Abstract
COPD exacerbations often lead to a downward spiral of physical activity. To compensate for the discomfort brought on by exertional dyspnea and the accompanying fatigue, patients with COPD will settle into a sedentary lifestyle that deconditions their bodies, serves to further aggravate breathlessness, and results in a further downward adjustment of physical activity. Progression of COPD imposes profound limitation on activities of daily living and gives rise to anxiety and depression. The distressing symptoms of breathlessness and the perception of these abnormalities by the patient lead to a reduction in health-related quality of life. The clinician's therapeutic interventions have to address these symptom and activity limitations with the goal of improving the patient's quality of life.
Keywords::
INTRODUCTION
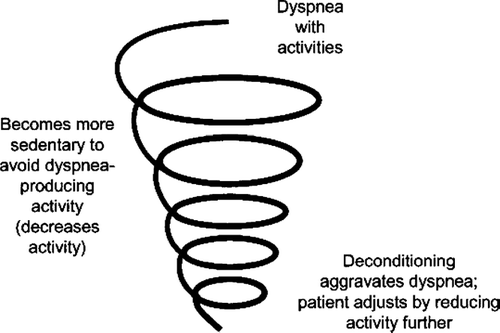
Exertional dyspnea usually becomes an important and distressing symptom as chronic obstructive pulmonary disease (COPD) progresses in severity (Citation[1]). Since exertional dyspnea cannot occur without exertion, COPD patients often subconsciously ratchet down their physical activity to reduce the intensity of this distressing symptom. Fatigue, another common and important symptom in COPD (Citation[2]) leads to a further reduction in physical activity. The reduction in daily activity levels from adopting a more sedentary lifestyle, in turn, leads to further deconditioning. This, in turn, aggravates dyspnea. This interplay has been depicted as a dyspnea-inactivity (downward) spiral or a vicious circle (Citation[3]) (). The remarkable extent to which patients with COPD limit their domiciliary activity has recently become evident with studies using tri-axial motion sensors. During the course of the day, patients with COPD spend considerably less time standing and walking than age-matched healthy individuals (Citation[4]).
Figure 1 The dyspnea inactivity downward spiral. Reprinted from Reardon JZ, Lareau S, ZuWallack R. Am J Med 2006; B119 (Suppl 1):32–37, with permission from Elsevier.
Everyday activities of daily living (ADL) can range from basic ADL—those required for daily life (such as eating, dressing, personal hygiene, and physical mobility) to instrumental ADL—those necessary to adapt independently within the environment (such as shopping, household chores, cooking, driving a car) (Citation[5], Citation[6]). Interference with physical activity in COPD usually results from associated breathlessness or fatigue; this can range from no limitation (but with distressing, accompanying symptoms), to a reduction in the activity, to actual elimination of the activity altogether. Basic activities, because of their necessity and their usual lower energy costs, are not usually eliminated by COPD patients.
However, as the disease progresses in severity or during the period following an exacerbation, these activities may be more profoundly affected. Those activities that require more energy expenditure and are more optional, such as many leisure activities, are more likely to be eliminated altogether since their accompanying symptoms might outweigh their benefits. Certain co-morbidities, such as reductions in fat-free mass related to nutritional depletion, increased levels of anxiety and depression, and side effects from medical therapy may enhance this decline in functional performance. Additionally, poor motivation and fear of dyspnea-producing activities aggravates the problem. Activity limitation is not static, but varies with the course of disease: it is especially impacted during and following COPD exacerbations (Citation[7]).
The distressing symptom of shortness of breath, decreased activity, and the perception of these abnormalities by the individual result in a reduction in health-related quality of life (HRQL). HRQL has been defined as “the gap between our expectations of health and our experience of it.” (Citation[8]). The perception of quality of life is unique to the individual, reflecting in part his/her ability to perform and enjoy activities of daily living (Citation[9], Citation[10]). Of clinical importance, physiologic markers such as the FEV1 cannot be used as surrogates for HRQL (Citation[11], Citation[12]). Additionally, HRQL is dynamic within the same individual, influenced by both changes in disease intensity and in the individual's expectations of what the desired level of health should be. As might be anticipated, exacerbations profoundly influence quality of life in COPD patients (Citation[13], Citation[14]).
The dynamic interactions between distressing symptoms and reduction in activity can interfere with the ability of the patient, family, or health-care provider to accurately assess the impact of COPD. Difficulties with assessment are made even worse because of the insidious onset and progression of the disease and the patient's (often unconscious) unwillingness to attribute these symptoms to COPD. Thus, the gradual increase in effort-intolerance is attributed to the effects of aging, or the abrupt increase in breathlessness and cough from an exacerbation is from a “chest cold.”
