ABSTRACT
Oxaliplatin (OXA) is a first-line chemotherapy drug for gastric cancer. We aimed to investigate the effect of circ 0008253, contained in M2 polarized macrophage-derived exosomes, on OXA resistance of gastric carcinoma cells. Flow cytometry was performed to detect the differentiation of macrophages and cell apoptosis. Cell Counting Kit-8 assay was conducted to examine the cell viability. Transmission electron microscopy, Nanoparticle Tracking Analysis, Western bolt, and Immunofluorescence were carried out. Cell proliferation was detected with a colony formation experiment. Levels of CD206, Arg1, IL-10, and TGF-β were increased in M2 polarized macrophages. Cell viability was decreased gradually with the increase of time and OXA concentration. Apoptosis of gastric carcinoma cells was decreased after co-culture with M2-polarized macrophages. Exosomes isolated from M2-polarized macrophages (M2-Exos) could be co-located with gastric carcinoma cells. M2-Exos enhanced drug resistance, reduced apoptosis and OXA resistance. Bioinformatics analysis showed that circ 0008253 could be transferred from M2-Exos to gastric carcinoma cells. Overexpressing circ 0008253 increased cell viability, tumor size, and ABCG2 levels, decreased OXA sensitivity. Circ 0008253, contained in M2-Exos, was directly transferred from tumor-associated macrophage to gastric carcinoma cells, finally enhancing OXA resistance.
Introduction
Gastric cancer is currently the fourth most common malignant tumor in the world and is also the third leading cause of cancer-related death [Citation1,Citation2]. The poor prognosis of gastric cancer is due to late discovery, susceptibility to recurrence and metastasis, and adverse reactions to existing therapies. Although preoperative and postoperative combination chemotherapy prolongs the survival time of patients, drug resistance remains to be the main barrier affecting the outcome of chemotherapy [Citation3]. Oxaliplatin (OXA) is the third generation of platinum anticancer drugs, which is equivalent to cisplatin [Citation4]. However, drug resistance frequently occurs [Citation5], and its mechanisms are not well understood, leading to cancer recurrence and poor survival. Therefore, it is vital to elucidate the molecular mechanism of OXA resistance to improve the survival rate of gastric cancer.
The tumor microenvironment contains a large number of nonmalignant stromal cells, which evolve together with tumor cells during tumor progression [Citation6–8]. Among them, tumor-associated macrophages (TAM) are the main component and play a significant role in tumor growth, angiogenesis, metastasis, as well as treatment resistance [Citation8]. In most solid tumors, TAM usually refers to M2 -polarized macrophages, and the association between poor prognosis and TAM density has been claimed in a variety of cancers, including gastric cancer [Citation9,Citation10]. More and more evidence indicates that TAM can regulate the therapeutic response of cancer cells, and an innovative combination therapy-immunotherapy targeting TAM has been proposed [Citation11]. Despite this, the detailed interactions between TAM anticancer therapies remain unclear.
Recent studies indicate that cells communicate with other cells by secreting exosomes, which are produced by multivesicular bodies and then released into the extracellular matrix. Exosomes range in size from 50 to 100 nm and contain lipids, proteins, mRNAs, and abundant non-coding RNAs [Citation12]. Multiple cell types can release exosomes, and exosome communication among cells in the tumor microenvironment can modulate the therapeutic resistance of cancer cells [Citation13]. Among these non-coding RNAs, circRNAs can be widely expressed in exosomes and can be transferred by exosomes, suggesting that exosomal circRNAs are crucial in the diagnosis, treatment, as well as prognosis of cancer [Citation14]. Previous literature has confirmed that exosome circRNAs play a crucial role in drug resistance to gastric cancer. For example, circRNA 0032821 can interfere with the OXA resistance of gastric carcinoma cells [Citation15].
In our study, we constructed M2 polarized macrophages and screened out a circRNA to explore the effect of exosomes carrying this circRNA on the OXA resistance of gastric carcinoma cells.
Materials and methods
Cell culture and treatment
Human monomyelocytic leukemia (THP-1) cells, gastric carcinoma cells KATO III and MKN45 were obtained from Procell (Wuhan, China). Cells were cultured in the RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (Gibco, USA), 100 U/mL penicillin (Gibco, USA), and 10 μg/mL streptomycin (Gibco, USA) in a 5% CO2 incubator at 37°C [Citation16].
THP-1 cells were treated with phorbol 12-myristate acetate (320 nM, Sigma, USA) for 24 h, followed by stimulation of interleukin-4 (20 ng/mL) and interleukin-13 (20 ng/mL) for 24 h to obtain M2 polarized macrophages [Citation17,Citation18].
Before OXA (15 μM) treatment, gastric carcinoma cells KATO III and MKN45 were co-cultured with M2-Exos (50 μg/ml) for 48 h using 0.4 µm Tanswell chambers (M2 in upper and gastric carcinoma cells below) [Citation18].
