Abstract
Objective: Driving under the influence of psychoactive drugs causes an increased risk for accidents. In combating this, sobriety tests at the roadside are common practice in most countries. Sampling of blood and urine for forensic investigation cannot be done at the roadside and poses practical problems associated with costs and time. An alternative specimen for roadside testing is therefore warranted and the aerosol particles in exhaled breath are one such alternative.
Methods: The present study investigated how the exhaled breath sample compared with the routine legal investigations of blood and urine collected from suspects of drugged driving at 2 locations in Sweden. Exhaled breath was collected using a simple filter collection device and analyzed with state-of-the-art mass spectrometry technique.
Results: The total number of cases used for this investigation was 67. In 54 of these cases (81%) the results regarding a positive or negative drug test result agreed and in 13 they disagreed. Out of these, the report from the forensic investigation of blood/urine was negative in 21 cases. In 6 of these, analytical findings were made in exhaled breath and these cases were dominated by the detection of amphetamine. In 7 cases a positive drug test from the forensic investigation was not observed in the breath sample and these cases were dominated by detection of tetrahydrocannabinol in blood. In total, 45 samples were positive with breath testing and the number of positives with established forensic methods was 46.
Conclusion: The promising results from this study provide support to exhaled breath as a viable specimen for testing of drugged driving. The rapid, easy, and convenient sampling procedure offers the possibility to collect a drug test specimen at the roadside. The analytical investigation must be done in a laboratory at present because of the need for a highly sensitive instrument, which is already in use in forensic laboratories. The analytical work is not more challenging than for blood or oral fluid and should not cause an increase in cost. However, more studies need to be done before exhaled breath drug testing can be applied routinely for drugged driving investigation.
Introduction
Driving under the influence of psychoactive drugs is generally acknowledged as causing an increased risk for accidents and is therefore considered a threat to public safety (Drummer et al. Citation2012; Lipari et al. Citation2016). Sobriety tests are mandatory in most countries and commonly entail a combination of a roadside screening test and a confirmation test performed at a laboratory.
In addition to breath tests for alcohol, some countries have implemented roadside drug testing using an oral fluid specimen, but most countries rely on signs and symptoms of impairment as a tool to require a sample for confirmation of drugs of abuse (Davey et al. Citation2017).
For many years, the gold standard for forensic laboratory investigations was a blood sample. However, in cases involving driving under the influence of psychoactive drugs, some countries now use oral fluid as both the screening and a confirmation matrix for drugs of abuse. Australia has successfully implemented a test protocol including methamphetamine, tetrahydrocannabinol (THC), and 3,4-methylenedioxymethamphetamine (MDMA) in oral fluid (Drummer et al. Citation2007). Oral fluid is collected by police at the roadside. The blood sampling procedure, on the other hand, cannot easily be done at the roadside but requires professional personnel and suitable facilities, which are costly and time consuming.
Over the years, technology for testing alcohol in breath has developed to the degree that evidential testing can be done without the need for professional medical and laboratory resources and has even allowed the development of alcolocks to hinder using a vehicle (Alvarez and Vanlaar Citation2005; Gullberg Citation2000). Alcohol breath testing is based on the volatility of ethanol, which allows it to be present in the gas phase of breath in measurable concentrations proportional to the legal limits for alcohol in blood (Jones Citation2010). Other drugs of abuse are generally not volatile and the relationship between a concentration in breath compared to blood is more complicated. However, research has shown that nonvolatile drugs of abuse are also present in exhaled breath (Beck et al. Citation2013).