The following case presentation of an individual with COPD, with assessments from different perspectives will hopefully illustrate this problem. The perspectives include: (Citation[1]) an experienced pulmonary clinician (not the author) after referral by a primary care physician, (Citation[2]) an in-depth interview by a registered nurse as part of the initial evaluation for pulmonary rehabilitation, and (Citation[3]) the patient herself, through completing pulmonary rehabilitation outcome assessments.
Case presentation
CC, a 74-year-old woman with COPD, was referred by her primary care physician to a pulmonary subspecialist in January 2006 for assessment and management of her disease. She has a 100 pack-year history of cigarette smoking, but quit in 2005 after she was hospitalized for an exacerbation of her disease. Other medical problems include peripheral vascular disease with claudication, and hypertension. She has a history of a right carotid endarterectomy. There is no history of cardiac disease. Her medications consist of cilostazol, metoprolol, inhaled fluticasone 250 mcg /salmeterol 50 mcg 1 inhalation twice-daily, albuterol/ipratropium via a metered dose inhaler 4 times daily, and bupropion for smoking cessation maintenance. She uses oxygen 2 liters per minute during sleeping hours. She had received influenza and pneumococcal vaccinations.
Her physical examination revealed a blood pressure of 180/80, pulse 90/minute, respirations 16/minute, oxygen saturation while breathing room air 95%. Her body-mass index (BMI) was 24 kg/m2. The A-P diameter of her chest was increased, she had a generalized decrease in breath sounds, and the expiratory phase of breathing was prolonged. There were no adventitious sounds. She had bilateral carotid bruits. The remainder of her physical examination was unremarkable. Her chest X-ray showed signs of hyperinflation.
Spirometry in January 2006 was consistent with severe to very severe COPD: (post-bronchodilator) FEV1/FVC = 0.39, and FEV1 = 0.76 L (32% of predicted). There was no demonstrable reversibility. Her oxygen saturation reached a nadir of 94% when walking in the hallway. The pulmonologist discontinued the albuterol/ipratropium inhaler, prescribed tiotropium, one inhalation daily in the morning, and an albuterol MDI for as-needed use, and referred her to outpatient pulmonary rehabilitation.
Pulmonologist's initial assessment of symptoms and activity
The patient was noted to have decreased exercise tolerance, which had gradually declined over several years. This had reached the point where she became breathless when walking faster than usual. She was able, however, to walk around the house at her own pace without undue difficulty. She had occasional wheezing episodes, and an occasional morning cough productive of small amounts of sputum. She did not have bothersome coughing paroxysms. She had lost an undefined amount of weight after an exacerbation several months earlier and had gained some of it back. There was no mention of activity limitation in the assessment.
The pulmonary physician made a diagnosis of severe COPD based on her symptoms and FEV1 (GOLD Stage III, almost Stage IV). At follow-up a few weeks later and after the above pharmacologic intervention, there was noted a “significant improvement in her pulmonary status, particularly improved exercise tolerance,” but again no mention of activity limitation.
Assessment of dyspnea and activity before pulmonary rehabilitation
This assessment was performed by a registered nurse pulmonary rehabilitation coordinator during the initial assessment for this intervention. The patient reported distressing levels of dyspnea with some activities in and out of the home. Specifically, she complained of breathlessness when trying to hurry getting dressed. She had to slow down when walking and tended to scuff her feet when walking any distance. She did not have to give up any particular activity. She did her own shopping, but used the grocery cart for support and as an aid in reducing dyspnea. She has a hairdressers' license but has been retired for a few years.
Outcome assessment during pulmonary rehabilitation
Her rating of dyspnea using the 5-point Medical Research Council (MRC) dyspnea scale () is 4: “Have to stop for breath every few minutes when walking even on level ground. The 6-minute walk distance is 222 m, which is about 40% of predicted (Citation[15]). HRQL is measured with the Chronic Respiratory Disease Questionnaire (CRQ); this has dimensions of dyspnea, fatigue, emotion, and mastery. For the dyspnea dimension, the patient must choose 5 most important activities performed in the past 2 weeks that have caused dyspnea. The level of dyspnea is then rated by the patient, using a seven-point scale where higher scores mean less dyspnea. The 5 most important dyspnea-producing activities chosen by the patient were: showering, dressing, hurrying, walking uphill, and meal preparation.