Flow cytometry
Anti-CD11b-PE antibody and anti-F4/80-FITC antibody (Abcam, USA) were used to detect macrophage differentiation by flow cytometry. The obtained M2 polarized macrophages were collected, resuspended in phosphate buffer saline (PBS), and then stained with fluorochrome-conjugated Anti-CD11b-PE antibody and anti-F4/80-FITC antibody at 4°C for 30 min. After that, cells were washed with PBS and analyzed on a flow cytometer (Thermo Fisher Scientific).
Clinical samples
The clinical samples (n = 10) were collected from gastric cancer patients in the First Affiliated Hospital of Zhengzhou University. All participants signed informed consent. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Quantitative real-time PCR (qRT-PCR)
RNA in tumor tissues, macrophages, and gastric carcinoma cells were extracted with Trizol (Beyotime Biotechnology, Shanghai) followed by reversed transcription into cDNA. Subsequently, the qPCR reaction was performed with a riboSCRIPT qRT-PCR Starter Kit (RiboBio Co., Ltd., Guangzhou, China) on an ABI 7500 fluorescence quantitative PCR instrument (Applied Biosystems, USA). Levels of CD206, Arg1, IL-10, TGF-β, circ 0008253, ABCG2, and ABCB1 were determined using the 2–ΔΔCt method.
Cell counting Kit-8 (CCK8) experiment
The cell viability was detected via a CCK-8 kit (ab228554, USA). Cells were treated with CCK-8 solution in a 37ºC, 5% CO2 incubator, followed by the addition of WST-8 Solution (10 µL/well). Then the absorbance was examined at 460 nm on an enzyme-labeled instrument (BioTek, USA).
Apoptosis detection
The apoptosis was determined with an apoptosis detection kit (YEASEN Biotech Co., Ltd, Shanghai, China) following its instructions. Cells were centrifuged at 300 g, 4ºC for 5 min. The collected cells were washed twice with cool PBS and centrifuged again. After that, PBS was discarded and 100 μL 1× Binding Buffer was added to resuscitate cells. Then, 10 μL PI Staining Solution and 5 μL Annexin V-FITC were added and mixed well. Fifteen min later, 400 μL 1× Binding Buffer was added and mixed thoroughly. The apoptosis was examined by flow cytometry (BD Biosciences, USA).
Exosome isolation and identification
The exosomes were isolated with ExoQuick-TC™ Exosome Precipitation Solution (SBI, USA). Macrophages were incubated in Foetal Bovine Serum medium without exosomes for 48 h. Then, the medium was collected, followed by centrifuging at 3000 g for 15 min to remove undesired cells. The supernatant was then transferred to a sterile vessel and the ExoQuick-TC was added. After full mixing, the mixture was cultured overnight at 4°C and centrifuged at 3000 g for 15 min. The exosomes appeared at the bottom of the vessel and were resuspended for later use. The exosomes were then observed through a transmission electron microscope (Philips, Holland) [Citation18]. Besides, the size of the exosomes was examined by Nanoparticle Tracking Analysis (NTA, ZetaVIEW, Germany) [Citation19].
Western blot
The M2-Exos or gastric cancer cell KATO III and MKN45 was lyzed with RIPA Lysis Buffer (Beyotime Biotechnology, Shanghai) on ice, followed by being subjected to a Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, USA) to quantify the protein concentration. Then, the proteins were electrophoresed and transferred to PVDF membranes (Millipore, USA). After that, the membranes were blocked with 5% bull serum albumin and incubated with primary antibodies anti-CD63 (ab134045, 1:1000), anti-CD9 (ab ab263019, 1:1000), anti-CD81 (ab109201, 1:1000), Anti-Calnexin (ab133615, 1:1000), anti-ABCB1 (ab170904, 1:1000), anti-ABCG2 (ab207732, 1:1000), and anti-GAPDH (ab8245, 1:1000) in a 4°C environment. On the second day, the membranes were washed with PBS and incubated with the secondary antibody for 2 h at room temperature. All plots were visualized by ECL reagents (Thermo Fisher Scientific, USA) on a protein exposure machine (Bio-Rad, USA) [Citation20].
Immunofluorescence
The exosomes derived from M2 macrophages were labeled with PKH67 (Umibio, Shanghai) according to its instructions. Subsequently, PKH67-labeled exosomes were co-cultured with (or without) gastric carcinoma cells KATO III and MKN45 for 24 h. Then, cells were fixed with 4% paraformaldehyde and incubated with Anti-F-actin antibody (Abcam, ab205) in a wet box for 1 h. Cells were further incubated with the DyLight 594 conjugated secondary antibody at room temperature. The cell nuclear was stained with 4’, 6-diamidino-2-phenylindole (Abcam, USA). The images were obtained through a microscope (Leica, Germany).
The tumor tissues collected from clinical samples of gastric cancer patients were fixed with 4% paraformaldehyde and incubated with F4/80 antibody (DF2789, 1:200) and CD206 antibody (sc -58,986, 200 μg/ml), followed by incubated with Goat Anti-Rabbit IgG H&L (Alexa Fluor® 647) or Goat Anti-Mouse IgG H&L (Alexa Fluor® 488). The cell nuclear was stained with 4’, 6-diamidino-2-phenylindole (Abcam, USA). The images were obtained through a microscope (Leica, Germany).