Studies on exhaled breath have demonstrated that human breath contains nonvolatile components (Beck et al. Citation2016; Kuban and Foret Citation2013). These components are carried out as part of aerosol particles that are formed during normal breathing (Schwarz et al. Citation2010). Regardless of breathing maneuver, these particles are always formed and present in exhaled breath and can be used for developing clinical and forensic biomarkers (Beck et al. Citation2016; Kuban and Foret Citation2013). The particles are composed of the airway lining fluid that are in close contact with the circulation and will contain components originating from the blood (Almstrand et al. Citation2009; Bredberg et al. Citation2012). It is therefore not surprising that drugs of abuse can be detected in the breath of drug users when these substances are present in the body. The first demonstration of this concept was published in 2010 and showed that amphetamine could be detected in breath of drug users (Beck et al. Citation2010). A commercial breath sampling device was subsequently used to demonstrate that abused drugs in general can be detected in breath while intoxicated (Beck et al. Citation2013). Several reports with controlled intake of different drugs have confirmed that exhaled breath is a viable matrix for drug testing (Coucke et al. Citation2016; Ellefsen et al. Citation2014; Himes et al. Citation2013; Kintz et al. Citation2017; Meyer et al. Citation2015). For tramadol, it was observed that the time versus concentration curve in breath paralleled that in blood (Meyer et al. Citation2015). Two other independent research groups have confirmed that exhaled breath is a viable specimen for clinical drug testing (Kintz et al. Citation2016, Citation2017) and for sports doping control (Thevis et al. Citation2016, Citation2017).
The aim of the present study was to collect breath samples for drug testing in addition to routine blood and urine samples that are collected from people suspected of drugged driving and determine how well the laboratory investigation of these breath samples compared with the routine legal investigations using blood and urine.
Method
Study subjects
The study was performed in collaboration with the local Swedish police authorities and dependence clinics in the cities of Stockholm and Örebro. These organizations participate in the SMADIT system (Forsman et al. Citation2011; Gustafsson et al. Citation2016), which is a unique Swedish project aimed at offering medical treatment to individuals with addiction syndromes. In practice, this means that drugged driving suspects are taken by the police to a dependence clinic for blood and urine sampling. The suspect is offered the opportunity to meet a staff member from the dependence clinic to discuss any health issues related to drug misuse. Subsequently, this can lead to the suspect entering a treatment program for a drug dependence problem. At the time of blood and urine collection the suspects were asked to also provide a sample of exhaled breath. The subjects were informed about the study and gave informed consent to participate. The logistics were arranged such that the collected breath sample was labeled with a unique code. The police document from the case was also labeled with a unique sample code. Upon receipt of the analytical report on investigation of the blood and urine samples from the National Board of Forensic Medicine, the police sent a copy of the report to the authors after removing any information on the subject’s identity. In this way, the results from the parallel investigations could be matched.
Ethical approval was obtained from the Stockholm Regional Ethics Committee (No 2008/1347-31).
Sampling of breath
A sample of exhaled breath was collected using the disposable SensAbues filter collection device, which has been described previously (Beck et al. Citation2013). The device collects particles on a low-resistance membrane filter. In brief, the device collects exhaled aerosol particles from ∼30 L of exhaled breath, which takes a few minutes during normal breathing. Once the sampling process is finished the device is capped and stored at −20 °C.
Analysis of exhaled breath
Upon analysis, the device is decapped and placed on top of a glass test tube. Methanol is poured through the filter and the methanol is collected, concentrated, and used for drug analysis using liquid chromatography–tandem mass spectrometry. The analytical procedure has been previously described (Beck et al. Citation2013; Ullah et al. Citation2018) and the method includes testing for amphetamine, methamphetamine, MDMA, methylphenidate, ritalinic acid, cocaine, benzoylecgonine, alprazolam, hydroxyalprazolam, diazepam, oxazepam, flunitrazepam, 7-aminoflunitrazepam, nitrazepam, 7-aminonitrazepam., clonazepam 7-aminoclonazepam, morphine, codeine, 6-acetylmorphine, methadone, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), tramadol, O-desmethyltramadol, buprenorphine, norbuprenorphine, hydromorphone, and tetrahydrocannabinol.
Analysis of blood and urine
Urine, whole blood, or serum specimens were sent by the police to the National Board of Forensic Medicine for routine investigation. The scope of testing varied between cases depending on the request from the police and the matrices provided.
The standard procedure was to screen for drugs of abuse in urine with immunoassays in urine. If no urine sample was provided, the immunoassay screening was performed in serum and if only whole blood was provided, screening was done in whole blood.
