Abstract
Dyspnea and activity limitation are the primary symptoms of chronic obstructive pulmonary disease and progress relentlessly as the disease advances. In COPD, dyspnea is multifactorial but abnormal dynamic ventilatory mechanics are believed to be important. Dynamic lung hyperinflation occurs during exercise in the majority of flow-limited patients with chronic obstructive pulmonary disease and may have serious sensory and mechanical consequences. This proposition is supported by several studies, which have shown a close correlation between indices of dynamic lung hyperinflation and measures of both exertional dyspnea and exercise performance. The strength of this association has been further confirmed by studies that have therapeutically manipulated this dependent variable. Relief of exertional dyspnea and improved exercise endurance following bronchodilator therapy correlate well with reduced lung hyperinflation. The mechanisms by which dynamic lung hyperinflation give rise to exertional dyspnea and exercise intolerance are complex. However, recent mechanistic studies suggest that dynamic lung hyperinflation-induced volume restriction and consequent neuromechanical uncoupling of the respiratory system are key mechanisms. This review examines, in some detail, the derangements of ventilatory mechanics that are peculiar to chronic obstructive pulmonary disease and attempts to provide a mechanistic rationale for the attendant respiratory discomfort and activity limitation.
Keywords::
INTRODUCTION
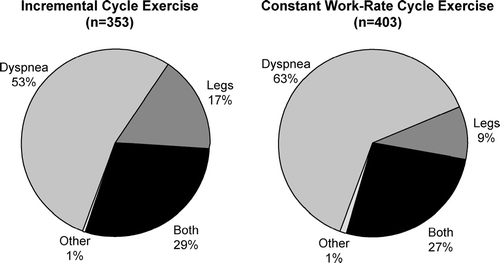
Dyspnea (i.e., perceived respiratory discomfort) is the primary symptom limiting exercise in the majority of patients with more advanced chronic obstructive pulmonary disease (COPD) and can lead to avoidance of activity with consequent skeletal muscle deconditioning (Citation[1], Citation[2], Citation[3], Citation[4], Citation[5], Citation[6]). In a study of 105 clinically stable patients (FEV1 = 37% predicted), severe breathing discomfort was the primary symptom limiting incremental cycle exercise in 61%; combined dyspnea and leg discomfort limited exercise in 19%, and 18% stopped primarily because of leg discomfort (Citation[3]). Patients who stopped exercise primarily because of dyspnea, had greater levels of dynamic lung hyperinflation, greater ventilatory constraints and poorer exercise performance than the minority who stopped mainly because of leg discomfort (Citation[3]). This frequency distribution of exercise limiting symptoms was almost similar to that found in a study in 125 symptomatic patients with COPD entering a pulmonary rehabilitation program (Citation[7]) and in a combined group of 353 COPD patients undertaking a symptom-limited incremental cycle exercise (Citation[5], Citation[8]) (). Similarly, in a group of 403 patients with moderate-to-severe COPD who undertook constant work-rate cycle exercise, dyspnea again was the main exercise-limiting symptom in the majority (63%), whereas leg discomfort alone and in combination with dyspnea accounted for 9% and 27%, respectively (Citation[5],Citation[8]) ().
Figure 1 Distribution of reasons for stopping exercise during symptom-limited incremental cycle exercise (n = 353) and constant work rate cycle exercise at 75% of maximal work capacity in COPD (n = 403). Data used to create pie graphs pooled from: Maltais F, Hamilton A, Marciniuk D, Hernandez P, Sciurba FC, Richter K, Kesten S, O'Donnell D. Chest 2005; 128: 1168-1178 and O'Donnell D, Flüge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Eur Respir J 2004; 23:832–840.
In COPD, exercise limitation is clearly multifactorial and ultimately reflects integrated abnormalities of the ventilatory, cardiovascular, peripheral muscle, metabolic and neurosensory systems in highly variable combinations. However, ventilatory limitation is the dominant contributor to exercise curtailment in more advanced disease and is the main focus of this review. In particular, we will review the evidence that lung hyperinflation provides a mechanistic link between expiratory flow-limitation, dyspnea and exercise intolerance.
Pathophysiology of lung hyperinflation in COPD
The volume of air remaining in the lung at the end of spontaneous expiration (i.e., end-expiratory lung volume (EELV)) is increased in COPD compared with health, indicating lung hyperinflation. While in health the EELV during relaxed resting breathing corresponds with the actual static equilibrium position of the respiratory system, this is often not the case in COPD (Citation[9]). In COPD, because of permanent destructive emphysema, the balance of static forces of the relaxed respiratory system is reset, such that EELV is elevated compared with health. During spontaneous resting breathing in patients with expiratory flow-limitation, EELV is “dynamically” determined and is maintained at a level above the statically determined relaxation volume of the respiratory system. In flow-limited patients, the mechanical time-constant for lung emptying is increased in many alveolar units, but the expiratory time available during quiet breathing (as dictated by the respiratory control centers) is often insufficient to allow EELV to decline to its normal relaxation volume, and gas accumulation (often termed “air trapping”) results. In other words, lung emptying during expiration becomes incomplete because it is interrupted by the next inspiration and EELV therefore exceeds the natural relaxation volume. EELV in COPD is therefore a continuous dynamic variable that differs with the extent of expiratory flow-limitation, the degree of time-constant abnormalities, and the breathing pattern.
Ventilatory response to activity in COPD
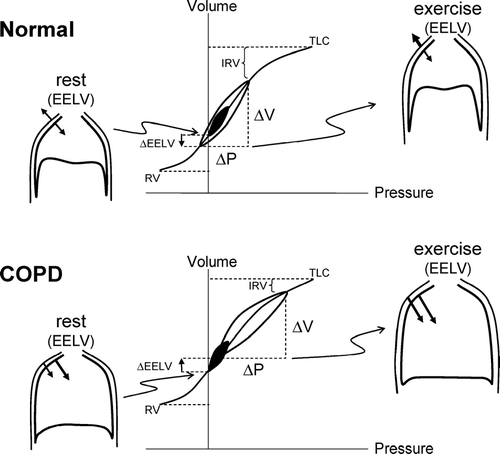
Ventilatory responses to exercise in COPD are increased relative to health. Stimuli for excessive ventilation in COPD include: increased chemo-stimulation as a result of the effects of extensive ventilation-perfusion abnormalities, early metabolic acidosis, feedback from activated mechanoreceptors/metaboreceptors in the working peripheral muscles or any combination of the above listed factors. During physical activity, healthy younger subjects tend to decrease EELV as a result of recruitment of abdominal and expiratoryrib cage muscles (Citation[10], Citation[11]). Active reduction of EELV in this manner assists the inspiratory muscles and ensures that tidal volume expansion occurs within the linear compliant portion of the respiratory system's pressure-volume relation ().