Animal experiment
Male 5-week nude mice were raised in the animal center of Zhengzhou University. All experiments have followed the guidelines of the Institutional Animal Care and Use Committee of The First Affiliated Hospital of Zhengzhou University. The exosomes (50 μg/mL) derived from M2 macrophages were co-cultured with (or without) MKN45 cells for 48 h. Then, cells were subcutaneously injected into nude mice (3 × 105 cells in 200 μl PBS). One week later, mice were injected with 5 mg/kg OXA. And mice were sacrificed two weeks after the cell injection and the tumor was collected.
Gastric carcinoma cells MKN45 transfected with circ 0008253 overexpression were subcutaneously injected into nude mice. One week later, mice were injected with 5 mg/kg OXA. And mice were sacrificed two weeks after the cell injection and the tumor was collected [Citation18,Citation21].
Bioinformatics analysis
Three of the GEO databases: “Gastric cancer vs healthy control” human circRNA microarray data (GSE78092, GSE83521, GSE93541) were reviewed and R package limma was used for differential expression analysis to find candidate circRNAs.
Cell transfection
The circ 0008253 overexpression vector or interference vector was synthesized by RiboBio (Guangzhou, China), followed by transfected into gastric carcinoma cells (KATO III and MKN45 M2) or M2 supernatant according to the instructions of Lipofectamine 3000 (Invitrogen, USA). After 24 h, the cells were collected for later analysis.
Colony formation experiment
The transfected KATO III and MKN45 cells were digested by trypsin and centrifuged to prepare the single-cell suspension. Cells were then seeded into 6-well plates (1 × 103 cells/well) and gently rotated to make the cells evenly dispersed. The 6-well plates were placed in a 37°C, 5% CO2 incubator with saturated humidity for 2 weeks. When visible clones appeared, cells were washed with PBS, and fixed with 4% paraformaldehyde, followed by staining with crystal violet. And the number of clones was counted with a microscope.
Verification of circ 0008253
The divergent (D) and convergent (C) primers were used for circ 0008253 verification through an agarose gel. The divergent primers can cause circRNA to loop only in cDNA, and convergent primers can be used for cDNA and genomic DNA (gDNA) amplification. We also used RNase R to further prove the existence of circ 0008253. Besides, the sequence of circ 0008253 was confirmed by loop sequencing.
Statistical analysis
The significance between the two groups was assessed using Paired t test, Unpaired t test, or Welch's unequal variances t-test. The significance among multiple groups was assessed using Brown-Forsythe and Welch's ANOVA test followed by Unpaired t with Welch's correction or Two-way ANOVA followed by Tukey's multiple comparisons test. Data were conducted via GraphPad Prism 8.0.1 (GraphPad Software, USA) and presented as mean ± SD. P < 0.05 was considered statistically significant.
Results
Co-culture with M2-polarized macrophages enhanced the resistance of gastric carcinoma cells to OXA
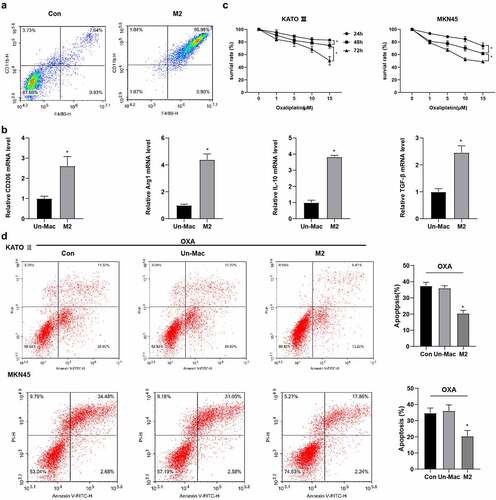
We first identified that the type of macrophages in the microenvironment of gastric cancer was M2-polarized macrophages from clinical samples of gastric cancer patients (Supplementary Figure S4). M2-polarized macrophages were obtained and CD11b and F4/80 were used for flow cytometry detection of macrophage differentiation (). Meanwhile, compared with unpolarized macrophages (Un-Mac), mRNA levels of CD206, Arg1, IL-10, and TGF-β were increased in M2-polarized macrophages (M2) (). To determine the optimal dose level of OXA, gastric cancer cell lines KATO III and MKN45 were treated with different concentrations of OXA. indicated that cell viability was decreased with the increase in concentration and time. Based on this observation, the dose used for resistance testing was 15 μM, 48 h. To confirm whether M2-polarized macrophages confer chemoresistance, gastric carcinoma cells were co-cultured with M2-polarized macrophages and then treated with OXA for 48 h. Flow cytometry results indicated that the apoptosis of cells after co-cultured with M2-polarized macrophages was significantly decreased than that of Un-Mac or control cells (). Data suggested that M2-polarized macrophages promoted OXA resistance in gastric carcinoma cells.