Confirmation of positive findings was done with a set of mass spectrometry methods, with the first choice being whole blood and, if negative, urine. Depending on the request from the police, either all positive findings were confirmed or confirmations were performed according to a priority list, including amphetamines, cannabis, cocaine, opiates, methadone, buprenorphine, benzodiazepines.
The applied cutoff limits are shown in Supplement Table 1 (see online supplement).
Results
No complaints regarding the breath sampling procedure were reported by any of the suspects or police personnel. The total number of cases used for analysis of how well exhaled breath and the reference blood/urine results compared for detecting drugged drivers was 67. In 54 of these 67 cases (81%) the results regarding a positive or negative drug test result agreed and in 13 they disagreed. An overview of results comparing the analytical investigations of blood and/or urine with the exhaled breath is given in . The report from the forensic investigation of blood/urine, except ethanol, was negative in 21 cases. In 6 of these, analytical findings were found in exhaled breath. The findings in these cases were mainly stimulants and are presented in detail in Supplement Table 2 (see online supplement). In 7 cases a positive drug test from the forensic investigation was not observed in breath and these cases are presented in the lower part of Supplement Table 2. In total, 45 samples were positive with breath testing, whereas the number of positives with established reference forensic methods was 46. Chromatograms from the analytical measurement of THC, amphetamine, and cocaine in breath from 3 of the cases are presented in . The identification of substances in the breath samples was performed with mass spectrometry meeting forensic criteria (Beck et al. Citation2013; Ullah et al. Citation2018).
Table 1. Analytical findings in the forensic investigation of blood and/or urine as compared with exhaled breath
Figure 1. Chromatograms from liquid chromatography-tandem masspectrometry analysis of an exhaled breath extract containing 380 pg amphetamine.
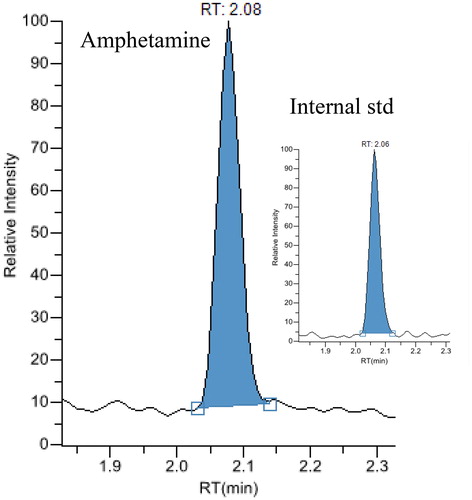
Figure 2. Chromatograms from liquid chromatography-tandem masspectrometry analysis of an exhaled breath extract containing 856 pg cocaine.
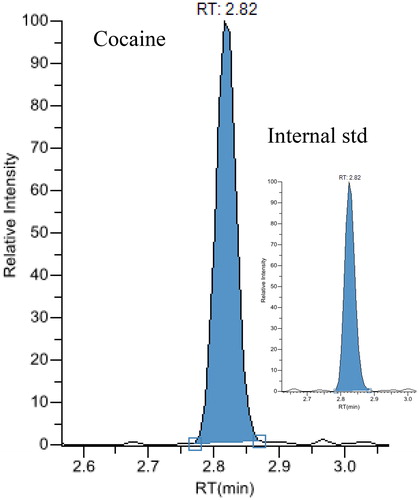
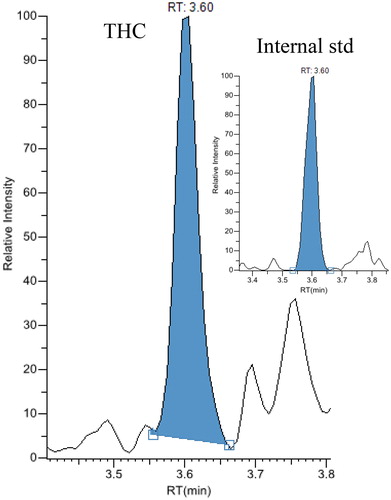
Figure 3. Chromatograms from liquid chromatography-tandem masspectrometry analysis of an exhaled breath extract containing 1,080 pg tetrahydrocannabinol.