Table 1 The British Medical Research Council (MRC) dyspnea scale
The mean, per-question score for the CRQ dyspnea domain is 3.8: between “quite a bit short of breath” (score of 3) and “moderate shortness of breath” (score of 4). Functional performance assessment is performed using the Pulmonary Functional Status Scale. Selected items and scores are given in . This indicates that she had given up several activities that require prolonged energy expenditure.
Table 2 Ms. CC's self-reported activity
Assessing the clinical morbidity from the pulmonologist's perspective
The pulmonologist usually must objectively identify the underlying disease or diseases, make some determination of their impact on the patient, and then decide on a therapeutic plan. To date, the only evidence-based intervention that modifies the course of COPD is smoking cessation, and the only life-prolonging interventions are smoking cessation, supplemental oxygen for hypoxemic patients, and lung volume reduction surgery for selected patients with emphysema. Consequently, most of the specific therapy for this disease is aimed at reducing symptoms, increasing function, and improving HRQL. Therefore, in order to rationally prescribe treatments the pulmonologist must in some way estimate symptoms, functional status, and HRQL in decision making.
The pulmonologist usually makes the above assessments using information gleaned from the history, in conjunction with objective findings from the physical examination and laboratory tests. This assessment which by necessity is done within a time frame that is all too often too short—is fraught with a couple of potential problems: (Citation[1]) usually more time is devoted to assessing symptoms than in determining activity limitation, and (Citation[2]) certain laboratory tests, such as spirometry, are often given excessive weight in clinical assessment, making them de facto equivalents of disease severity.
Too often, we ask the question, “How are you doing?” rather than, “What are you doing?” Thus, we ask the patient if he/she is short of breath or has wheezing, but do not ask if there has been any limitation in activities resulting from the disease, its co-morbidity, or its treatments. For example, in the case described above, the pulmonologist accurately determined that the patient had exercise intolerance, but neglected to ask if the patient about the impact of their disease, i.e., whether she had decreased activity levels.
Determination of changes in activity levels further defines the impact of the disease on the patient, complementing symptom assessment. Activity level, as determined by a functional status questionnaire, is a significant, independent marker for mortality in COPD (Citation[16]). Unfortunately, we do not have a validated, ultra-quick method of measuring activity. However, in essence, the 5-point MRC, which is usually considered a dyspnea instrument, does estimate some forms of activity (walking, dressing) as limited by dyspnea. This, at a minimum, should be considered in the initial and subsequent clinical evaluations until a better method appears. The MRC score has demonstrated importance is an independent predictor of survival in COPD as a component of the multi-dimensional BODE (Body mass index, Obstruction (FEV1), Dyspnea (MRC), Exercise (6-minute walk distance) score (Citation[17]).
The nurse's assessment prior to pulmonary rehabilitation did include a more direct activity analysis and determined that the patient did have to dress slower to avoid dyspnea, had to slow down, and had some trouble with a grocery cart. Actually, the fact that the patient had to dress slower because of dyspnea might categorize this patient as MRC 5. But the patient did not admit to having eliminated any activities, and it was only from the structured questionnaire format did it become apparent this had also happened. It is not practical to administer questionnaires to all patients with COPD, but this does suggest that our clinical evaluations in the hurried office setting may at times underestimate functional limitations. While we do not know why the patient stopped going to the movies and doing other activities, it is probable that dyspnea, fatigue, or fear of symptom-producing activity led to this situation. Of course, other possibilities such as cardiac disease or anemia must also be considered as potential etiologies. It is not clear whether the numerical results from a health status or functional status questionnaire would be useful in the one-on-one clinical setting, since they have been validated only in large groups of patients. However, the responses to these structured questionnaires can provide some insight into what is going on with the individual patient.
The pulmonologist also uses laboratory tests in making decisions. This process uses available disease markers to make inferences on the clinical outcomes of the patient. Jones and Agusti (Citation[18]) define clinical outcomes as consequences of the disease as experienced by the patient. Examples include symptoms, weight loss, exercise intolerance, exacerbations, quality of life, health resource utilization, and death. Disease markers, which are usually easier to measure and quantify, serve as surrogates to clinical outcomes. Examples include spirometric measurements such as the FEV1, exercise capacity measured in the laboratory, the 6-minute walk distance, dyspnea scores such as the MRC, and standardized health-status questionnaires, which are designed to measure health-related quality of life.
