2009;122(14):1666–1673.
- Paimela T, Hyttinen JM, Viiri J, et al. Celastrol regulates innate immunity response via NF-kappaB and Hsp70 in human retinal pigment epithelial cells. Pharmacol Res. 2011;64(5):501–508.
- Friedman RC, Farh KK, Burge CB, et al. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105.
- Plastino F, Pesce NA, André H. MicroRNAs and the HIF/VEGF axis in ocular neovascular diseases. Acta Ophthalmol. 2021;99(8):e1255–e1262.
- Desjarlais M, Rivera JC, Lahaie I, et al. MicroRNA expression profile in retina and choroid in oxygen-induced retinopathy model. PLoS One. 2019;14(6):e0218282.
- Di Stefano AB, Massihnia D, Grisafi F, et al. Adipose tissue, angiogenesis and angio-MIR under physiological and pathological conditions. Eur J Cell Biol. 2019;98(2–4):53–64.
- Connor KM, Krah NM, Dennison RJ, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4(11):1565–1573.
- Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111.
- Ahmed NE, Murakami M, Kaneko S, et al. The effects of hypoxia on the stemness properties of human dental pulp stem cells (DPSCs). Sci Rep. 2016;6(1):35476.
- Solly K, Wang X, Xu X, et al. Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev Technol. 2004;2(4):363–372.
- Yao B, Wang S, Xiao P, et al. MAPK signaling pathways in eye wounds: multifunction and cooperation. Exp Cell Res. 2017;359(1):10–16.
- Guo J, Mei Y, Li K, et al. Downregulation of miR-17-92a cluster promotes autophagy induction in response to celastrol treatment in prostate cancer cells. Biochem Biophys Res Commun. 2016;478(2):804–810.
- Kim YH, Chung IY, Choi MY, et al. Triamcinolone suppresses retinal vascular pathology via a potent interruption of proinflammatory signal-regulated activation of VEGF during a relative hypoxia. Neurobiol Dis. 2007;26(3):569–576.
- Rivera JC, Madaan A, Zhou TE, et al. Review of the mechanisms and therapeutic avenues for retinal and choroidal vascular dysfunctions in retinopathy of prematurity. Acta Paediatrica. 2016;105(12):1421–1433.
- Huang S, Tang Y, Cai X, et al. Celastrol inhibits vasculogenesis by suppressing the VEGF-induced functional activity of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun. 2012;423(3):467–472.
- Wu F, Han M, Wilson JX. Tripterine prevents endothelial barrier dysfunction by inhibiting endogenous peroxynitrite formation. Br J Pharmacol. 2009;157(6):1014–1023.
- Zhang DH, Marconi A, Xu LM, et al. Tripterine inhibits the expression of adhesion molecules in activated endothelial cells. J Leukoc Biol. 2006;80:309–319.
- Karlstetter M, Scholz R, Rutar M, et al. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res. 2015;45:30–57.
- Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31(5):377–406.
- Bucher F, Stahl A, Agostini HT, et al. Hyperoxia causes reduced density of retinal astrocytes in the central avascular zone in the mouse model of oxygen-induced retinopathy. Mol Cell Neurosci. 2013;56:225–233.
- Checchin D, Sennlaub F, Levavasseur E, et al. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006;47(8):3595–3602.
- Xu Y, Lu X, Hu Y, et al. Melatonin attenuated retinal neovascularization and neuroglial dysfunction by inhibition of HIF-1alpha-VEGF pathway in oxygen-induced retinopathy mice. J Pineal Res. 2018;64(4):e12473.
- Davies MH, Eubanks JP, Powers MR. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Mol Vis. 2006;12:467–477.
- Bian M, Du X, Cui J, et al. Celastrol protects mouse retinas from bright light-induced degeneration through inhibition of oxidative stress and inflammation. J Neuroinflammation. 2016;13(1):50.
- Ma J, Han LZ, Liang H, et al. Celastrol inhibits the HIF-1alpha pathway by inhibition of mTOR/p70S6K/eIF4E and ERK1/2 phosphorylation in human hepatoma cells. Oncol Rep. 2014a;32(1):235–242.
- Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem. 2009;284(38):25602–25611.
- Kaelin WG Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402.
- Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol. 2014;76(1):39–56.
- Bonello S, Zahringer C, BelAiba RS, et al. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol. 2007;27(4):755–761.
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732.
- Shrivastava S, Jeengar MK, Reddy VS, et al. Anticancer effect of celastrol on human triple negative breast cancer: possible involvement of oxidative stress, mitochondrial dysfunction, apoptosis and PI3K/Akt pathways. Exp Mol Pathol. 2015;98(3):313–327.
- Huang LE, Arany Z, Livingston DM, et al. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271(50):32253–32259.
- Yuan Y, Hilliard G, Ferguson T, et al. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J Biol Chem. 2003;278(18):15911–15916.
- Kimura H, Weisz A, Kurashima Y, et al. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95(1):189–197.
- Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297.
- Soufi-Zomorrod M, Hajifathali A, Kouhkan F, et al. MicroRNAs modulating angiogenesis: miR-129-1 and miR-133 act as angio-miR in HUVECs. Tumour Biol. 2016;37(7):9527–9534.
- Wang S, Aurora AB, Johnson BA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–271.
- Doebele C, Bonauer A, Fischer A, et al. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood. 2010;115(23):4944–4950.
- Chen Z, Pan X, Sheng Z, et al. miR-17 regulates the proliferation and apoptosis of endothelial cells in coronary heart disease via targeting insulin-like-growth factor 1. Pathol Res Pract. 2019;215(9):152512.
- Pickering MT, Stadler BM, Kowalik TF. miR-17 and miR-20a temper an E2F1-induced G1 checkpoint to regulate cell cycle progression. Oncogene. 2009;28(1):140–145.
- Zhao J, Xiao A, Liu C, et al. The HIF-1A/miR-17-5p/PDCD4 axis contributes to the tumor growth and metastasis of gastric cancer. Signal Transduct Target Ther. 2020;5:46.
- Ma J, Han LZ, Liang H, et al. Celastrol inhibits the HIF-1α pathway by inhibition of mTOR/p70S6K/eIF4E and ERK1/2 phosphorylation in human hepatoma cells. Oncol Rep. 2014b;32(1):235–242.
- Alizadeh E, Mammadzada P, André H. The different facades of retinal and choroidal endothelial cells in response to hypoxia. Int J Mol Sci. 2018;19(12):3846.
- Bonauer A, Carmona G, Iwasaki M, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324(5935):1710–1713.
- Hao MX, Wang X, Jiao KL. MicroRNA-17-5p mediates hypoxia-induced autophagy and inhibits apoptosis by targeting signal transducer and activator of transcription 3 in vascular smooth muscle cells. Exp Ther Med. 2017;13(3):935–941.
- Yang S, Fan T, Hu Q, et al. Downregulation of microRNA-17-5p improves cardiac function after myocardial infarction via attenuation of apoptosis in endothelial cells. Mol Genet Genomics. 2018;293(4):883–894.
- Gong F, Zhao F, Gan XD. Celastrol protects TGF-β1-induced endothelial-mesenchymal transition. J Huazhong Univ Sci Technol. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban. 2017;37:185–190.
- Han XB, Tan Y, Fang YQ, et al. Protective effects of celastrol against γ irradiation-induced oxidative stress in human umbilical vein endothelial cells. Exp Ther Med. 2018;16(2):685–694.
- Li M, Liu X, He Y, et al. Celastrol attenuates angiotensin II mediated human umbilical vein endothelial cells damage through activation of Nrf2/ERK1/2/Nox2 signal pathway. Eur J Pharmacol. 2017;797:124–133.
- Ng SW, Chan Y, Chellappan DK, et al. Molecular modulators of celastrol as the keystones for its diverse pharmacological activities. Biomed Pharmacothe. 2019;109:1785–1792.
- Cascão R, Fonseca JE, Moita LF. Celastrol: a spectrum of treatment opportunities in chronic diseases. Front Med (Lausanne). 2017;4:69.