When examining the results in detail, other differences were observed. Supplement Table 3 (see online supplement) lists the results concerning cannabis intake. In 12 cases the finding of THC in breath was supported by results from blood, serum, or urine analysis. However, in 9 cases the breath sample was negative for THC despite a finding of THC in blood. The median blood concentration value of the THC-positive subgroup was 3.4 ng/mL (n = 9) and the median value for the THC-negative group was 1.9 ng/mL (n = 7). A calculation of the difference between the groups using the Mann-Whitney test gave a P value of .0903. Taking the results from the routine forensic investigation as a reference, a sensitivity of 57% for breath testing was calculated for cannabis.
For amphetamines the number of positive cases in blood was 21 (Supplement Table 3). All of these cases were also positive for amphetamines in breath, giving a sensitivity of 100%. In addition, another 5 cases were positive in breath but not in blood, serum, or urine. The median breath concentration of amphetamine in the blood-positive subgroup was 7,870 pg/sample, and in the blood-negative group it was 380 pg/sample. A calculation of the difference between the groups using the Mann-Whitney test gave a P value of .0006. The difference might indicate that the blood concentrations in the 5 negative samples were low and therefore undetected. In 3 of the cases the analysis for amphetamine was done with immunoassay in blood with a low sensitivity. In the remaining 2 cases no blood analysis was done due to negative urine screening.
Eleven cases concerned cocaine (Supplement Table 3). In 6 out of 7 cases where the blood, serum, or urine data revealed cocaine intake, the breath analysis were also positive, giving a sensitivity of 86%. When both cocaine and benzoylecgonine were detected in blood, the concentration of cocaine in breath was the highest of all cases, and in the case of only benzoylecgonine in blood the breath concentration of cocaine was the next highest. In all cases the concentration of cocaine in breath was higher than that of the metabolite, benzoylecgonine, with a median concentration ratio of 8.6 (n = 6). In 2 cases with low cocaine concentrations in breath, the results were in contrast with the negative results from the urine screening.
Seven cases concerned benzodiazepines (Supplement Table 3). Breath testing was positive in 3 of these cases, giving a sensitivity of 43%. In one of the 3 cases the blood screening was negative but was done with a method with poor sensitivity. In the third case diazepam was detected in breath but several more substances were detected in blood.
The results from the cases with other drugs are also presented in Supplement Table 3. Additional compounds detected in breath were 6-acetylmorphine, buprenorphine, codeine, methylphenidate, and tramadol.
Discussion
The present study provides support that exhaled breath drug testing can be used to investigate cases of suspected driving under the influence of drugs. When the outcome of the breath drug test was evaluated with respect to a positive or negative result, the breath testing resulted in 45 positives, whereas the routine investigation resulted in 46. The breath sampling procedure was well accepted by the suspected individuals and police officers. One advantage with exhaled breath drug testing is the convenient sampling procedure that can be conducted at the roadside and without the need for medical professionals. This can result in cost savings and a possibility to collect samples in more cases.
An important observation was that the detection rate for cannabis in breath was lower than that in blood. There was a tendency for the group of cases with a positive breath test to have higher median blood concentrations than the negative cases. A previous clinical study of cannabis users compared blood and breath testing for THC and demonstrated statistically significantly higher blood concentrations in subjects with a positive breath test (Skoglund et al. Citation2015). Three studies with controlled smoking of cannabis have indicated that the detection time of THC in breath is short but at least 6 h (Coucke et al. Citation2016; Himes et al. Citation2013; Kintz et al. Citation2017). It is also known that chronic cannabis smokers can have THC concentrations remaining in the blood on the order of 1 ng/mL after a week of abstinence (Bosker et al. Citation2013) but that this concentration is reached shortly after intake by occasional users (Hartman et al. Citation2016). It is therefore possible that a positive breath test may be more strongly associated with the time of being intoxicated and under the influence from a recent smoking event than a positive blood test. In many countries, there is an applied legal concentration limit for THC in blood regarding drugged driving (Hartman et al. Citation2016; Rooney et al. Citation2017). It has been advocated, however, that the reporting limit should be lower than the concentrations that can remain as a base level in blood after more frequent use (Ferrari et al. Citation2018). Another advantage with the breath specimen is that when taken directly at the roadside there is no time delay that may hamper the interpretation of results and influence detection rates, as has been pointed out before (Hartman et al. Citation2016). For on-site analysis of oral fluid the detection time was reported as 2–3 h after smoking (Kintz et al. Citation2009) and <12 h with a more sensitive laboratory analysis (Van der Linden et al. Citation2014). Kintz et al. (Citation2017) noted that detection time in breath is similar to detection time in oral fluid.