Figure 2 Pressure-volume (P-V) relationships of the total respiratory system in health and in COPD. Tidal pressure-volume curves during rest (filled area) and exercise (open area) are shown. In COPD, because of resting and dynamic hyperinflation (a further increased EELV), exercise tidal volume (VT) encroaches on the upper, alinear extreme of the respiratory system's P-V curve where there is increased elastic loading. In COPD, the ability to further expand VT is reduced, i.e., inspiratory reserve volume (IRV) is diminished. In contrast to health, the combined recoil pressure of the lungs and chest wall in hyperinflated patients with COPD is inwardly directed during both rest and exercise; this results in an inspiratory threshold load on the inspiratory muscles. Abbreviations: EELV = end-expiratory lung volume; RV = residual volume; TLC = total lung capacity. Reprinted from Mahler DA, O'Donnell DE (eds). Dyspnea: Mechanisms, Measurement, and Management, 2nd edition. Lung Biology in Health and Disease Series, Volume 208, Chapter 3. New York: Taylor & Francis Group, 2005; pp. 29–58, used with permission.
With advancing age, changes in the lungs' connective tissue matrix can lead to expiratory flow-limitation at higher levels of ventilation and therefore the ability to reduce EELV is curtailed during exercise. Although patients with COPD progressivelyrecruit their expiratory muscles during exercise, EELV usually increases as a consequence of expiratory flow-limitation (Citation[3],Citation[12], Citation[13], Citation[14], Citation[15], Citation[16], Citation[17]). This temporary and variable increase in EELV above its baseline value is termed “dynamic” lung hyperinflation (DH). Thus, under any condition of increased ventilation in flow-limited patients (e.g., exercise or voluntary hyperventilation), inspiratory tidal volume increases and expiratory time diminishes further as breathing frequency increases above the baseline value, causing further acute-on-chronic DH (Citation[3], Citation[12], Citation[18], Citation[19], Citation[20]).
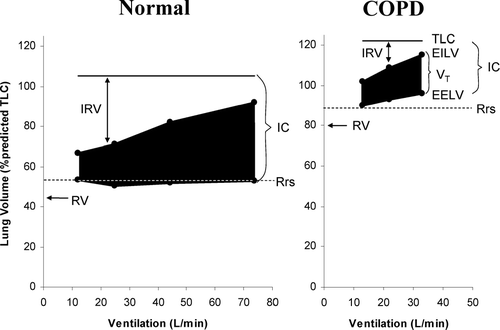
It has been established for some time that DH occurs in flow-limited patients under conditions of increased ventilatory demand during exercise (Citation[21], Citation[22], Citation[23], Citation[24]) (). The rate and magnitude of DH during exercise is generally measured in the laboratory setting by serial inspiratory capacity (IC) measurements (Citation[3], Citation[14], Citation[25]). Since total lung capacity (TLC) does not change during activity (Citation[23], Citation[26]), the change (decrease) in IC reflects the change (increase) in dynamic EELV, or the extent of DH. This simple method has been shown to be reliable and recent multi-centre clinical trials have confirmed its reproducibility and responsiveness (Citation[8], Citation[14], Citation[25]). The use of change in IC to track DH is further validated by studies that have used esophageal manometry to demonstrate that even severely dyspneic patients are capable of generating maximal inspiratory pressures at the end of exhaustive exercise (Citation[2], Citation[27]). This implies that the reductions in IC seen during exercise in COPD are not due to submaximal efforts, and indeed reflect changes in underlying EELV.
Figure 3 Changes in operating lung volumes are shown as ventilation increases with exercise in COPD (n = 105) and in age-matched normal subjects (n = 25). “Restrictive” constraints on tidal volume (VT, solid area) expansion during exercise are significantly greater in the COPD group from both below (reduced inspiratory capacity (IC)) and above (minimal inspiratory reserve volume (IRV). Other abbreviations: EELV = end-expiratory lung volume; EILV = end-inspiratory lung volume; Rrs = relaxation volume of the respiratory system; RV = residual volume; TLC = total lung capacity. With permission from O'Donnell DE, Webb KA. Mechanisms of dyspnea in COPD. In: Mahler DA, O'Donnell DE (eds). Dyspnea: Mechanisms, Measurement, and Management, 2nd edition. Lung Biology in Health and Disease Series, Volume 208, Chapter 3. New York: Taylor & Francis Group, 2005; pp. 29–58.
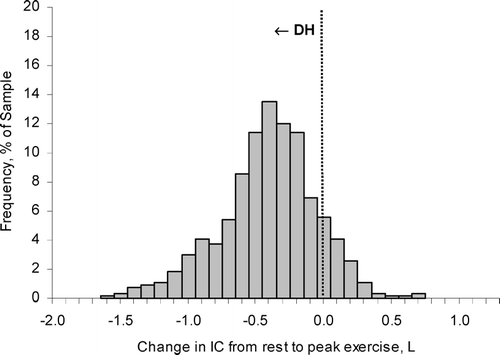
In combined studies conducted in over 500 patients with moderate-to-severe COPD, the change in EELV during cycle ergometry averaged 0.4 L, representing a reduction in IC by ∼ 20% of the resting value, but with wide variation in the range (Citation[3], Citation[5], Citation[8]) (). Eighty-five percent of this population sample showed increases in EELV from rest to peak exercise, confirming the presence of significant DH (Citation[3], Citation[5], Citation[8]). The minority of patients who showed little reduction in IC with exercise demonstrated the most severe resting lung hyperinflation (Citation[3]). The rate of rise of DH was steeper in patients with the most severe expiratory flow-limitation (as estimated by the FEV1/FVC ratio), the lowest diffusing capacity for carbon monoxide and the highest ventilatory demand (reflecting greater ventilation-perfusion abnormalities), and generally reached a maximal value early in exercise (Citation[3]).
Figure 4 The distribution of the extent of change in inspiratory capacity (IC) during exercise is shown in moderate-to-severe COPD (n = 534). A reduction (negative change) in IC reflects dynamic hyperinflation (DH) during exercise. Each bar width corresponds to a change in IC range of 0.10 L. The majority of patients with COPD experienced significant DH during exercise. Graphs represent cumulative data from O'Donnell DE, Revill SM, Webb KA. Am J Respir Crit Care Med 2001; 164: 770–777; O'Donnell DE, Voduc N, Fitzpatrick M, Webb KA. Eur Respir J 2004; 24: 86–94; Maltais F, Hamilton A, Marciniuk D, Hernandez P, Sciurba FC, Richter K, Kesten S, O'Donnell D. Chest 2005; 128:1168–1178; O'Donnell D, Flüge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Eur Respir J 2004; 23:832–840.
Lung hyperinflation and activity limitation in COPD: The evidence
The presence of expiratory flow-limitation appears to be an important predictor of exercise tolerance in patients with COPD. In a cohort of 52 patients, all of those who had evidence of significant expiratory flow-limitation at rest (measured by the negative expiratory pressure technique) showed a reduction of both peak workload and peak oxygen uptake (V'O2) (Citation[28]). By contrast, the achieved peak V'O2 during exercise was within normal limits in 35% of the subjects who were not flow-limited at rest. Moreover, all of the patients with significant expiratory flow-limitation had evidence of resting lung hyperinflation: IC was reduced to < 80% predicted. In contrast, IC was preserved in the majority of patients without flow-limitation. This supports the findings of Koulouris et al. (Citation[15]) and suggests that: (Citation[1]) reduced IC is a good and validated marker of flow-limitation and the propensity to develop worsening DH during exercise (Citation[2], Citation[27]); (Citation[2]) resting IC represents the operating limits for tidal volume (VT) expansion during the increased ventilation of exercise (Citation[3], Citation[28]); and (Citation[3]) resting IC can predict the peak symptom-limited V'O2 in patients with expiratory flow-limitation at rest (Citation[3], Citation[28], Citation[29]).