Figure 1. Co-culture with M2-polarized macrophages enhanced the resistance of gastric carcinoma cells to OXA. Human monomyelocytic leukemia (THP-1) cells were stimulated to obtain M2 polarized macrophages. (A) the differentiation of macrophages was detected by flow cytometry. (B) the mRNA expression levels of CD206, Arg1, IL-10, and TGF-β in macrophages were detected by qRT-PCR. *P <0.05 vs. Un-Mac. (C) the cell viability was determined by CCK8. *P <0.05 vs. 24 h or 48 h. (D) the cell apoptosis was detected by flow cytometry. *P <0.05 vs. Un-Mac. B:Unpaired t test; C: Two-way ANOVA followed by Tukey's multiple comparisons test; D: Brown-Forsythe and Welch's ANOVA test followed by Unpaired t with Welch's correction.
M2-Exos can be absorbed by gastric carcinoma cells
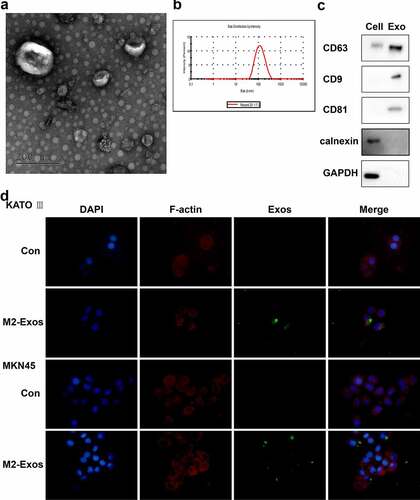
To confirm the role of M2-polarized macrophage-derived exosomes (M2-Exos) in OXA resistance of gastric cancer, exosomes were isolated from M2 macrophages. Besides, the morphology and size distribution intensity were displayed in . Meanwhile, the expression of exosome markers was detected, and results showed that CD9, CD63, and CD81 were highly expressed, Calnexin was lowly expressed in exosomes (). To investigate whether M2-Exos could be absorbed by gastric carcinoma cells, PKH67 labeled M2-Exos were incubated with gastric carcinoma cells. Confocal microscopy showed that green fluorescence (exosomes), red fluorescence (gastric carcinoma cell marker F-actin), and blue fluorescence (DAPI) can be co-located, indicating the absorption of M2-Exos by gastric carcinoma cells ().
Figure 2. Exosomes derived from M2-polarized macrophages can be absorbed by gastric carcinoma cells. Exosomes were isolated from M2 macrophages. (A) the morphology of exosomes was observed in transmission electron microscopy. (B) the size of exosomes was assessed by nanoparticle tracking analysis. (C) the markers of exosomes were detected using Western blot. (D) Immunofluorescence staining was performed.
M2-Exos induced OXA resistance in gastric carcinoma cells
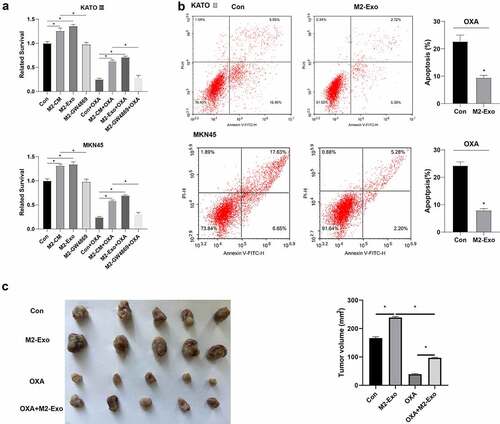
To further investigate the role of M2-Exo in OXA resistance of gastric carcinoma cells, M2-Exo or conditioned medium (CM) were co-cultured with gastric carcinoma cells and then treated with 15 μM OXA for 48 h. indicated that co-culture with M2 supernatant or M2-Exo could reduce OXA sensitivity, improve the relative survival rate, and promote drug resistance of gastric carcinoma cells, and M2-Exo had a more obvious effect. In addition, we also found that Exo release inhibitor GW4869 treatment (40 μM) has no effect on OXA sensitivity and relative survival rate of gastric carcinoma cells (). We further tested the effect of M2-Exo on apoptosis resistance of gastric carcinoma cells treated with OXA, flow cytometry results indicated that co-culture with M2-Exo reduced apoptosis (). To confirm the role of M2-Exo in chemical resistance in gastric carcinoma cells in vivo, the exos (50 μg/mL) derived from M2 macrophages were co-cultured with gastric carcinoma cells MKN45 and subcutaneously injected into nude mice, and then treated with OXA. showed that M2-Exo significantly promoted tumor growth compared to the con group. Besides, OXA + M2-Exo promoted tumor growth and inhibited the OXA chemotherapy effect.
