Abstract
Inadequately regulated proteolytic activity is responsible for the chronic lung tissue degeneration and irreversible loss of pulmonary function that define emphysema. In this study, we show that an inhaled broad-spectrum matrix metalloprotease inhibitor, ilomastat, can provide protection against the development of emphysema in cigarette smoke-treated mice. Control animals were exposed to daily cigarette smoke for 6 months. As has been reported previously, cigarette smoke was seen to increase significantly the recruitment of macrophages into the lungs of these animals, leading to concomitant alveolar airspace enlargement and emphysema. In animals treated daily with nebulized ilomastat for 6 months, lung macrophage levels were greatly reduced, and neutrophil accumulation was also inhibited. Corresponding reductions in airspace enlargement of up to 96% were observed. These striking observations suggest that delivery of ilomastat directly into the lungs of smoke-treated mice can not only inhibit lung tissue damage mediated by metalloproteases, but may also reduce that component of tissue degeneration mediated by excess neutrophil-derived products. Our data also suggest that the matrix metalloprotease inhibitors may represent a class of drugs that, when delivered by inhalation, could be used practically to treat cigarette smoking-related chronic obstructive pulmonary disease by modifying the course of the disease.
Key words: :
Introduction
It is well established that inflammatory cell-derived serine proteases and matrix metalloproteases are involved in the development of emphysema Citation[1-4]. In individuals with the inherited form of emphysema, greatly reduced levels of circulating alpha 1-antitrypsin (AAT), the major physiological inhibitor of neutrophil elastase (NE), lead to uncontrolled proteolytic activity and the progressive loss of lung function Citation[5-7]. In emphysema associated with cigarette smoking, a significant role for matrix metalloproteases (MMPs) in lung tissue degradation has also been established Citation[8-10]. This class of proteases may be pivotal in the development of smoke-induced emphysema as well as other forms of chronic obstructive pulmonary disease (COPD). For example, transgenic knockout mice that are deficient in macrophage elastase (MMP-12) production do not develop emphysema upon prolonged exposure to cigarette smoke Citation[11]. Similarly, marked attenuation of emphysema has been observed in wild-type mice treated orally Citation[12] and in guinea pigs treated subcutaneously Citation[13] with broad-spectrum MMP inhibitors (MMPIs). Mouse model systems have been used to great effect in rationalizing important roles for both MMPs and NE in disease progression Citation[14-16]. These studies have also implicated tumor necrosis factor-α (TNF-α) as a central mediator of this process. Indeed, Churg et al. Citation[17] have shown that MMP-12 promotes smoke-induced inflammation by releasing TNF-α from macrophages, with subsequent endothelial cell activation, neutrophil influx, and proteolytic breakdown of elastin by excess NE activity.
A number of MMPs have been implicated in the breakdown of elastin, collagen and other extracellular matrix components during the development of emphysema. Most prominent of these in human COPD is MMP-9 Citation[9&10]. Other MMPs that are elevated in human COPD include MMP-1, -2 and -8 Citation[8&9] and, although there is less evidence for its expression in human alveolar macrophages Citation[10]Citation[18], MMP-12 has also been implicated genetically in the rate of decline of lung function in human COPD Citation[19]. Furthermore, a member of the ADAM family of metalloproteases, TNF-α converting enzyme (TACE; ADAM-17) is well known to release active TNF-α from its precursor form Citation[20]. Accordingly, therapeutic strategies that aim to redress the proteolytic imbalance in human COPD will likely have to take into account this multiplicity of protease targets.