Indices of lung hyperinflation have repeatedly emerged as predictors of exercise intolerance in patients with COPD. For example, using symptom-limited peak V'O2 as the dependent variable, O'Donnell and colleagues (Citation[3]) found that peak VT (standardized as % predicted vital capacity) emerged as the strongest predictor of exercise tolerance in patients with COPD (r = 0.68, p < 0.0005). In turn, peak VT was most strongly predicted by the peak IC during exercise (r = 0.791, p < 0.0005) and by the resting IC (r = 0.75, p < 0.0005), expressed as % predicted (). Furthermore, the relationship between peak VT during exercise and peak IC was particularly strong in the patients who showed an IC < 70% predicted (r = 0.87, p < 0.0005) and presumably significant expiratory flow-limitation, but was not significant in the patients who had preserved IC (r = 0.27, p = 0.244). In addition, the ratio of peak VT to peak IC (taken as an index of the mechanical constraint on VT expansion) was the best correlate of the level of ventilatory limitation (i.e., peak ventilation as a fraction of maximal ventilatory capacity) during exercise (Citation[3]).
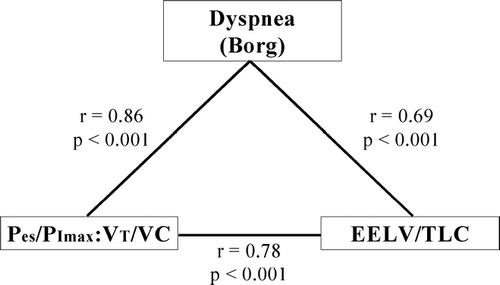
Figure 5 Significant intercorrelations between dyspnea intensity, neuromechanical dissociation and hyperinflation. Dyspnea, as assessed by the Borg scale, correlates significantly with the effort-displacement ratio (which is given by the ratio of Pes/PImax:VT/VC, where Pes is esophageal pressure, PImax is the maximal inspiratory pressure, VT is tidal volume, and VC is vital capacity) as an index of neuromechanical coupling. Dyspnea intensity is also significantly predicted by hyperinflation, as assessed by the end-expiratory lung volume (EELV) as a proportion of total lung capacity (TLC). The effort-displacement ratio and hyperinflation are also strongly correlated. From O'Donnell DE, Bertley JC, Chau LK, Webb KA. Am J Respir Crit Care Med 1997; 155:109–115 and adapted from Mahler DA, O'Donnell DE (eds). Dyspnea: Mechanisms, Measurement, and Management, 2nd edition. Lung Biology in Health and Disease Series, Volume 208, Chapter 3. New York: Taylor & Francis Group, 2005; pp. 29–58, used with permission.
Similarly, Diaz and colleagues found that IC was a significant spirometric correlate of both peak workload (r = 0.48) and peak V'O2 (r = 0.63) in 52 patients with COPD (Citation[28]). Recently, Puente-Maestu (Citation[29]) and colleagues showed a good correlation between the resting IC (expressed as % predicted) and the peak V'O2 in 27 severe COPD during constant work-rate exercise at different intensities (i.e., 65%, 75%, 85%, and 95% of the peak incremental work rate; r = 0.64 to 0.69 between the IC as % predicted and peak V'O2 at the 4 work rates). Collectively, these studies support the idea that lung hyperinflation, dynamic mechanical restriction and exercise limitation are mechanistically linked in COPD.
Negative effects of acute dynamic hyperinflation during exercise
Although DH serves to optimise expiratory flow rates by avoiding expiratory flow-limitation at lower lung volumes, it has the deleterious effect of forcing VT to operate on the upper, flatter part of the respiratory system's compliance curve where increases in pressure no longer generate significant incremental volume change (). With worsening DH, the ability of the VT to increase during exercise is reduced, imposing “restrictive” mechanics. In fact, in 105 patients with COPD, the end inspiratory lung volume (EILV) was found to be 94 ± 5% of TLC at a peak symptom-limited V'O2 of only 12.6 ± 5.0 mL/kg/min; this corresponded to a reduction in the inspiratory reserve volume (IRV) to a minimum of 0.42 ± 0.33 L (Citation[3]). In contrast, when breathing at a minute ventilation (V'E) similar to that of COPD patients at peak exercise, healthy age-matched individuals had significantly less constraints imposed on VT expansion, with IRV measured at 1.75 ± 1.16 L (). DH reduces the ability of VT to expand appropriately during exercise and this leads to early mechanical limitation of V'E (Citation[16]). The consequence of this “saturation” of VT is that further increases in V'E must rely on increases in breathing frequency (Citation[30]). Unfortunately, in these already flow-limited patients, increases in breathing frequency may further aggravate DH in a vicious cycle and, in addition, contribute to reduced dynamic lung compliance and increased flow-resistive work.
DH results in sudden increases in the elastic and threshold loads on the inspiratory muscles, thus increasing the work and oxygen cost of breathing. The inspiratory threshold load (ITL) reflects the force that the inspiratory muscles must generate to counterbalance the inward (expiratory) recoil of the lung and chest wall at end-expiration and can be substantial in COPD (Citation[2]). DH results in functional inspiratory muscle weakness by maximally shortening the muscle fibers in the diaphragm (Citation[31]). The combination of excessive mechanical loading and increased velocity of shortening of the inspiratory muscles can also predispose them to fatigue. In some patients, this mechanical constraint on VT expansion, in the setting of severe ventilation-perfusion abnormalities (i.e., high fixed physiological dead space), leads to carbon dioxide retention and arterial oxygen desaturation during exercise (Citation[16]). Finally, DH adversely affects dynamic cardiac function by contributing to pulmonary hypertension, by reducing right ventricular pre-load (reduced venous return) and, in some cases, by increasing left ventricular afterload (Citation[32], Citation[33], Citation[34]). It has recently been postulated that competition between the overworked ventilatory muscles with the active peripheral muscles for a reduced cardiac output may compromise blood flow and oxygen delivery to the latter, with negative consequences for exercise performance (Citation[35], Citation[36], Citation[37]). All the above factors are clearly interdependent and contribute in a complex, integrated manner to dyspnea and exercise limitation in COPD.
Lung hyperinflation and exertional dyspnea in copd: The evidence
Dyspnea, is a complex multifaceted and highly personalized sensory experience, the source and mechanisms of which are incompletely understood. The notion that DH contributes to perceived exertional dyspnea has been bolstered by a number of studies that have shown a consistent statistical association between dyspnea intensity (assessed by the Borg scale) and various indices of DH during exercise (Citation[2], Citation[3], Citation[14]). Using multiple regression analysis, subjective Borg ratings of dyspnea intensity were found to be most strongly correlated with changes in EILV (expressed as % TLC; r = 0.63, p = 0.001) during exercise in 23 patients with advanced COPD (average FEV1 = 36% predicted). Furthermore, the measured change in EELV, and the subsequent constraint of VT expansion, also emerged as independent significant contributors to exertional dyspnea in these patients (Citation[1]). In another study (Citation[2]), exertional Borg dyspnea ratings correlated well with the ratio of EELV to TLC (r = 0.69, p < 0.001) (). Similarly, in a larger study of 105 patients with moderate-to-severe COPD, the VT/IC ratio, as an index of VT constraint, emerged as the strongest predictor of exertional dyspnea (p < 0.0005) (Citation[3]). Less important contributing variables included V'E/MVC, breathing frequency, and IRV/pred TLC, each accounting for 25% of the variance in Borg dyspnea ratings (p < 0.0005) (Citation[3]). Poor correlation has also repeatedly been found between the FEV1 and/or the FVC and measures of disability such as dyspnea and exercise capacity (Citation[3]).