Figure 3. Exosomes derived from M2-polarized macrophages induced OXA resistance in gastric carcinoma cells. Gastric carcinoma cells (KATO III and MKN45) were incubated with exosomes (50 μg/ml) from M2 macrophages for 48 h, followed by treatment with OXA (15 μM) for 48 h. (A) the cell viability was determined by CCK8. (B) the cell apoptosis was detected by flow cytometry. Gastric carcinoma cells were co-cultured with exosomes from M2 macrophages, followed by injected subcutaneously to male nude mice as well as OXA treatment. mice were grouped: Con, M2-Exo, OXA, and OXA + M2-Exo. N = 5. (C) the tumor growth was analyzed. *P <0.05. A/C: Brown-Forsythe and Welch's ANOVA test followed by Unpaired t with Welch's correction; B: Unpaired t test.
Circ 0008253 was transferred from M2-Exos to gastric carcinoma cells
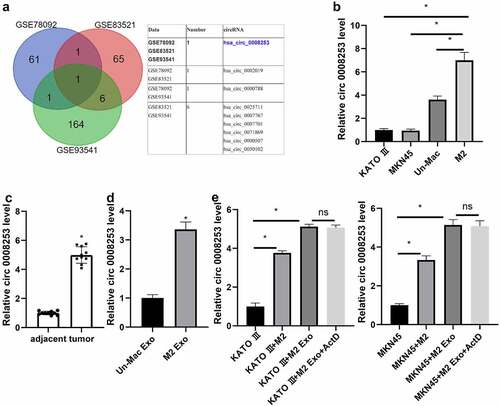
We used bioinformatics analysis to search for candidate circRNA and found 64, 73, and 172 up-regulated circRNAs, respectively. By taking the intersection of these circRNAs, we got the candidate circRNA: hsa_circ_0008253 (). Agarose gel electrophoresis showed that divergent primers derived from gDNA produced no amplification of circ 0008253 (Supplementary Figure S1E). QRT-PCR results indicated that compared with gastric carcinoma cells, circ 0008253 was much higher in macrophages, especially in M2 polarized macrophages (). The clinical samples (n = 10) of gastric cancer patients were collected. It was found that tumor tissue contained higher levels of circ 0008253 than para cancer tissue (n = 10) (). Besides, the level of circ 0008253 in M2-Exos was higher than that in non-activated macrophages (). To further confirm whether the up-regulated circ 0008253 level in gastric carcinoma cells was caused by the transport of M2 macrophages into gastric carcinoma cells through exosomes rather than the tumor cells themselves, qRT-PCR was conducted to examine circ 0008253 level in gastric carcinoma cells after co-culture with M2-Exos or M2-polarized macrophages. The level of circ 0008253 was significantly increased, while after the addition of ActD (2 μg/mL, 8 h) inhibited endogenous RNA production [Citation22,Citation23], there was no significant difference. These results indicated that the changes of circ 0008253 were mainly caused by exosomes after the co-culture of tumor cells and M2 exosomes ().
Figure 4. Circ 0008253 was transferred from exosomes from M2 macrophages to gastric carcinoma cells. (A) the candidate circRNA hsa_circ_0008253 was screened by bioinformatics analysis. (B) Levels of circ 0008253 in cells were detected using qRT-PCR. *P <0.05 vs. KATO III, or MKN45, or Un-Mac. (C) Levels of circ 0008253 in clinical samples (n = 10) of gastric cancer patients were detected using qRT-PCR. *P <0.05 vs. adjacent. (D) Levels of circ 0008253 in macrophages were detected using qRT-PCR. *P <0.05 vs. Un-Mac Exo. Gastric carcinoma cells were co-cultured with M2-polarized macrophages or M2-Exos, followed by treatment with or without ActD (an inhibitor of endogenous RNA production). (E) Levels of circ 0008253 in cells were detected using qRT-PCR. *P <0.05 vs. KATO III or MKN45. B/E: Brown-Forsythe and Welch's ANOVA test followed by Unpaired t with Welch's correction; C: Paired t test; D: Unpaired t test.