Although protease inhibitor therapeutics are now commonly used to treat a variety of diverse human disease, including hereditary emphysema, the clinical development of MMPIs has been hampered severely by their musculoskeletal toxicities when delivered by oral or parenteral routes Citation[21&22]. One approach to abrogating MMPI toxicity has been to develop highly selective inhibitor molecules aimed at specific MMP targets Citation[22]. Another approach, and one that is described in the present work, is to target delivery of the MMPI directly to the lung. Inhalation delivery of respiratory drugs is well established, and allows for greatly reduced therapeutic doses when compared with systemic delivery mechanisms. Such delivery, therefore, gives consequent reductions in real or potential systemic toxicities. Here we show that low doses of the potent, broad spectrum MMPI, ilomastat Citation[23&24] when delivered directly to the lung by inhalation, can reduce significantly the degradation of lung tissue induced by chronic exposure to cigarette smoke. Our data suggest that, when delivered by inhalation, MMPIs may represent a class of drugs that could treat a major underlying cause of smoking-related emphysema. A portion of these results have been published in abstract form Citation[25].
Materials and Methods
Materials
Ilomastat (GM 6001) was purchased from AMS Scientific, Inc. (Concord, CA). The MMP peptide substrate Ac-Pro-Leu-Gly-[2-mercapto-4-methyl-pentanoyl]-Leu-Gly-OEt (Catalog #H-7145) and the neutrophil elastase peptide substrate Suc-Ala-Ala-Pro-Val-pNA (Catalog #L-1770) were purchased from Bachem (Torrance, CA). Active MMP-9 (Catalog #PF024-5UG) was purchased from Oncogene Research Products (San Diego, CA).
Preparation of Solutions for Aerosol Delivery
A stock solution of ilomastat was prepared by dissolving an excess amount of lyophilized ilomastat (3 mg) in 1X phosphate-buffered saline (PBS), pH 7.4 (10 ml) overnight at room temperature with constant mixing. The resulting solution was filtered (0.45 µm Acrodisc; Gelman Inc.) to remove particles and the concentration (ug/ml) of dissolved ilomastat calculated based on the molar extinction coefficient of ilomastat at 280 nm of 5500 M− 1cm− 1. This stock solution was further diluted with sterile PBS to make solutions of 100 ug/ml, 50 ug/ml and 10 ug/ml ilomastat. Aliquots (20 ml) of these solutions were prepared and frozen at − 70°C.
Aerosol Generation and Delivery System Characterization
Aerosols containing different concentrations of ilomastat were generated using an Aeroneb Pro nebulizer (Aerogen Inc., Mountain View, CA). The nebulizer was integrated into a single chamber plenum with 5 ports for exposing animals to aerosol and an inlet for airflow. Aerosols were generated at 1 end of the plenum and carried to the 5 exposure ports by a constant airflow (2.5 L/min) into and across the plenum. The amount of drug delivered to each port was calculated by placing cotton balls into each exposure port and capturing and quantitating the amount of drug deposited on each cotton ball over predefined time periods. Captured drug was eluted from the cotton balls in PBS and quantitated by reversed phase high-performance liquid chromatography (HPLC). This analysis showed that the spatial uniformity of drug delivery to each port had a CV of 13%. On average 3.5% of the total drug nebulized was delivered to each port. Temporal uniformity was also consistent over periods of up to 1 minute. Beyond this time frame, recoveries declined due to saturation of the cotton balls. The aerosol exposure chamber was designed to integrate with the 5-port smoke exposure chamber so that animal handling was minimized.
Test Animal Exposure Times and Groups
Studies were performed on A/J female mice, 12 weeks of age at study initiation. To deliver drug or buffer control, animals (5 at a time) were placed in the aerosol delivery system. Then 10 mL of sterile saline or drug was placed in the nebulizer and delivered to each group of 5 animals. Delivery proceeded until the nebulizer ran dry (approximately 30 minutes). Between deliveries and after completion of the study the nebulizer was rinsed thoroughly with warm water. Drug was delivered 6 days per week, just prior to smoking. After delivery of drug, each smoking group was subjected to smoke from 2 non-filtered cigarettes per day (University of Kentucky research cigarettes), 6 days per week for 6 months. Non-smoking age-matched littermates were used as controls.