Qualitative aspects of exertional dyspnea in COPD
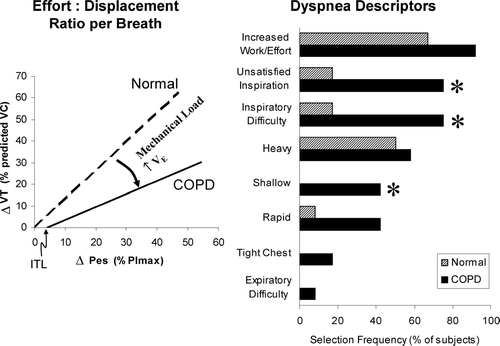
Further insights into the link between dyspnea and DH in COPD have arisen from studies that have explored the qualitative aspects of respiratory discomfort at a point where it reaches intolerable levels at the end of exercise. In qualitative terms, patients with COPD consistently select descriptor clusters that allude to both “increased effort” and “unsatisfied inspiration” at the break-point of cycle exercise ().
Figure 6 The relationship between tidal swings of respiratory effort (Pes/PImax) and tidal volume (VT) at the end of symptom-limited peak exercise in health and in COPD (left panel). Note the inspiratory threshold load (ITL) and the disparity between effort and VT. Descriptors of dyspnea at the end of exercise are also shown in health and in COPD (right panel). From O'Donnell DE, Bertley JC, Chau LK, Webb KA. Am J Respir Crit Care Med 1997; 155:109–115 with permission. OFFICIAL JOURNAL OF THE AMERICAN THORACIC SOCIETY. © AMERICAN THORACIC SOCIETY.
Dyspnea and perceived increased respiratory effort
Recent theories on the mechanisms of dyspnea have emphasized the central importance of the perception of increased contractile inspiratory muscle effort (Citation[38], Citation[39], Citation[40], Citation[41], Citation[42], Citation[43], Citation[44], Citation[45]). When skeletal muscles are mechanically loaded, weakened or fatigued, increased electrical activation of the muscle is required to generate a given force, and motor output to these muscles is amplified. It is hypothesized that increased motor output is accompanied by increased corollary discharge to the sensory cortex where it is directly perceived as a heightened sense of effort (Citation[43], Citation[44], Citation[45], Citation[46], Citation[47]). In COPD, inspired effort and central motor command output are increased compared with health, reflecting the relatively higher ventilation, the increased loading and functional weakness of the inspiratory muscles. Altered afferent information from activated mechanoreceptors in the overworked and shortened inspiratory muscles (secondary to DH) in COPD may contribute to an increased sense of work or effort, but this remains conjectural (Citation[47]). Beyond a certain threshold, increased effort may be consciously registered as respiratory discomfort (Citation[38], Citation[39], Citation[40], Citation[41], Citation[42], Citation[43]). Qualitative descriptors at end-exercise that allude to increased effort or work of breathing are pervasive across health and disease and increased corollary discharge remains a plausible mechanistic explanation for this (Citation[2]) ().
However, it must be remembered that increased sense of effort is only one component of this multi-dimensional symptom, and it is acknowledged that dyspnea can rise to severe levels even in the absence of increases in contractile muscle effort (Citation[48], Citation[49], Citation[50], Citation[51], Citation[52], Citation[53], Citation[54]). Mechanical ventilation, which successfully unloads the ventilatory muscles (thereby reducing effort), may not fully alleviate dyspnea (Citation[55], Citation[56]). Chemoreceptor stimulation (by adding carbon dioxide) can induce breathing discomfort, described as air hunger, even in the absence of ventilatory muscle activation. Finally, increasing breathing effort to a high fraction of the maximal possible effort is not necessarily perceived as discomfort in all circumstances.
Unsatisfied inspiration
In many respects, the sensory experience in COPD differs fundamentally from that of age-matched healthy individuals at peak V'O2 (Citation[2]). While the sense of increased effort, work or heaviness of breathing is common to both groups, only COPD patients consistently select descriptors that allude to unsatisfied inspiration (i.e., “can't get enough air in”), and it is reasonable to assume that these different qualitative dimensions of exertional dyspnea in COPD reflect different underlying mechanisms (Citation[2]).
The physiological events that occur at the end of exercise, when dyspnea becomes intolerable, are well understood. The neural drive to breathe reaches near maximal values, driven by the elevated carbon dioxide production (V'CO2) that accompanies exercise and the early metabolic acidosis that may occur in many deconditioned COPD patients.
In some patients, critical arterial oxygen desaturation, sympathetic nervous system over-activation and altered feedback from peripheral muscle metaboreceptors may additionally stimulate ventilation. As already outlined, however, the ventilatory output in response to the increased drive is often markedly diminished because of derangements of dynamic ventilatory mechanics. It is noteworthy that, in contrast to health, the effort-displacement ratio (the ratio of inspired effort (tidal esophageal pressure relative to maximum inspiratory pressure, i.e., Pes/PImax) to volume displacement (tidal volume expressed as a percentage of predicted vital capacity, i.e., VT/VC)) continues to rise in COPD as exercise proceeds. This increased ratio, which crudely reflects the position of the operating tidal volume on the respiratory system's pressure-volume relation (and thus the degree of neuromechanical dissociation), correlates well with perceived intensity of inspiratory difficulty. For example, in 12 patients with severe COPD (FEV1 = 37% predicted), the effort-displacement ratio was the strongest correlate of dyspnea intensity during exercise (r = 0.86, p < 0.001), and also correlated strongly with dynamic hyperinflation (EELV/TLC; r = 0.78, p < 0.001) (Citation[2]).
A recent mechanistic study in our laboratory has attempted to reconcile the beneficial effects of DH in early exercise with its deleterious sensory effects that ultimately contribute to exercise limitation. Thus, DH early in exercise allowed flow-limited patients to increase V'E while minimizing respiratory discomfort (Citation[30]). As a result of this early DH, the airways are maximally stretched at the higher lung volumes (close to TLC) and expiratory flow-limitation is attenuated allowing patients to maximize expiratory flow rates. Thus, patients with severe COPD could abruptly increase V'E commensurate with increased metabolic demand, to approximately 40 L/min and generate tidal inspiratory pressures exceeding 40% of the maximal possible pressure generation while experiencing minimal increases in dyspnea (modified Borg ratings Citation[1], Citation[2]).