For amphetamine, more positive cases were detected in breath compared to blood. In some instances when the blood analysis was negative this could be explained by a low sensitivity in the immunoassay screening method used. There were significantly lower measured values in breath in the 5 cases with a negative blood/urine amphetamine result, supporting this assumption. In addition, cocaine showed high sensitivity in breath testing. In addition to cannabis, benzodiazepines showed low sensitivity. This has been noted before and is explained by the high protein binding of these compounds in blood (Altamura et al. Citation2013; Vajda and Eadie Citation2014) and will also influence the detection rate for oral fluid testing. Other substances with positive breath tests were codeine, buprenorphine, 6-acetylmorphine, and tramadol, but such few cases do not allow for an estimation of sensitivity. Tramadol has previously been studied in a controlled clinical trial and gave a 100% detection rate in breath for 24 h (Meyer et al. Citation2015). The better detection rate of 6-acetylmorphine compared to morphine has been noted before (Beck et al. Citation2013).
A limitation of this study was that the forensic investigations were performed for blood and urine with several different procedures/methods and according to a priority list. Therefore, a straightforward comparison between the breath test and the reference forensic investigation could not be performed, but details of the comparisons have been provided.
There is a need to develop effective methods for drug testing to deter drugged driving. One strategy is to use alternative specimens to venous blood, because sampling of blood requires health professionals and is time consuming. Blood has been the standard specimen for drugged driving investigations and was also required for alcohol determinations. With time, breath alcohol measurement has been developed to the point that it is now considered evidential and can be conducted at the roadside. A similar solution for drug testing might be a future possibility based on the present study. However, this would require use of a new detection technology and legal acceptance of the results. One possibility is a miniature mass spectrometer or the use of surface-enhanced Raman spectroscopy, which has the sensitivity needed and has provided promising results (Massarini et al. Citation2015) and is already available as a handheld device. The possibility to measure drugs in exhaled breath in real time has been demonstrated using mass spectrometry techniques (Berchtold et al. Citation2014, Ng et al. Citation2014). However, those techniques are not yet suitable for use at the roadside.
Other possible specimens include oral fluid and sweat, for which immunoassay technology exists for rapid screening at the roadside (Samyn and van Haeren Citation2000). A possible use of exhaled breath in a roadside system could also be used as a confirmatory specimen. For example, in the system used in Belgium, which uses the examination of signs of being under influence of drugs followed by on-site oral fluid screening, exhaled breath could be used as a confirmatory specimen (Van der Linden et al. Citation2015).
In conclusion, the promising results from this study provide support that exhaled breath is a viable specimen for testing for drugged driving and that a future study evaluating breath sampling at the roadside for detection of drugs with more comprehensive analytical investigations of blood is warranted. One aspect that needs future attention is the issue of passive exposure when sampling is done at the roadside. The rapid, easy, and convenient sampling procedure offers a possibility to collect drug test specimens at the roadside.
Supplemental Material
Download PDF (344.6 KB)Acknowledgments
We acknowledge support from the police authorities and the dependence clinics in Stockholm and Örebro. We are especially grateful for the support and help from Officer Nina Gual.
References
- Almstrand AC, Ljungström E, Lausmaa J, Bake B, Sjövall P, Olin AC. Airway monitoring by collection and mass spectrometric analysis of exhaled particles. Anal Chem. 2009;81:662–668.