Pilot Studies
Two pilot studies were carried out to establish that smoke induces MMP activity in lung lavage fluid and that the production of this activity could be inhibited by ilomastat. The first pilot study (1 week) was designed to treat 2 groups of 5 animals each with smoke alone or drug plus smoke. At the end of the study the lungs of each animal were excised and the pulmonary architecture of one lobe lavaged with 1 mL of normal saline. These bronchoalveolar lavage (BAL) samples were centrifuged and tested for the presence of MMP activity as described below. The second pilot study was identical to the first except 10 animals/group were treated and the study duration was 2 weeks.
Six-Month Study
This study was comprised of 5 groups of mice. Group 1 animals (thirty-two) were control non-smoking animals. Group 2 animals (thirty-two) were administered aerosolized PBS (10 ml/5 animals) followed by cigarette smoke. Group 3 animals (thirty-two, Dose 1) were administered low-dose nebulized ilomastat (10 ug/ml in PBS; 10 ml/5 animals) followed by cigarette smoke. Group 4 animals (thirty-two, Dose 2) were administered mid-dose nebulized ilomastat (50 ug/ml in PBS; 10 ml/5 animals) followed by cigarette smoke. Group 5 animals (thirty-two, Dose 3) received high-dose nebulized ilomastat (100 ug/ml in PBS; 10 ml/5 animals) followed by cigarette smoke.
Time Points for Data Collection
Within each treatment group there were 3 time points for analysis: 1 week, 3 months, and 6 months. After 1 week, 3 months, and 6 months on study, 10 animals, 10 animals, and 12 animals, respectively, from each group were sacrificed and analytical work on lungs performed and BAL samples collected. Saline was perfused through the right ventricle to remove blood from the lungs. The left lung was ligated and removed for BAL sampling and inflammatory cell analysis. The right lung was prepared for morphological analysis.
Tissue Processing and Morphometry
The right lung was inflated to a fixed pressure (25 cm H2O) by instilling 10% buffered formalin (for 15 minutes), and it was then ligated and removed. Inflated lungs were fixed for 48 hours and then serially dehydrated before being embedded in paraffin. Serial sagittal sections were obtained for morphological studies and histological analysis. Lm, an indicator of airspace size, was calculated for each mouse by a single, blinded observer (SDS) from 7 random fields at X200 as described previously Citation[11]Citation[26].
Inflammatory Cell Analysis
BAL samples were prepared as described for the pilot studies and analyzed for neutrophil and macrophage content. BAL return volume was measured and 100 ul was cytospun onto slides. Slides were stained with Leukostat Stain Kit. The numbers of each cell type were identified by nuclear shape and staining pattern and quantified by counting a 100-cell field. The total cell count and differential count was calculated.
Statistics
All graphs show mean value ± standard error. p values were determined by Student's unpaired t-test using STATVIEW 5.0. p values < 0.05 were considered significant.
MMP and NE Assays
MMP activity in the centrifuged murine BAL samples was detected by adding the assay reagent mix (50 mM HEPES, pH 7.0, 10 mM CaCl2, 2.5 mM Ellmans Reagent, 0.85 mM MMP substrate) directly to serial dilutions of BAL samples and monitoring color development at 405 nm over a 3-hour time period. Negative and positive control assays were also performed, using PBS or 0.54 pM recombinant MMP-9 (active fragment) respectively. Neutrophil elastase activity was determined essentially as described previously Citation[27].