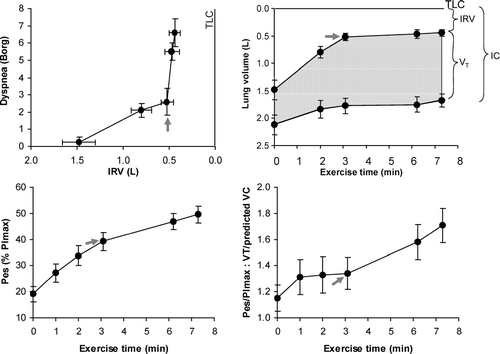
Effort-displacement ratios are therefore well maintained early in exercise even in advanced COPD. However, this advantage of DH was quickly negated when VT expanded to reach a critically low IRV of approximately 0.5 L (or 10% predicted TLC) below TLC (). At this “threshold,” VT becomes fixed on the upper less compliant extreme of the respiratory system's sigmoid-shaped pressure-volume relation, where there is increased elastic loading of the inspiratory muscles. At this operating volume, the diaphragm muscle fibers are maximally shortened and the increased breathing frequency leads to increased velocity of shortening and significant reductions in dynamic lung compliance. After reaching this minimal IRV, dyspnea (described as unsatisfied inspiration) soon rose to intolerable levels and reflected the widening disparity between inspiratory effort (reaching near maximal central neural drive) and the simultaneous VT response, which becomes essentially fixed, i.e., increased effort-displacement ratio (Citation[30]). Consistent with a previous study (Citation[2]), dyspnea intensity again correlated well with the increase in this effort-displacement ratio during exercise in COPD (Citation[30]).
Figure 7 The mechanical threshold of dyspnea is indicated by the abrupt rise in dyspnea after a critical “minimal” inspiratory reserve volume (IRV) is reached, which prevents further expansion of tidal volume (VT) during exercise. Beyond this dyspnea/IRV inflection point during exercise, dyspnea intensity, respiratory effort (Pes/PImax), and the ratio of Pes/PImax to tidal volume displacement (VT standardized as a % of predicted vital capacity (VC)) all continued to rise. Arrows indicate the dyspnea/IRV inflection point. Values are expressed as means ± SEM. IC = inspiratory capacity. Modified from O'Donnell DE, Hamilton AL, Webb KA. J Appl Physiol 2006; 101:1025–1035, with permission.
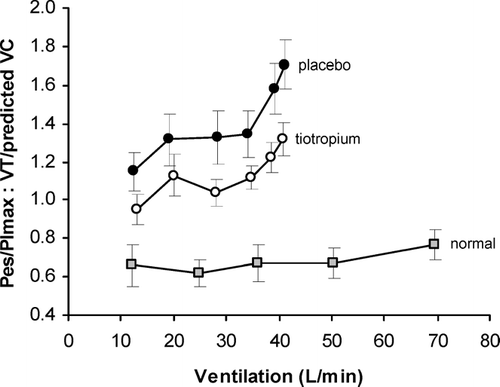
The contention that DH contributes importantly to exercise limitation in COPD has also been bolstered by numerous studies that have shown that pharmacological and surgical lung volume reduction are associated with consistent improvements in dyspnea and exercise endurance (Citation[4], Citation[8], Citation[14], Citation[30], Citation[57], Citation[58], Citation[59], Citation[60], Citation[61], Citation[62], Citation[63]). Furthermore, reduced dyspnea ratings following bronchodilator therapy (i.e., tiotropium) were associated with consistent improvements in the effort-displacement ratio throughout exercise (Citation[30]) ().
Figure 8 The ratio between respiratory effort (Pes/PImax) and tidal volume displacement (VT standardized as a fraction of predicted vital capacity (VC)), an index of neuromechanical dissociation, is shown during exercise after tiotropium and placebo in COPD (n = 11) compared to a previously studied group of age-matched normal subjects (n = 12). The effort-displacement ratio is increased in COPD compared with normal throughout exercise, with an upward trend after a ventilation of approximately 30 L/min that did not occur in the normal subjects. Compared with placebo, tiotropium reduced this ratio throughout exercise in COPD. From O'Donnell DE, Hamilton AL, Webb KA. J Appl Physiol 2006; 101: 1025-1035, with permission.
Neurophysiology of exertional dyspnea
In health, during resting spontaneous breathing and during exercise, the mechanical output of the respiratory system, measured as V'E, changes in accordance with the level of central neural drive. Complex proprioceptive information (obtained from muscle spindles, Golgi tendon organs, and joint receptors), as well as sensory information pertaining to respired airflows and volume displacement (from mechanosensors located in the lung parenchyma and airways), provide simultaneous feedback to the central nervous system that ventilatory output is appropriate for the prevailing drive (Citation[46], Citation[47], Citation[64], Citation[65], Citation[66], Citation[67], Citation[68]). Physiological adaptations during exercise, which include precise control of operating lung volumes and airway (intra- and extra-thoracic) resistance together with breathing pattern adjustments, ensure harmonious neuromechanical coupling of the respiratory system and avoidance of respiratory discomfort (Citation[69], Citation[70], Citation[71]). Effort-displacement ratios therefore remain remarkably constant throughout exercise in health. Although the perceived effort of breathing may increase as V'E increases during exercise, medullary output remains appropriately rewarded, and participants generally do not describe inspiratory difficulty or unsatisfied respiratory effort, even at peak exercise (Citation[2]).
The situation is markedly different in COPD, where DH during exercise constrains VT expansion and results in maximal shortening of the inspiratory muscles. Once VT expands to reach a critical IRV ceiling, further increases in neural output to the respiratory system are unrewarded in terms of increased mechanical output. We have argued that this mechanical (volume) restriction is a primary mechanism by which DH induces exertional dyspnea and its dominant qualitative dimension of unsatisfied inspiration (Citation[2], Citation[30], Citation[72]). It is possible, therefore, that sensory feedback from a multitude of mechanoreceptors throughout the respiratory system (in the muscles, chest wall, airways and lung parenchyma) collectively convey the information to consciousness that the mechanical output achieved is inadequate for the prevailing respiratory drive. In the final phase of exercise, central drive had likely reached near maximal levels yet the VT response was essentially fixed at only 30% of the predicted vital capacity (Citation[30]). Respiratory mechanoreceptors are ideally placed to detect any disparity between the volume displacement achieved and that which is expected (Citation[73]).
Dyspnea and volume restriction in COPD
Several previous studies in resting healthy humans have shown that when chemical drive is increased in the face of voluntary suppression or imposed restriction of the spontaneous breathing response (i.e., VT expansion), dyspnea quickly escalates to intolerable levels (Citation[48], Citation[49], Citation[50], Citation[72], Citation[74], Citation[75]). Moreover, resumption of spontaneous breathing was associated with immediate improvement in respiratory discomfort, despite persistent (or even increased) chemical loading. During exercise in health, mechanical restriction of VT (by chest strapping) induced severe dyspnea (described as unsatisfied inspiration) in the setting of added chemical loading (Citation[72]). We postulate that in COPD, a similar mismatch between central drive and a restricted mechanical response (as a result of DH) is fundamental to the origin of dyspnea. This hypothesis is supported by a number of controlled therapeutic studies that have shown a correlation between reduced dyspnea intensity ratings and the extent of release of tidal volume restriction following pharmacological lung volume reduction (Citation[4], Citation[8], Citation[14], Citation[30], Citation[57], Citation[58], Citation[59], Citation[60]).