Circ 0008253 induced OXA resistance in gastric carcinoma cells
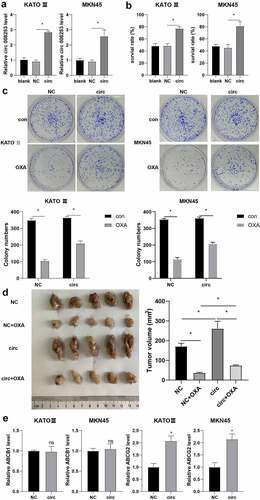
To further verify whether circ 0008253 could induce OXA resistance in gastric carcinoma cells, we directly infected gastric carcinoma cells with circ 0008253 overexpression or negative control. As shown in , overexpressing circ 0008253 increased the levels of circ 0008253 both in KATO III and MKN45. Besides, overexpression of circ 0008253 showed significantly reduced sensitivity to OXA (). Therefore, we further assessed whether the effect of circ 0008253 affected cell proliferation and the results of the Colony formation experiment proved that this effect did exist (). Next, we studied the functional role of circ 0008253 in OXA resistance of gastric carcinoma cells in vivo. The infected gastric carcinoma cells overexpressed by circ 0008253 were injected subcutaneously into nude mice, and then OXA was injected intraperitoneally. The final tumor size of the overexpressed circ 0008253 group was much larger than that of the control group (), indicating that circ 0008253 overexpression significantly inhibited the OXA chemotherapy effect. On the contrary, supernatant from M2 with circ 0008253 interference could inhibit tumor growth and enhanced the OXA chemotherapy effect (Supplementary Figure S2). The ABC (ATP binding box) membrane transporter family plays an important role in cancer chemoresistance [Citation24]. Therefore, we would like to investigate whether circ 0008253 affects the expression of the ABC transporter gene. We detected two major ABC transporters with clear reports of OXA resistance in gastric cancer by qRT-PCR: ATP binding cassette subfamily G member 2 (ABCG2) and ATP Binding Cassette Subfamily B Member 1 (ABCB1) [Citation25], the results showed that ABCG2 was significantly up-regulated in both gastric cancer cell lines after overexpression of circ 0008253 (). Protein expression changes of ABCG2 and ABCB1 with or without circ 0008253 overexpression in gastric carcinoma cells KATO III and MKN45 were also detected. Results showed that protein levels of ABCG2 and ABCB1 were both up-regulated in different amplitudes after overexpression (Supplementary Figure S3). It seems that circ 0008253 regulates ABCG2 and ABCB1 in different ways. To sum up, these results suggest that circ 0008253 reduced OXA sensitivity as well as the apoptosis of gastric carcinoma cells.
Figure 5. Circ 0008253 induced OXA resistance in gastric carcinoma cells. Gastric carcinoma cells (KATO III and MKN45) were transfected with circ 0008253 overexpression vector or its negative control. (A) Levels of circ 0008253 in gastric carcinoma cells were detected using qRT-PCR. (B) CCK-8 assay was performed. (C)the colony formation assay was performed in gastric carcinoma cells KATO III and MKN45. Gastric carcinoma cells MKN45 were transfected with circ 0008253 overexpression vector or its negative control, followed by subcutaneously injected into nude mice. One week later, mice were injected with 5 mg/kg OXA. Mice were grouped: NC, NC + OXA, circ, circ + OXA. N = 5. (D) the tumor growth was analyzed. (E) Levels of ABCB1 and ABCG2 in gastric carcinoma cells KATO III and MKN45 were determined. ns P >0.05; *P <0.05. A/B: Brown-Forsythe and Welch's ANOVA test followed by Unpaired t with Welch's correction; C/D: Two-way ANOVA followed by Tukey's multiple comparisons test; E: Unpaired t test or Unpaired Welch's t test.
Discussion
Gastric cancer has a high morbidity and mortality rate [Citation26], resulting in more than 780,000 deaths per year. When patients with gastric cancer are inoperable or have metastasis, OXA is usually used for chemotherapy, but the prognosis is poor and is easy to develop drug resistance [Citation27,Citation28]. In addition to genetic changes in the tumor cells themselves that lead to increased drug outflow or strengthened anti-apoptosis ability, drug resistance may be due to the protection of tumor cells by the tumor microenvironment [Citation29]. In the present study, we discovered that M2-exos expressed higher levels of circ 0008253 than non-activated macrophages, and the transfer of exosomes carrying circ 0008253 from M2 macrophages to gastric carcinoma cells conferred OXA resistance. This study may provide new insight to elucidate the molecular mechanism of OXA resistance and improve the survival rate of gastric cancer.
The tumor microenvironment is very important in tumor growth, invasion and metastasis. Meanwhile, it can also be used as a therapeutic target to reduce drug resistance [Citation7,Citation29]. Tumor-associated macrophages (TAM) are abundant in the tumor microenvironment and can regulate the chemotherapeutic resistance in tumor cells [Citation30]. M2-polarized macrophages have been reported to induce cisplatin resistance in gastric carcinoma cells [Citation18]. Similarly, in our study, co-culture with M2-polarized macrophages reduced the apoptosis rate and enhanced the resistance of gastric carcinoma cells to OXA.
Exosomes are secreted by eukaryotes and belong to extracellular vesicles, which are important in cell communication and tissue microenvironment regulation [Citation31]. Exosomes derived from cancer cells can regulate neighboring cells to affect the progression of tumors [Citation32,Citation33]. It has been reported that M2-exos are involved in gastric cancer progression and cisplatin resistance [Citation34]. In our experiments, we isolated the exosomes from M2-polarized macrophages and found that they could be absorbed by gastric carcinoma cells. Besides, we also found that M2-exos could promote OXA resistance in gastric carcinoma cells. In vivo experiments also indicated that M2-exos significantly inhibited the chemotherapy effect of OXA.