Results
To determine whether inhibitory levels of ilomastat could be delivered to the mouse lung via nebulization, we performed initial short-term pilot studies. These studies demonstrated that inhaled ilomastat delivered at 100 µg/ml (2 ml/mouse/day) for 1 week in one cohort (5 animals), and for 2 weeks in a second cohort (10 animals) of cigarette smoke-treated mice was capable of inhibiting matrix metalloprotease activity in lavage fluids. shows that although MMP activity was detected in only one-third of control animals, MMP activity could not be detected in any ilomastat-treated animals. It is important to note that although the assay used is configured primarily for the detection of MMP-9 activity, it is known that the substrate is also cleaved by MMP-12, a MMP that has been shown to have great importance in the development of emphysema in this mouse model Citation[11]. Furthermore, we have shown that ilomastat inhibits MMP-12 with a similar sub-nanomolar Ki to that observed for its inhibition of MMP-9 and other MMPs involved in COPD [Refs. Citation[8]Citation[23] and these authors, unpublished results].
Table 1. Detection of MMP activity in lung lavage fluids of ilomastat-treated mice
Based upon the above observations, we initiated longer-term studies, adopting this level of daily drug administration as the highest dose (dose 3). Additional, lower delivered doses (doses 1 and 2) were 10-fold and 2-fold lower respectively.
shows the effect on neutrophil levels of administering cigarette smoke to groups of mice for time periods of 1 week, 3 months and 6 months, with or without concomitant treatment with inhaled ilomastat at the dose levels noted above. Lavage neutrophil levels were highly variable and no clear trend was observed at the one week and three month time points. At 6 months, however, lavage neutrophil levels were elevated significantly in the smoking group compared to the non-smoking animals. In each of the 3 ilomastat-treated groups, lavage neutrophil levels were reduced significantly at the 6-month time point compared with the smoking group. Despite the elevated levels of neutrophils, however, measurement of changes in NE levels in the BAL samples was not possible as, in all samples measured, NE levels were below the limit of detection. The high level of variability in neutrophils is unlikely to be related to methodology artifacts, such as sample collection, as the measured levels of macrophages in the identical BAL samples exhibited a low level of variability between test animals ().
Figure 1 The effects of inhaled ilomastat on numbers of lavage neutrophils at 1 week (A), 3 months (B), and 6 months (C). The groups included control animals, age-matched cigarette smoke-treated animals (Smoke) and 3 groups of animals that received both smoke exposure and inhaled ilomastat at three dose levels, see “Materials and Methods.” There was a significant increase in lavage neutrophils in the smoking group at six months when compared with control animals. At this time point, inhaled ilomastat reduced neutrophil levels well below that of untreated smoking animals at all dose levels tested (dose 1, dose 2, dose 3).
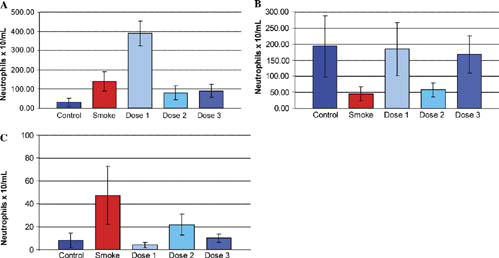
Figure 2 The effects of inhaled ilomastat on numbers of lavage macrophages (MAC) at 1 week (A), 3 months (B), and 6 months (C). The groups included control animals, age-matched cigarette smoke-treated animals (Smoke) and 3 groups of animals that received both smoke exposure and inhaled ilomastat at 3 dose levels, see “Materials and Methods.” There was a significant increase in lavage macrophages in the smoking group at all 3 time points when compared with control animals. Although at 1 week, the smoke and low-dose ilomastat-treated animals had elevated levels of macrophages when compared with smoke alone, in general, there was a dose-dependent reduction in macrophage counts at all 3 time points. At 6 months, lavage macrophages were reduced considerably in all 3 dose groups (dose 1, dose 2, dose 3) when compared with both control, and smoke-treated animals.