It is certainly possible that acute DH could result in fatigued or excessively shortened inspiratory muscle fibers that fail to respond appropriately to increased electrical activation. Accordingly, as originally proposed by Campbell (Citation[76]), spindles in the ventilatory muscles (which accurately sense the disparity between length and tension development) are ideally suited to serve as the proximate peripheral source of this sensory information (Citation[42], Citation[46], Citation[47], Citation[70], Citation[71], Citation[73], Citation[74]). However, based on the existing literature, it is not clear whether overt inspiratory muscle fatigue consistently occurs in the setting of symptom-limited exercise, even in severe COPD (Citation[77], Citation[78], Citation[79], Citation[80]). Moreover, it is highly unlikely, given the considerable redundancy in the neurosensory system, that muscle spindles are the only mechanosensors that provide sensory feedback with respect to the appropriateness of the ventilatory output for the prevailing drive.
Dyspnea: The emotional dimension
We have seen that DH-associated dyspnea is likely to arise in flow-limited patients under conditions of abruptly increased neural drive and ventilatory demand. Common provocative situations include: physical activity, episodes of transient hypoxemia and anxiety. Recent studies have confirmed that daily fluctuations in DH can reflect circadian variability in airway smooth muscle tone that is not fully reversed by long-acting bronchodilators. More sustained DH occurs in the setting of exacerbations and this has recently been linked to dyspnea, the dominant presenting symptom (Citation[18],Citation[19]). It is reasonable to assume that when perceived respiratory discomfort exceeds a certain threshold (which varies between individuals), it will elicit behavioral or affective responses. This affective dimension, in many instances encompasses feelings of fear that can quickly escalate to panic and helplessness, which are key components of perceived respiratory distress. Sudden fear or overt panic will elicit neuro-humoral responses (via pathways in the amygdala, adrenals and sympathetic nervous system), which will trigger patterned ventilatory and circulatory responses that can further amplify respiratory discomfort.
Recently, the use of functional imaging techniques such as positron-emission tomography (PET) scanning and functional magnetic resonance imaging (fMRI) have been utilized to investigate the mechanisms underlying the central processing and perception of dyspnea (Citation[81], Citation[82], Citation[83], Citation[84], Citation[85], Citation[86]). These studies have shown activation of central limbic structures including the anterior insula, pars opercularis, anterior cingulate gyrus, amygdala, putamen, and caudate. These phylogenetically ancient areas of the central nervous system have an integral role in the perception and genesis of primal emotions, and it has been suggested that air hunger and dyspnea evoke programmed neurohumoral and behavioral responses similar to those that occur in response to pain (Citation[87], Citation[88], Citation[89]), extreme hunger (Citation[90]) or thirst (Citation[91]). Other data suggest that the anterior insula is also activated in the setting of panic attacks (Citation[92]), which may provide a common pathway for the disabling sensations of panic, anxiety and fear that often accompany severe dyspnea (Citation[93]).
SUMMARY
Severe dyspnea is a major exercise-limiting symptom in moderate-to-severe COPD and every effort should be made to alleviate this distressing symptom. Although exercise limitation is multifactorial, there is considerable evidence that deranged ventilatory mechanics, specifically dynamic lung hyperinflation, may represent the proximate mechanical limit to exercise performance in patients with more advanced disease. Dynamic lung hyperinflation occurs during activity in the vast majority of flow-limited patients with COPD and has been shown repeatedly to correlate with dyspnea intensity ratings. Dynamic lung hyperinflation stresses the already limited cardiopulmonary reserves of patients with COPD and greatly constrains their ability to expand tidal volume appropriately in response to the increased neural drive of exercise. Recent studies have proposed that this acute neuromechanical dissociation of the respiratory system may form the basis for the perception of respiratory discomfort, which ultimately triggers intolerable respiratory distress. Dynamic lung hyperinflation therefore represents an important therapeutic target in COPD and several studies have now shown that pharmacological lung deflation is associated with clinically important improvements of dyspnea and exercise endurance, even in advanced disease.
REFERENCES
- O'Donnell D E, Webb K A. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis 1993; 148: 1351–1357
- O'Donnell D E, Bertley J C, Chau L K, Webb K A. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med 1997; 155: 109–115
- O'Donnell D E, Revill S M, Webb K A. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 770–777
- O'Donnell D E, Voduc N, Fitzpatrick M, Webb K A. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J 2004; 24: 86–94
- Maltais F, Hamilton A, Marciniuk D, Hernandez P, Sciurba F C, Richter K, Kesten S, O'Donnell D. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest 2005; 128: 1168–1178
- Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, Carone M, Celli B, Engelen M, Fahy B, Garvey C, Goldstein R, Gosselink R, Lareau S, MacIntyre N, Maltais F, Morgan M, O'Donnell D, Prefault C, Reardon J, Rochester C, Schols A, Singh S, Trooster T. ATS/ERS Pulmonary Rehabilitation Writing Committee. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006; 173: 1390–1413
- O'Donnell D E, Webb K A. Exercise reconditioning in patients with chronic airflow limitation. Current Therapy in Sports Medicine, 3rd ed, J S Torg, R J Shepherd. Mosby Year Book Inc., St. Louis, Missouri 1995; 678–684
- O'Donnell D E, Flüge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23: 832–840
- Pride N B, Macklem P T. Lung mechanics in disease. Handbook of Physiology, Section 3, Vol III, Part 2: The Respiratory System, A P Fishman. American Physiological Society, Bethesda, MD 1986; 659–692
- Henke K G, Sharratt M, Pegelow D, Dempsey J A. Regulation of end-expiratory lung volume during exercise. J Appl Physiol 1988; 64: 135–146
- Druz W S, Sharp J T. Activity of respiratory muscles in upright and recumbent humans. J Appl Physiol 1981; 51: 1522–1561
- O'Donnell D E, D'Arsigny C, Webb K A. Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163: 892–898
- O'Donnell D E. Ventilatory limitations in chronic obstructive pulmonary disease. Med Sci Sports Exerc 2001; 33: S647–655