CircRNAs are non-coding RNA molecules, which are mainly located in the cytoplasm or exosomes, expressed stably and not easily degraded [Citation35]. CircRNAs are involved in multiple types of cancer. For example, circ_0003258 could accelerate the metastasis of prostate cancer [Citation36]. The up-regulation of circRNA_103809 enhanced the cisplatin resistance in non-small cell lung cancer [Citation37]. CircRNA_100269 has been reported to suppress the development of gastric cancer [Citation38]. To investigate the mechanism of M2-Exos endows gastric carcinoma cells with chemotherapy resistance, we searched for candidate circRNA by Bioinformatics analysis. However, the public data on circRNA before and after the polarization of human macrophage M2 with OXA resistance is lacking. Considering that the up-regulation of differentially sequenced genes in gastric cancer tissues may be caused by exosomes of donor cells, we used three circRNA chip data in the GEO database and R packet limma was used for differential expression analysis. By taking the intersection of these up-regulated circRNAs, we obtained a candidate circRNA: HSA_circ_0008253. GSE78092 and GSE83521 were gastric cancer clinical samples, and GSE93541 was a plasma sample. The difference of HSA_circ_0008253 in tumor tissue and para cancer tissue was consistent with that in the plasma sample, so it was inferred that this circRNA might come from exosomes. The information of circ 0008253 was shown in Supplementary Figure S1.
In the present study, circ 0008253 was highly expressed in M2-polarized macrophages, tumor tissues, and M2-exos. Besides, the elevated level of circ 0008253 in gastric carcinoma cells was due to the transport of M2 macrophages into gastric carcinoma cells through exosomes. Furthermore, the overexpression of circ 0008253 significantly reduced the sensitivity of OXA and promoted tumor progression. We also detected two ABC transporters (ABCG2 and ABCB1), which are important in cancer chemical resistance and have been reported to be connected with OXA resistance in gastric cancer, and found that ABCG2 was significantly up-regulated in both gastric cancer cell lines after overexpression of circ 0008253.
Conclusively, our research proved that the transfer of M2-polarized macrophages to gastric carcinoma cells through exosomes had a regulatory effect on OXA resistance, and the transfer of exosomes carrying circ 0008253 into gastric carcinoma cells also affected OXA resistance. In addition, circ 0008253 interfered with the growth and metastasis of gastric carcinoma cells. Apart from that, circ 0008253 carried by exosomes may promote OXA resistance by up-regulating ABCG2 levels. Next, we will investigate this finding in more detail.
Supplemental Material
Download Zip (6.1 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384101.2022.2146839.
Additional information
Funding
References
- Ferro A, Rosato V, Rota M, et al. Meat intake and risk of gastric cancer in the Stomach cancer Pooling (StoP) project. Int J Cancer. 2020;147(1):45–55. DOI:10.1002/ijc.32707
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. DOI:10.3322/caac.21262
- Orditura M, Galizia G, Sforza V, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635–1649. DOI:10.3748/wjg.v20.i7.1635
- Lordick F, Lorenzen S, Yamada Y et al. Optimal chemotherapy for advanced gastric cancer: is there a global consensus? Gastric Cancer. 2014;17(2):213–225. DOI:10.1007/s10120-013-0297-z
- Liu C, Liu Q, Yan A, et al. Metformin revert insulin-induced oxaliplatin resistance by activating mitochondrial apoptosis pathway in human colon cancer HCT116 cells. Cancer Med. 2020;9(11):3875–3884. DOI:10.1002/cam4.3029
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022 doi:10.1038/ni.2703.
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437 doi:10.1038/nm.3394.
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61 doi:10.1016/j.immuni.2014.06.010.
- Mantovani A, Allavena P, Sica A et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. DOI:10.1038/nature07205
- Yuan M, Zou X, Liu S, et al. Modified Jian-pi-yang-zheng decoction inhibits gastric cancer progression via the macrophage immune checkpoint PI3Kγ. Biomed Pharmacother. 2020;129:110440 doi:10.1016/j.biopha.2020.110440.
- Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27(4):462–472 doi:10.1016/j.ccell.2015.02.015.
- Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232 doi:10.1016/j.cell.2016.01.043.
- Boelens MC, Wu T, Nabet B, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159(3):499–513. DOI:10.1016/j.cell.2014.09.051
- Dai J, Su Y, Zhong S, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5(1):145. DOI:10.1038/s41392-020-00261-0
- Zhong Y, Wang D, Ding Y, et al. Circular RNA circ_0032821 contributes to oxaliplatin (OXA) resistance of gastric cancer cells by regulating SOX9 via miR-515-5p. Biotechnol Lett. 2021;43(2):339–351. DOI:10.1007/s10529-020-03036-3
- Njau F, Haller H. Calcium dobesilate modulates PKCδ-NADPH oxidase- MAPK-NF-κB signaling pathway to reduce CD14, TLR4, and MMP9 expression during monocyte-to-macrophage differentiation: potential therapeutic implications for atherosclerosis. Antioxidants (Basel). 2021;10(11):1798 doi:10.3390/antiox10111798.