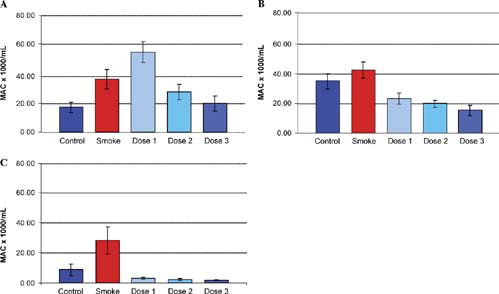
shows the effect on lavage macrophage levels of the above treatment. A marked increase in lavage macrophage levels was observed in the smoking group versus the control non-smoking group, particularly at the 1-week and 6-month time points. At the 1-week time point, lavage macrophage levels were higher in the low-dose ilomastat-treated animals than in the group exposed to smoke alone. At each time point in the ilomastat-treated groups, lavage macrophage levels appeared to decrease in a dose-dependent manner, becoming significantly reduced in all treated groups at the 6-month time point.
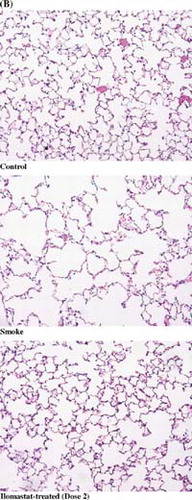
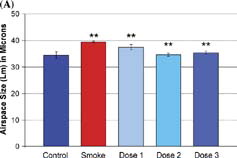
Airspace size was increased by 14.5% in the smoke-exposed animals when compared with non-smoking controls (Control, 34.4 ± 3.4 µm, Smoke-treated, 39.4 ± 1.4 µm; p value = 0.0006). shows the reduction in airspace enlargement (Lm) obtained by treating cigarette smoke-treated mice with inhaled ilomastat at the dose levels described above. There was a striking reduction of airspace enlargement at 6 months in the mid-dose group (group 2) of 96% (34.6 ± 3.4 µm; p value < 0.0001 vs. smoke-treated group). Large reductions in airspace enlargement were also noted in the low and high dose groups of 42% and 83% respectively (37.4 ± 3.4 µm and 35.3 ± 3.4 µm; p values = 0.003 and 0.001 vs. smoke-treated group). Representative images of histological sections are shown in , corresponding to the lungs of non-smoking controls, smoke-treated animals, and smoke- and ilomastat-treated animals (dose 2). Airspace enlargement by cigarette smoke and its inhibition by inhaled ilomastat are clearly apparent in these images.
Figure 3 Airspace enlargement in response to cigarette smoke exposure. (A) Smoke-treated mice (Smoke) have increased emphysema when compared with age-matched, non-smoke-exposed control mice (Control). Ilomastat-treated mice exhibited significantly less airspace enlargement in all 3 dose groups (dose 1, dose 2, dose 3). Double asterisks represent p values < 0.005 vs. control (smoke-treated group), and vs. smoke-treated group (dose 1, dose 2, dose 3), see “Results.” (B) Representative mid-sagittal slices, stained with H&E, as described in “Materials and Methods,” from control, smoke-treated, and smoke and inhaled mid-dose ilomastat-treated mice (dose 2). Note high level of protection against the development of emphysema in ilomastat-treated sample.
Discussion
In this paper, we have shown that the MMPI, ilomastat, when delivered by inhalation, can abrogate completely the effects of chronic cigarette smoke on airspace enlargement in susceptible mice. Similarly quantitative results have been obtained previously by knockout of the MMP-12 gene Citation[11], and by treatment of rodents orally or subcutaneously with MMPIs Citation[12&13]. Thus, a pivotal role for matrix metalloproteases in the development of COPD is now well established for both animal model systems and, more recently, for the human disease. Genetic linkage studies in humans have identified susceptibility loci in the genes for MMP-1 and MMP-12, with additional polymorphisms also located in the MMP-9 gene Citation[19]. Furthermore, empirical observations of smoker's lung tissue have also identified elevated levels of MMP-2 and MMP-8 in such diseased samples Citation[8]. Ilomastat is a potent inhibitor of all of the MMPs that have been implicated in smoke-induced emphysema, in both mice and humans. This broad-spectrum inhibitor has also been shown to inhibit members of the ADAM family of metalloproteases, another group of enzymes that are known to be involved in inflammation and lung disease Citation[28&29].