- O'Donnell D E, Lam M, Webb K A. Measurement of symptoms, lung hyperinflation and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158: 1557–1565
- Koulouris N G, Dimopoulou I, Valta P, Finkelstein R, Cosio M G, Milic-Emili J. Detection of expiratory flow limitation during exercise in COPD patients. J Appl Physiol 1997; 82: 723–731
- O'Donnell D E, D'Arsigny C, Fitzpatrick M, Webb K A. Exercise hypercapnia in advanced chronic obstructive pulmonary disease: the role of lung hyperinflation. Am J Respir Crit Care Med 2002; 166: 663–668
- Dempsey J A. Exercise carbon dioxide retention in chronic obstructive pulmonary disease: a case for ventilation/perfusion mismatch combined with hyperinflation. Am J Respir Crit Care Med 2002; 166: 634–635
- Stevenson N J, Walker P P, Costello R W, Calverley P M. Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 1510–1516
- O'Donnell D E, Parker C M. COPD exacerbations · 3: Pathophysiology. Thorax 2006; 61: 354–361
- Gelb A F, Guitierrez C A, Weisman I M, Newsom R, Taylor C F, Zamel N. Simplified detection of dynamic hyperinflation. Chest 2004; 126: 1855–1860
- Grimby G, Bunn J, Mead J. Relative contribution of rib cage and abdomen to ventilation during exercise. J Appl Physiol 1968; 24: 159–166
- Potter W A, Olafsson S, Hyatt R E. Ventilatory mechanics and expiratory flow limitation during exercise in patients with obstructive lung disease. J Clin Invest 1971; 50: 910–919
- Stubbing D G, Pengelly L D, Morse J LC, Jones N L. Pulmonary mechanics during exercise in subjects with chronic airflow obstruction. J Appl Physiol 1980; 49: 511–515
- Dodd D S, Brancatisano T, Engel L A. Chest wall mechanics during exercise in patients with severe chronic airway obstruction. Am Rev Respir Dis 1984; 129: 33–38
- O'Donnell D, He Z, Lam M, Webb K, Flüge T, Hamilton A. Reproducibility of measurements of inspiratory capacity, dyspnea intensity and exercise endurance in multicentre trials in COPD (abs). Eur Respir J 2004; 24, Suppl. 48, 323s, Poster 2053
- Vogiatzis I, Georgiadou O, Golemati S, Aliverti A, Kosmas E, Kastanakis E, Geladas N, Koutsoukou A, Nanas S, Zakynthinos S, Roussos C. Patterns of dynamic hyperinflation during exercise and recovery in patients with severe chronic obstructive pulmonary disease. Thorax 2005; 60: 723–729
- Yan S, Kaminski D, Sliwinski P. Reliability of inspiratory capacity for estimating end-expiratory lung volume changes during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997; 156: 55–59
- Diaz O, Villafranca C, Ghezzo H, Borzone G, Leiva A, Milic-Emil J, Lisboa C. Role of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitations at rest. Eur Respir J 2000; 16: 269–275
- Puente-Maestu L, Garcia de Pedro J, Martinez-Abad Y, Ruiz de Ona J M, Llorente D, Cubillo J M. Dyspnea, ventilatory pattern, and changes in dynamic hyperinflation related to the intensity of constant work rate exercise in COPD. Chest 2005; 128: 651–656
- O'Donnell D E, Hamilton A L, Webb K A. Sensory-mechanical relationships during high-intensity, constant-work-rate exercise in COPD. J Appl Physiol 2006; 101: 1025–1035
- Sinderby C, Spahija J, Beck J, Kaminski D, Yan S, Comtois N, Sliwinski P. Diaphragm activation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163: 1637–1641
- Montes de Oca M, Rassulo J, Celli B R. Respiratory muscle and cardiopulmonary function during exercise in very severe COPD. Am J Respir Crit Care Med 1996; 154: 1284–1289
- Light R W, Mintz W M, Linden G S, Brown S E. Hemodynamics of patients with severe chronic obstructive pulmonary disease during progressive upright exercise. Am Rev Respir Dis 1984; 130: 391–395
- Vizza C D, Lynch J P, Ochoa L L, Richardson G, Trulock E P. Right and left ventricular dysfunction in patients with severe pulmonary disease. Chest 1998; 113: 576–583
- Simon M, Leblanc P, Jobin J, Desmeules M, Sullivan M J, Maltais F. Limitation of lower limb and VO2 during cycling exercise in COPD patients. J Appl Physiol 2001; 90: 1013–1019
- Harms G A, Babcock M A, McClaren S R, Pegelow D F, Nickele G A, Nelson W B, Dempsey J A. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 1997; 82: 1573–1583
- Richardson R S, Sheldon J, Poole D C, Hopkins S R, Ries A L, Wagner P D. Evidence of skeletal muscle metabolic reserve during whole body exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159: 881–885
- Killian K J, Gandevia S C, Summers E, Campbell E JM. Effect of increased lung volume on perception of breathlessness, effort and tension. J Appl Physiol 1984; 57: 686–691
- Campbell E J, Gandevia S C, Killian K J, Mahutte C K, Rigg J R. Changes in the perception of inspiratory resistive loads during partial curarization. J Physiol 1980; 309: 93–100
- Supinski G S, Clary S J, Bark H, Kelsen S G. Effect of inspiratory muscle fatigue on perception of effort during loaded breathing. J Appl Physiol 1987; 62: 300–307
- el-Manshawi A, Killian K J, Summers E, Jones N L. Breathlessness during exercise with and without resistive loading. J Appl Physiol 1986; 61: 896–905
- Gandevia S C. The perception of motor commands or effort during muscular paralysis. Brain 1982; 105: 151–159
- Chen Z, Eldridge F L, Wagner P G. Respiratory-associated rhythmic firing of midbrain neurones in cats: relation to level of respiratory drive. J Physiol 1991; 437: 305–325
- Chen Z, Eldridge F L, Wagner P G. Respiratory-associated thalamic activity is related to level of respiratory drive. Respir Physiol 1992; 90: 99–113
- Davenport P W, Friedman W A, Thompson F J, Franzen O. Respiratory-related cortical potentials evoked by inspiratory occlusion in humans. J Appl Physiol 1986; 60: 1843–1848
- Gandevia S C, Macefield G. Projection of low-threshold afferents from human intercostal muscles to the cerebral cortex. Respir Physiol 1989; 77: 203–214
- Homma I, Kanamara A, Sibuya M. Proprioceptive chest wall afferents and the effect on respiratory sensation. Respiratory Psychophysiology, C von Euler, M Katz-Salamon. Stockton Press, New York 1988; 161–166
- Chonan T, Mulholland M B, Cherniack N S, Altose M D. Effects of voluntary constraining of thoracic displacement during hypercapnia. J Appl Physiol 1987; 63: 1822–1828
- Schwartzstein R M, Simon P M, Weiss J W, Fencl V, Weinberger S E. Breathlessness induced by dissociation between ventilation and chemical drive. Am Rev Respir Dis 1989; 139: 1231–1237
- Harty H R, Corfield D R, Schwartzstein R M, Adams L. External thoracic restriction, respiratory sensation, and ventilation during exercise in men. J Appl Physiol 1999; 86: 1142–1150
- Schwartzstein R M, Manning H L, Weiss J W, Weinberger S E. Dyspnea: a sensory experience. Lung 1990; 168: 185–199
- Manning H L, Molinary E J, Leiter J C. Effect of inspiratory flow rate on respiratory sensation and pattern of breathing. Am J Respir Crit Care Med 1995; 151: 751–757
- Sibuya M, Yamada M, Kanamaru A, Tanaka K, Suzuki H, Noguchi E, Altose M D, Homma I. Effect of chest wall vibration on dyspnea in patients with chronic respiratory disease. Am J Respir Crit Care Med 1994; 149: 1235–1240