- Yamaguchi T, Fushida S, Yamamoto Y, et al. Low-dose paclitaxel suppresses the induction of M2 macrophages in gastric cancer. Oncol Rep. 2017;37(6):3341–3350. DOI:10.3892/or.2017.5586
- Zheng P, Chen L, Yuan X, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36(1):53. DOI:10.1186/s13046-017-0528-y
- Sun D, Cao H, Yang L, et al. MiR-200b in heme oxygenase-1-modified bone marrow mesenchymal stem cell-derived exosomes alleviates inflammatory injury of intestinal epithelial cells by targeting high mobility group box 3. Cell Death Dis. 2020;11(6):480. DOI:10.1038/s41419-020-2685-8
- Huang Z, Xie C, Huang Z, et al. Anti-tumor effect of pcDNA3.1(-)/rmetase-shUCA1 hyaluronic acid-modified G5 polyamidoamine nanoparticles on gastric cancer. Aging Pathobiology Ther. 2020;2(2):86–95. DOI:10.31491/APT.2020.06.019
- Zhang P, Shi L, Zhang T, et al. Piperlongumine potentiates the antitumor efficacy of oxaliplatin through ROS induction in gastric cancer cells. Cell Oncol (Dordr). 2019;42(6):847–860. DOI:10.1007/s13402-019-00471-x
- Tang R, Zhang Z, Han W. CircLRRK1 targets miR-223-3p to inhibit the proliferation, migration and invasion of trophoblast cells by regulating the PI3K/AKT signaling pathway. Placenta. 2021;104:110–118 doi:10.1016/j.placenta.2020.12.003.
- Chen H, Gu B, Zhao X, et al. Circular RNA hsa_circ_0007364 increases cervical cancer progression through activating methionine adenosyltransferase II alpha (MAT2A) expression by restraining microRNA-101-5p. Bioengineered. 2020;11(1):1269–1279. DOI:10.1080/21655979.2020.1832343
- Szakács G, Annereau J-P, Lababidi S, et al. Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell. 2004;6(2):129–137. DOI:10.1016/j.ccr.2004.06.026
- Yashiro M, Nishii T, Hasegawa T, et al. A c-Met inhibitor increases the chemosensitivity of cancer stem cells to the irinotecan in gastric carcinoma. Br J Cancer. 2013;109(10):2619–2628. DOI:10.1038/bjc.2013.638
- Xia T, Pan Z, Zhang J. CircSMC3 regulates gastric cancer tumorigenesis by targeting miR-4720-3p/tjp1 axis. Cancer Med. 2020;9(12):4299–4309 doi:10.1002/cam4.3057.
- An O, Song Y, Ke X, et al. “3G” trial: an RNA editing signature to guide gastric cancer chemotherapy. Cancer Res. 2021;81(10):2788–2798. DOI:10.1158/0008-5472.CAN-20-2872
- Sun Y, Xie Y, Tang H, et al. In vitro and in vivo evaluation of a novel estrogen-targeted PEGylated oxaliplatin liposome for gastric cancer. Int J Nanomedicine. 2021;16:8279–8303 doi:10.2147/ijn.S340180.
- Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25(4):198–213 doi:10.1016/j.tcb.2014.11.006.
- Weizman N, Krelin Y, Shabtay-Orbach A, et al. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene. 2014;33(29):3812–3819. DOI:10.1038/onc.2013.357
- Ruivo CF, Adem B, Silva M, et al. The biology of cancer exosomes: insights and new perspectives. Cancer Res. 2017;77(23):6480–6488. DOI:10.1158/0008-5472.CAN-17-0994
- Malla RR, Shailender G, Kamal MA. Exosomes: critical mediators of tumour microenvironment reprogramming. Curr Med Chem. 2021;28(39):8182–8202 doi:10.2174/0929867328666201217105529.
- Alharbi M, Lai A, Guanzon D, et al. Ovarian cancer-derived exosomes promote tumour metastasis in vivo: an effect modulated by the invasiveness capacity of their originating cells. Clin Sci (Lond). 2019;133(13):1401–1419. DOI:10.1042/CS20190082
- Cui HY, Rong J-S, Chen J, et al. Exosomal microRNA-588 from M2 polarized macrophages contributes to cisplatin resistance of gastric cancer cells. World J Gastroenterol. 2021;27(36):6079–6092. DOI:10.3748/wjg.v27.i36.6079
- Sun J-F, Wu, S.L., Wang, G.J, et al. Roles of circular RNAs and their interactions with microRNAs in human disorders. Clin Surg Res Commun. 2018;2(2):1–8. DOI:10.31491/CSRC.2018.6.012
- Yu YZ, Lv D-J, Wang C, et al. Hsa_circ_0003258 promotes prostate cancer metastasis by complexing with IGF2BP3 and sponging miR-653-5p. Mol Cancer. 2022;21(1):12. DOI:10.1186/s12943-021-01480-x
- Zhu X, Han J, Lan H, et al. A novel circular RNA hsa_circrna_103809/mir-377-3p/got1 pathway regulates cisplatin-resistance in non-small cell lung cancer (NSCLC). BMC Cancer. 2020;20(1):1190. DOI:10.1186/s12885-020-07680-w
- Zhang Y, Liu H, Li W, et al. CircRNA_100269 is downregulated in gastric cancer and suppresses tumor cell growth by targeting miR-630. Aging (Albany NY). 2017;9(6):1585–1594. DOI:10.18632/aging.101254