Previous strategies involving the use of MMPIs to reduce the development of emphysema in animal models have been focused on the use of oral or injectable inhibitors. Such strategies in humans, in other disease indications, have invariably been restricted due to the inherent toxicity associated with the requisite high dose therapy. A potential way around this problem, is in the development of highly selective, orally active MMPIs designed to inhibit specific target proteolytic enzymes Citation[22]. Because of the multitude of potential metalloproteases involved in COPD, however, we have adopted an approach whereby low-dose therapy of a broad-spectrum and potent MMPI is used to control the elevated lung degenerative activities of such enzymes.
The serine protease NE is clearly involved in lung tissue degradation in cigarette smoke-treated mice Citation[16]. Our striking observation that lung degradation can be inhibited virtually quantitatively suggests strongly that broad-spectrum MMPI therapy can also inhibit NE activity, albeit by an indirect mechanism. At least 2 mechanisms can be envisaged to account for this indirect inhibition. First, inhibition of the MMPs protects against the well known MMP-mediated degradation of AAT, the major physiological inhibitor of NE Citation[16]. Such protection would serve to provide continued balance in this critical protease/antiprotease equilibrium. Also, reduced activation of TNF-α by inhibition of MMPs and ADAM family members that are capable of pro-TNF-α conversion would also lead to reduced recruitment of neutrophils, thereby also decreasing significantly the expression of active NE within lung interstitium Citation[15]Citation[17]. In [after Churg et al. Citation[17]] is shown a proposed mechanism describing the interplay between the known protease mediators of lung inflammation and matrix degradation. In this scheme, it can be seen how a potent MMPI could inhibit such degradation both directly, and by inhibiting the NE-mediated breakdown of elastin by an indirect mechanism. It is interesting to note, however, that similar strategies using potent NE inhibitors, NE knockout mice, or TNF-α receptor knockout mice, while giving levels of protection in the 60–70% range, do not lead to the almost quantitative, i.e., close to 100%, reduction in airspace enlargement seen in the present study Citation[16]Citation[30-32]. Further study on the effects of inhaled MMPIs on NE levels measured directly in the smoking mouse lung will, however, require a more sensitive assay for NE.
Figure 4 Schematic representation of the proposed role for ilomastat in interrupting the protease/protease inhibitor imbalance and resulting inflammatory cascade provoked by cigarette smoke. First, ilomastat inhibits directly the degradation of AAT, thereby increasing protection of elastin from degradation by neutrophil elastase. In addition, the inhibition of TNF-α release leads to reduced neutrophil recruitment and consequent lowering of the neutrophil elastase burden within the cigarette smoke-treated lung tissue. Additional protection against matrix degradation is likely to occur through lowering of both neutrophil and macrophage recruitment by reducing or possibly even eliminating the production of chemotactic matrix breakdown products such as elastin-derived peptides [From Ref. Citation[33]].
![Figure 4 Schematic representation of the proposed role for ilomastat in interrupting the protease/protease inhibitor imbalance and resulting inflammatory cascade provoked by cigarette smoke. First, ilomastat inhibits directly the degradation of AAT, thereby increasing protection of elastin from degradation by neutrophil elastase. In addition, the inhibition of TNF-α release leads to reduced neutrophil recruitment and consequent lowering of the neutrophil elastase burden within the cigarette smoke-treated lung tissue. Additional protection against matrix degradation is likely to occur through lowering of both neutrophil and macrophage recruitment by reducing or possibly even eliminating the production of chemotactic matrix breakdown products such as elastin-derived peptides [From Ref. Citation[33]].](/cms/asset/a780f867-9b32-4dc2-bbbe-fe26f57c5f9d/icop_a_121800_uf0005_b.gif)
In summary, therefore, the dramatic effects of ilomastat on the reduction of smoke-mediated airspace enlargement could occur at a multiplicity of levels (). In addition to ilomastat's direct and potent inhibition of the MMPs responsible for extracellular matrix degradation, inhibitory effects on the multiple cellular processes that lead to an increased imbalance in the serine elastase/inhibitor equilibrium are highly likely to be significant factors. This apparent upstream inhibition of NE activity by ilomastat further supports the potential of this and similar broad-spectrum MMPIs in the treatment of human COPD. Indeed, the low doses of ilomastat used in the present work suggest that inhalation delivery, a well-established method of drug therapy in human respiratory disease, may represent a practical method for the treatment of COPD using protease inhibitors.