- Hong H, Webb K A, O'Donnell D E. Effects of chest wall restriction and dead space loading on exercise tolerance and dyspnea in healthy normals. Am J Respir Crit Care Med 1999; 159: A787
- Wijkstra P J. Non-invasive positive pressure ventilation (NIPPV) in stable patients with chronic obstructive pulmonary disease (COPD). Respir Med 2003; 97: 1086–1093
- Kyroussis D, Polkey M I, Hamnegard C H, Mills G H, Green M, Moxham J. Respiratory muscle activity in patients with COPD walking to exhaustion with and without pressure support. Eur Respir J 2000; 15: 649–655
- O'Donnell D E, Lam M, Webb K A. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160: 542–549
- Belman M J, Botnick W C, Shin J W. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 153: 967–975
- O'Donnell D E, Sciurba F, Celli B, Mahler D A, Webb K A, Kalberg C J, Knobil K. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest 2006; 130: 647–656
- Peters M M, Webb K A, O'Donnell D E. Combined physiological effects of bronchodilators and hyperoxia on exertional dyspnoea in normoxic COPD. Thorax 2006; 61: 559–567
- O'Donnell D E, Webb K A, Bertley J C, Chau L K, Conlan A A. Mechanisms of relief of exertional breathlessness following unilateral bullectomy and lung volume reduction surgery in emphysema. Chest 1996; 110: 18–27
- Martinez F J, de Oca M M, Whyte R I, Stetz J, Gay S E, Celli B R. Lung-volume reduction improves dyspnea, dynamic hyperinflation, and respiratory muscle function. Am J Respir Crit Care Med 1997; 155: 1984–1990
- Laghi F, Jurban A, Topeli A, Fahey P J, Garrity E, Jr., Archids J M, De Pinto D J, Edwards L C, Tobin M J. Effect of lung volume reduction surgery on neuromechanical coupling of the diaphragm. Am J Respir Crit Care Med 1998; 157: 475–483
- Banzett R B, Lansing R W, Reid M B, Adams L, Brown R. ‘Air hunger’ arising from increased PCO2 in mechanically ventilated quadriplegics. Respir Physiol 1989; 76: 53–67
- Altose M D, Syed I, Shoos L. Effects of chest wall vibration on the intensity of dyspnea during constrained breathing (abs). Proc Int Union Physiol Sci 1989; 17: 288
- Matthews P B. Where does Sherrington's ‘muscular sense’ originate? Muscles, joints, corollary discharges?. Annu Rev Neurosci 1982; 5: 189–218
- Roland P E, Ladegaard-Pedersen H. A quantitative analysis of sensations of tension and of kinaesthesia in man. Evidence for a peripherally originating muscular sense and for a sense of effort. Brain 1977; 100: 671–692
- Noble M IM, Eisele J H, Trenchard D, Guz A. Effect of selective peripheral nerve blocks on respiratory sensations. Breathing: Hering-Breyer Symposium, R Porter. Churchill, London 1970; 233–246
- O'Donnell D E, Webb K A. Mechanisms of dyspnea in COPD. Dyspnea: Mechanisms, Measurement, and Management, 2nd edition, D A Mahler, D E O'Donnell. Taylor & Francis Group, New York 2005; 29–58, Lung Biology in Health and Disease Series, Volume 208, Chapter 3
- Killian K J, Campbell E JM. Dyspnea. Lung Biology in Health and Disease, vol 29, part B: The Thorax, C Roussos, P T Macklem. Marcel Dekker, New York 1985; 787–828
- Altose M, Cherniack N, Fishman A P. Respiratory sensations and dyspnea: perspectives. J Appl Physiol 1985; 58: 1051–1054
- O'Donnell D E, Hong H H, Webb K A. Respiratory sensation during chest wall restriction and dead space loading in exercising men. J Appl Physiol 2000; 88: 1859–1869
- Killian K J, Jones N L. Respiratory muscles and dyspnea. Clin Chest Med 1988; 9: 237–248
- Fowler W S. Breaking point of breath-holding. J Appl Physiol 1954; 6: 539–545
- Xu F, Taylor R F, McLarney T, Lee L Y, Frazier D T. Respiratory load compensation. I Role of the cerebrum. J Appl Physiol 1993; 74: 853–858
- Campbell E JM, Howell J BL. The sensation of breathlessness. Br Med Bull 1963; 19: 36–40
- Grassino A, Gross D, Macklem P T, Roussos C, Zagelbaum G. Inspiratory muscle fatigue as a factor limiting exercise. Bull Eur Pathophysiol Respir 1979; 15: 105–111
- Bye P T, Esau S A, Levy R D, Shiner R J, Macklem P T, Martin J G, Pardy R L. Ventilatory muscle function during exercise in air and oxygen in patients with chronic air-flow limitation. Am Rev Respir Dis 1985; 32: 236–240
- Kyroussis D, Polkey M I, Keilty S EJ, Mills G H, Hamnegard C H, Moxham J, Green M. Exhaustive exercise slows inspiratory muscle relaxation rate in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 153: 787–793
- Polkey M I, Kyroussis D, Mills G H, Hamnegard C H, Keilty S EJ, Green M, Moxham J. Inspiratory pressure support reduces slowing of inspiratory muscle relaxation rate during exhaustive treadmill walking in severe COPD. Am J Respir Crit Care Med 1996; 154: 1146–1150
- Banzett R B, Dempsey J A, O'Donnell D E, Wamboldt M Z. Symptom perception and respiratory sensation in asthma. Am J Respir Crit Care Med 2000; 162: 1178–1182
- Kukorelli T, Namenyi J, Adam G. Visceral afferent projection areas in the cortex. II. Representation of the carotid sinus receptor area. Acta Physiol Acad Sci Hung 1969; 36: 261–263
- Evans K C, Banzett R B, Adams L, McKay L, Frackowiak R S, Corfield D R. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol 2002; 88: 1500–1511
- Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, Robillard R, Lancaster J, Zamarripa F E, Fox P T, Denton D. Brain responses associated with consciousness of breathlessness (air hunger). Proc Natl Acad Sci USA 2001; 98: 2035–2040
- Peiffer C, Poline J B, Thivard L, Aubier M, Samson Y. Neural substrates for the perception of acutely induced dyspnea. Am J Respir Crit Care Med 2001; 163: 951–957
- Banzett R B, Mulnier H E, Murphy K, Rosen S D, Wise R J, Adams L. Breathlessness in humans activates insular cortex. Neuroreport 2000; 11: 2117–2120
- Casey K L. Forebrain mechanisms of nociception and pain: analysis through imaging. Proc Natl Acad Sci USA 1999; 96: 7668–7674
- Coghill R C, Sang C N, Maisog J M, Iadarola M J. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol 1999; 82: 1934–1943
- Hsieh J C, Hannerz J, Ingvar M. Right-lateralised central processing for pain of nitroglycerin-induced cluster headache. Pain 1996; 67: 59–68
- Tataranni P A, Gautier J F, Chen K, Uecker A, Bandy D, Salbe A D, Pratley R E, Lawson M, Reiman E M, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 1999; 96: 4569–4574
- Denton D, Shade R, Zamarippa F, Egan G, Blair-West J, McKinley M, Lancaster J, Fox P. Neuroimaging of genesis and satiation of thirst and an interoceptor-driven theory of origins of primary consciousness. Proc Natl Acad Sci USA 1999; 96: 5304–5309
- Javanmard M, Shlik J, Kennedy S H, Vaccarino F J, Houle S, Bradwejn J. Neuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time points. Biol Psychiatry 1999; 45: 872–882
- Banzett R B, Lansing R W, Evans K C, Shea S A. Stimulus-response characteristics of CO2-induced air hunger in normal subjects. Respir Physiol 1996; 103: 19–31