In the case presented, the reduction in FEV1 (32% of predicted) is considered severe bordering on very severe (Citation[19]), based on the American Thoracic Society—European Respiratory Society Statement on COPD. In this instance, the FEV1 severity is arguably greater than the overall severity based on the impact upon the individual. In other cases, it is the other way around. The 6-minute walk distance, which is reduced to about 40% of predicted (Citation[20]), gives important information that complements the spirometric data. However, the validity of using the 6-minute walk distance to direct care of individual patients, although intuitively reasonable, does not have much evidence-based support.
SUMMARY
Symptom levels and activity limitation are interrelated in COPD patients: dyspnea is frequently associated with physical activity in patients and activity levels are often decreased to reduce this distressing symptom. This leads to adoption of a more sedentary lifestyle, which in turn leads to more effort-intolerance. The pulmonologist should appreciate that both symptoms (especially dyspnea) and functional status limitation, besides being common in COPD, are major factors in impairment in HRQL. A more targeted inquiry by the pulmonologist, stressing assessing activity limitation in addition to dyspnea assessment will better define the impact of COPD on the individual patient. At present, our therapeutic interventions have to focus in large part on addressing symptoms and activity limitation, with the ultimate goal of improving quality of life.
REFERENCES
- ZuWallack R, Lareau S, Meek P. The effect of pulmonary rehabilitation on dyspnea. Lung Biology in Health and Disease. Dyspnea: Mechanisms, Measurement and Management. 2nd Edition, D Mahler, D O'Donnell. Taylor and Francis Group, Boca Raton, FL 2004; 301–320
- Meek P M, Lareau S C. Critical outcomes in pulmonary rehabilitation: assessment and evaluation of dyspnea and fatigue. Rehabil Res Dev Sep–Oct;, 2003; 40: 13–24, (5 Suppl 2)
- Agusti A G. COPD, a multicomponent disease: implications for management. Med 2005; 99: 670–682
- Pitta F, Troosters T, Probst V S, Spruit M A, Decramer M, Gosselink R. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur Respir J 2006; 27: 1040–1055
- Guccione A A. Functional assessment. Physical Medicine and Rehabilitation: Assessment and Treatment. Philadelphia, PA 1994; 193–208, Chapter 11
- Spector W D, Katz S, Murphy J B, Fulton J P. The hierarchical relationship between activities of daily living and instrumental activities of daily living. J Chron Dis 1987; 40: 481–489
- Pitta F, Troosters T, Probst V S, Spruit M A, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest 2006; 129: 536–544
- Jones P W. Issues concerning health-related quality of life in COPD. Chest 1995; 107: 187s–193s
- Jones P W. Health status measurement in chronic obstructive pulmonary disease. Thorax 2001; 56: 880–887
- ZuWallack R, Haggerty M, Jones P. Clinically meaningful outcomes for patients with COPD. Amer J Med 2004; 117: 49S–59S, (Suppl 12A)
- Zu Wallack R L. Clinical interpretation of health-related quality of life outcomes in COPD: application to clinical care. Eur Respir Rev 2002; 12: 92–97
- ZuWallack R, Haggerty M, Jones P. Clinically meaningful outcomes for patients with COPD. Amer J Med 2004; 117: 49S–59S, (Suppl 12A)
- Seemungal T A, Donaldson G C, Paul E A, Bestall J C, Jeffries D J, Wedzicha J A. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157: 1418–1422
- Aaron S D, Vandemheen K L, Clinch J J, Ahuja J, Brison R J, Dickinson G, Hebert P C. Measurement of short-term changes in dyspnea and disease-specific quality of life following an acute COPD exacerbation. Chest 2002; 121: 688–696
- Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J 1999; 14: 270–274
- Bowen J B, Votto J J, Thrall R S, Campbell M, Stockdale-Woolley R, Bandyopadhyay T, Zu Wallack R. Functional status and survival following pulmonary rehabilitation. Chest 2000; 118: 697–703
- Celli B R, Cote C G, Marin J M, Casanova C, Montes de Oca M, Mendez R A, Pinto Plata V, Cabral H J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005–1012
- Jones P W, Agusti A GN. Outcomes and markers in the assessment of chronic obstructive pulmonary disease. Eur Respir J 2006; 27: 822–832
- American Thoracic Society and the European Respiratory Society. Standards for the diagnosis and management of patients with COPD. 20 June, 2006, http://www-test.thoracic.org/copdAccessed
- Gibbons W J, Fruchter N, Sloan S, Levy R D. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil 2001; 21: 87–93