References
- Stockley R A. Neutrophils and protease/antiprotease imbalance.Am J Respir Crit Care Med 1999; 160:S49–S52. [PUBMED], [INFOTRIEVE], [CSA]
- Barnes P J. Medical progress: chronic obstructive pulmonary disease.N Engl J Med 2000; 343:269–280. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Barnes P J, Shapiro S D, Pauwels R A. Chronic obstructive pulmonary disease: molecular and cellular mechanisms.Eur Respir J 2003; 22:672–688. [PUBMED], [INFOTRIEVE], [CSA]
- Shapiro S D. Proteolysis in the lung.Eur Respir J 2003; 22(suppl 44):30s–32s. [CSA]
- Laurell C-B, Eriksson S. The electrophoretic α-1-globulin pattern of serum in α-1 antitrypsin deficiency.Scand J Clin Lab Invest 1963; 15:132–140. [CSA]
- Tobin M J, Cook P JL, Hutchison D CS. Alpha-1 antitrypsin deficiency: the clinical and physiological features of pulmonary emphysema in patients homozygous for Pi type Z.Br J Dis Chest 1983; 77:14–27. [PUBMED], [INFOTRIEVE], [CSA]
- American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency.Am J Respir Crit Care Med 2003; 168:818–900. [CSA], [CROSSREF]
- Segura-Valdez L, Pardo A, Gaxiola M, Uhal B D, Becerril C, Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD.Chest 2000; 117(3):684–694. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Russell R E, Culpitt S V, De Matos C, Donnelly L, Smith M, Wiggins J, Barnes P J. Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary disease.Am J Respir Cell Mol Biol 2002; 26:602–609. [PUBMED], [INFOTRIEVE], [CSA]
- Finlay G A, O'Driscoll L R, Russell K J, D'Arcy E M, Masterson J B, FitzGerald M X, O'Connor C M. Matrix metalloprotease expression and production by alveolar macrophages in emphysema.Am J Respir Crit Care Med 1997; 156:240–247. [PUBMED], [INFOTRIEVE], [CSA]
- Hautamaki R D, Kobayashi D K, Senior R M, Shapiro S D. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice.Science 1997; 277:2002–2004. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Martin R L, Shapiro S D, Tong S E, Van Wart H E. Macrophage metalloelastase inhibitors.Prog Respir Res 2001; 31:177–180. [CSA]
- Selman M, Cisneros-Lira J, Gaxiola M, Ramíerez R, Kudlaez E M, Mitchell P G, Pardo A. Matrix metalloproteinases inhibition attenuates tobacco smoke-induced emphysema in guinea pigs.Chest 2003; 123:1633–1641. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Churg A, Zay K, Shay S, Xie C, Shapiro S D, Hendricks R, Wright J L. Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice.Am J Respir Cell Mol Biol 2002; 27:368–374. [PUBMED], [INFOTRIEVE], [CSA]
- Churg A, Dai J, Tai H, Xie C, Wright J. Tumor necrosis factor-α is central to acute cigarette smoke-induced inflammation and connective tissue breakdown.Am J Respir Crit Care Med 2002; 166:849–854. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Shapiro S D, Goldstein N M, McGarry Houghton A, Kobayashi D K, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice.Am J Pathol 2003; 163:2329–2335. [PUBMED], [INFOTRIEVE], [CSA]
- Churg A, Wang R D, Tai H, Wang X, Xie C, Dai J, Shapiro S D, Wright J L. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via TNF-alpha release.Am J Respir Crit Care Med 2003; 167:1083–1089. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Barnes P J. Alveolar macrophages as orchestrators of COPD.COPD 2004; 1:59–70., [CSA]