- Altamura AC, Moliterno D, Paletta S, Maffini M, Mauri MC, Bareggi S. Understanding the pharmacokinetics of anxiolytic drugs. Expert Opin Drug Metab Toxicol. 2013;9:423–440.
- Alvarez J, Vanlaar W. Research on alcolock implementation in the European Union. Addiction. 2005;100:136.
- Beck O, Leine K, Palmskog G, Franck J. Amphetamines detected in exhaled breath from drug addicts: a new possible method for drugs-of-abuse testing. J Anal Toxicol. 2010;34:233–237.
- Beck O, Olin AC, Mirgorodskaya E. Potential of mass spectrometry in developing clinical laboratory biomarkers of nonvolatiles in exhaled breath. Clin Chem. 2016;62:84–91.
- Beck O, Stephanson N, Sandqvist S, Franck J. Detection of drugs of abuse in exhaled breath using a device for rapid collection: comparison with plasma, urine and self-reporting in 47 drug users. J Breath Res. 2013;7(2):1–11.
- Berchtold C, Bosilkovska M, Daali Y, Walder B, Zenobi R. Real-time monitoring of exhaled drugs by mass spectrometry. Mass Spectrom Rev. 2014;33:394–413.
- Bosker WM, Karschner EL, Lee D, et al. Psychomotor function in chronic daily cannabis smokers during sustained abstinence. PLoS One. 2013;8(1):1–7.
- Bredberg A, Gobom J, Almstrand AC, et al. Exhaled endogenous particles contain lung proteins. Clin Chem. 2012;58:431–440.
- Coucke L, Massarini E, Ostij Z, Beck O, Verstraete AG. Δ(9)-tetrahydrocannabinol concentrations in exhaled breath and physiological effects following cannabis intake—a pilot study using illicit cannabis. Clin Biochem. 2016;49:1072–1077.
- Davey J, Armstrong K, Freeman J, Sheldrake M. Roadside drug testing scoping study. 2017. Available at: http://roadsafety.gov.au/projects/files/Roadside-Drug-Testing.pdf. Accessed October 7, 2018.
- Drummer OH, Gerostamoulos D, Chu M, Seann P, Boorman M, Cairns I. Drugs in oral fluid in randomly selected drivers. Forensic Sci Int. 2007;170:105–110.
- Drummer OH, Kourtis I, Beyer J, Tayler P, Boorman M, Gerostamolous D. The prevalence of drugs in injured drivers. Forensic Sci Int. 2012;215:14–17.
- Ellefsen KN, Concheiro M, Beck O, Gorelick DA, Pirard S, Huestis MA. Quantification of cocaine and metabolites in exhaled breath by liquid chromatography-high-resolution mass spectrometry following controlled administration of intravenous cocaine. Anal Bioanal Chem. 2014;406:6213–6223.
- Ferrari D, Manca M, Banfi G, Locatelli M. Alcohol and illicit drugs in drivers involved in road traffic crashes in the Milan area. A comparison with normal traffic reveals the possible inadequacy of current cut-off limits. Forensic Sci Int. 2018;282:127–132.
- Forsman Å, Hrelja R, Henriksson P, Wiklund M. Cooperation between police and social treatment services offering treatment to drink and drug drivers—experience in Sweden. Traffic Inj Prev. 2011;12:9–17.
- Gullberg RG. Methodology and quality assurance in forensic breath alcohol analysis. Forensic Sci Rev. 2000;12:49–68.
- Gustafsson S, Nyberg J, Hrelja R. The Swedish joint action method against drink driving—a study of suspected drink drivers’ own experiences. Traffic Inj Prev. 2016;17:558–563.
- Hartman RL, Brown TL, Milavetz G, et al. Effect of blood collection time on measured Δ9-tetrahydrocannabinol concentrations: implications for driving interpretation and drug policy. Clin Chem. 2016;62:367–377.
- Himes SK, Scheidweiler KB, Beck O, Gorelick DA, Desrosiers NA, Huestis MA. Cannabinoids in exhaled breath following controlled administration of smoked cannabis. Clin Chem. 2013;59:1780–1789.