- Joos L, He J Q, Shepherson M B, Connett J E, Anthonisen N R, Pare P D, Sandford A J. The role of matrix metalloproteinase polymorphisms in the rate of decline of lung function.Hum Mol Genet 2002; 11:569–576. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gearing A J, Beckett P, Christodoulou M, Churchill M, Clements J, Davison A H, Drummond A H, Galloway W A, Gilbert R, Gordon J L. Processing of tumor necrosis factor-α precursor by metalloproteinases.Nature 1994; 370:555–557. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Bramhall S R, Rosemurgy A, Brown P D, Bowry C, Buckels J A. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial.J Clin Oncol 2001; 19:3447–3455. [PUBMED], [INFOTRIEVE], [CSA]
- Brown P D. Ongoing trials with matrix metalloproteinase inhibitors.Expert Opin Investig Drugs 2000; 9:2167–2177. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Levy D E, Lapierre F, Liang W, Ye W, Lange C W, Li X, Grobelny D, Casabonne M, Tyrrell D, Holme K, Nadzan A, Galardy R E. Matrix metalloproteinase inhibitors: a structure-activity study.J Med Chem 1998; 41:199–223. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Solorzano C C, Ksontini R, Pruitt J H, Auffenberg T, Tannahill C, Galardy R E, Schultz G P, MacKay S L, Copeland E M III, Moldawer L L. A matrix metalloproteinase inhibitor prevents processing of tumor necrosis factor alpha (TNF alpha) and abrogates endotoxin-induced lethality.Shock 1997; 7:427–431. [PUBMED], [INFOTRIEVE], [CSA]
- Pemberton P A, Cantwell J S, Kim K M, Sundin D J, Kobayashi D, Fink J, Shapiro S D, Barr P J. An inhaled MMP inhibitor blocks cigarette-induced lung damage in the smoking mouse model.Eur Respir J, Suppl 2003; 45:A1317. [CSA]
- Dunnill M S. Quantitative methods in the study of pulmonary pathology.Thorax 1962; 17:320–328. [CSA]
- Feinstein G, Kupfer A, Sokolovsky M. N-acetyl-(l-Ala)3-p-nitroanilide as a new chromogenic substrate for elastase.Biochem Biophys Res Commun 1973; 50:1020–1026. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ito N, Nomura S, Iwase A, Ito T, Kikkawa F, Tsujimoto M, Ishiura S, Mizutani S. ADAMs, a disintegrin and metalloproteinases, mediate shedding of oxytocinase.Biochem Biophys Res Commun 2004; 314:1008–1013. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Shapiro S D, Owen C A. ADAM-33 surfaces as an asthma gene.N Engl J Med 2002; 347:936–938. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wright J L, Farmer S G, Churg A. A neutrophil elastase inhibitor reduces cigarette smoke-induced remodeling of lung vessels.Eur Respir J 2003; 22:77–81. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Churg A, Wang R D, Xie C, Wright J L. Alpha-1-antitrypsin ameliorates cigarette smoke-induced emphysema in the mouse.Am J Respir Crit Care Med 2003; 168:199–207. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Churg A, Wang R D, Tai H, Wang X, Xie C, Wright J L. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse.Am J Respir Crit Care Med 2004; 170:492–498. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Senior R M, Griffin G L, Mecham R P. Chemotactic activity of elastin-derived peptides.J Clin Invest 1980; 66:859–862. [PUBMED], [INFOTRIEVE], [CSA]