- Jones AW. The Relationship Between Blood Alcohol Concentration (BAC) and Breath Alcohol Concentration (BrAC): A Review of the Evidence. London, UK: Department for Transportation; 2010. Road Safety Web Publication No. 15.
- Kintz P, Brunet B, Muller JF, et al. Evaluation of the Cozart DDSV test for cannabis in oral fluid. Ther Drug Monit. 2009;31:131–134.
- Kintz P, Mathiaux F, Villéger P, Gaulier JM. Testing for drugs in exhaled breath collected with ExaBreath in a drug dependence population: comparison with data obtained in urine after liquid chromatographic–tandem mass spectrometric analyses. Ther Drug Monit. 2016;38:135–139.
- Kintz P, Mura P, Jamey C, Raul JS. Detection of Δ9-tetrahydrocannabinol in exhaled breath after cannabis smoking and comparison with oral fluid. Forensic Toxicol. 2017;35:173–178.
- Kuban P, Foret F. Exhaled breath condensate: determination of non-volatile compounds and their potential for clinical diagnosis and monitoring. A review. Anal Chim Acta. 2013;805:1–18.
- Lipari RN, Hughes A, Bose J. Driving Under the Influence of Alcohol and Illicit Drugs. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2016.
- Massarini E, Wästerby P, Landström L, Lejon C, Beck O, Andersson PO. Methodologies for assessment of limit of detection and limit of identification using surface enhanced Raman spectroscopy. Sens Actuators B Chem. 2015;207;437–446.
- Meyer MR, Rosenborg S, Stenberg M, Beck O. First report on the pharmacokinetics of tramadol and O-desmethyltramadol in exhaled breath compared to plasma and oral fluid after a single oral dose. Biochem Pharmacol. 2015;98:502–510.
- Ng KM, Tang HW, Man SH, Mak PY, Choi YC, Wong MYM. Direct analysis of large living organism by megavolt electrostatic ionization mass spectrometry. J Am Soc Mass Spectrom. 2014;25:1515–1520.
- Rooney B, Gouveia GJ, Isles N, et al. Drugged drivers blood concentrations in England and Wales prior to the introduction of per se limits. J Anal Toxicol. 2017;41:140–145.
- Samyn N, van Haeren C. On-site testing of saliva and sweat with Drugwipe and determination of concentrations of drugs of abuse in saliva, plasma and urine of suspected users. Int J Legal Med. 2000;113:150–154.
- Schwarz K, Biller H, Windt H, Koch W, Hohlfeld JM. Characterization of exhaled particles from the healthy human lung—a systematic analysis in relation to pulmonary function variables. J Aerosol Med Pulm Drug Deliv. 2010;23:371–379.
- Skoglund C, Hermansson U, Beck O. Clinical trial of a new technique for drugs of abuse testing: a new possible sampling technique. J Subst Abuse Treat. 2015;48:132–136.
- Thevis M, Geyer H, Tretzel L, Schänzer W. Sports drug testing using complementary matrices: advantages and limitations. J Pharm Biomed Anal. 2016;130:220–230.
- Thevis M, Krug O, Geyer H, Schänzer W. Expanding analytical options in sports drug testing: mass spectrometric detection of prohibited substances in exhaled breath. Rapid Commun Mass Spectrom. 2017;31:1290–1296.
- Ullah S, Sandqvist S, Beck O. A liquid chromatography and tandem mass spectrometry method to determine 28 non-volatile drugs of abuse in exhaled breath. J Pharm Biomed Anal. 2018;148:251–258.
- Vajda FJ, Eadie MJ. The clinical pharmacology of traditional antiepileptic drugs. Epileptic Disord. 2014;16:395–408.
- Van der Linden T, Silverans P, Verstraete AG. Comparison between self-report of cannabis use and toxicological detection of THC/THCCOOH in blood and THC in oral fluid in drivers in a roadside survey. Drug Test Anal. 2014;6:137–142.
- Van der Linden T, Wille SM, Ramírez-Fernandez M, Verstraete AG, Samyn N. Roadside drug testing: comparison of two legal approaches in Belgium. Forensic Sci Int. 2015;249:148–155.
