Abstract
Historically, spirometry has been the objective measure used to confirm a symptom-based clinical suspicion of COPD. The third National Health and Nutrition Examination Survey (NHANES III) created a strong rationale for early identification and intervention in COPD by documenting the ability of spirometry to detect mild airflow problems in many asymptomatic smokers. Predicted values for spirometry, however, must be adjusted to account for variations in age, gender, height, ethnicity and race. Many experts agree that NHANES III reference equations are much better suited to COPD practice than most other predicted value standards. However, standards other than NHANES III have been adopted in current medical guidelines; standards that may inappropriately classify younger adults as normal and older adults as abnormal, potentially leading to widespread misdiagnosis and mis-directed therapies in clinical practice. Despite the shortcomings of established diagnostic predicted values, spirometry remains the best available tool for early and accurate diagnosis of COPD in those at risk for the disease, and is also useful in conjunction with other modalities in patients with established disease to determine prognosis and assessing therapeutic benefits. In the clinical trial settings, as well as in day-to-day practice, spirometry results should be combined with other endpoints in order to better reflect overall patient status. This review highlights key medical evidence surrounding both usefulness and limitations of FEV1 in the setting of COPD.
Key words: :
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a preventable illness characterized by airflow limitation that is progressive and partially reversible, associated with an abnormal inflammatory response of the lungs to noxious particles or gases (Citation[1], Citation[2]). More than 85% of COPD cases in the United States develop as a direct result of prolonged exposure of the lungs to tobacco smoke, both primary and secondhand exposure, while the majority of the remaining 15% is secondary to genetic predispositions (e.g., alpha-1 antitrypsin deficiency), chronic environmental/occupational exposures, and chronic inhalation of biomass fuels. Millions of tobacco users who do not yet have COPD are at high risk for developing it during their lifetime.
While mortality rates for most chronic illnesses such as heart disease, malignancy, and stroke have seen declines of between 7% and 64% in the past 3 decades, those for COPD have risen by 163% (Citation[3]). In a recent survey conducted by the Centers for Disease Control (CDC), an estimated 10 million U.S. adults self-reported a diagnosis of COPD. Epidemiologic estimates, however, place the prevalence of COPD at 24 million plus, indicating that COPD is vastly unrecognized, underdiagnosed and/or perhaps misdiagnosed (Citation[4]). The fourth-leading cause of death and second-leading cause of disability (per Social Security statistics) in the United States, COPD accounts for more than 125,000 mortalities each year (Citation[5]). Although COPD is stereotypically considered to be a “male” disease, the number of women who died from COPD surpassed the number of men for the first time in 2000, and it is estimated that the incidence of COPD will be greater in women verses men in the near future (Citation[4]).
The physiological impairment in COPD is characterized by airflow limitation, air trapping, and hyperinflation – depending on the severity of the disease. These abnormalities can lead to dyspnea, which is unpleasant and often severely limits the activities patients can or want to undertake, prompting them to avoid situations that demand physical activity. Beginning at the mildest stage of the disease, COPD patients slowly modify their lifestyle over years to decades and often avoid activities that lead to dyspnea; accordingly, they frequently do not present with respiratory complaints to clinicians until the disease is severe. The classic time course of COPD is a gradual downward spiral of lung function and the level daily activities, often progressing to deconditioning during disease progression. Most patients experience some frequency of exacerbations and perhaps hospitalizations, often triggered by respiratory tract infections. As COPD worsens, patients are more likely to experience increasingly severe exacerbations, compromised quality of life, and, ultimately, respiratory failure, co-morbid cardiovascular disease, and death.
Misdiagnosis, “silent” unrecognized and/or unacknowledged early signs and symptoms of COPD, or coexistent conditions or co-morbidities are all believed to contribute to underdiagnosis, despite the availability of clinical practice guidelines for appropriate early diagnosis and appropriate treatment of COPD. The most recent comprehensive guidelines for identification and management of COPD, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (Citation[1]) and the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines (Citation[2]), offer the optimistic perspective that the disease is partially reversible and treatable with sustained smoking cessation, initially maximizing maintenance bronchodilator therapy, and the addition of other pharmacologic and nonpharmacologic therapies as COPD progresses. Both the GOLD and ATS/ERS COPD guidelines recommend measurement of pre- and post-bronchodilator lung function to diagnose and stage COPD, with the latter guideline putting more emphasis on symptoms. Airflow limitation is defined as a post-bronchodilator FEV1/FVC ratio of less than 0.70. The severity of airflow obstruction is stratified based on the post-bronchodilator FEV1 (Citation[1]).
Historically, and reaffirmed by the above guidelines, spirometry has been the objective measure used to confirm a symptom-based clinical suspicion of COPD. Once spirometry establishes airflow obstruction (an FEV1/FVC < 70%), both the GOLD and ATS/ERS criteria stratify severity based on the degree of FEV1 impairment: for mild COPD (stage 1) an FEV1 of ≥ 80% predicted, for moderate COPD (stage 2), an FEV1 < 80% of predicted but ≥ 50% predicted, for severe COPD (stage 3) an FEV1 < 50% of predicted but ≥ 30% predicted, and for very severe COPD, an FEV1 < 30% of predicted (or an FEV1 < 50% predicted plus chronic respiratory failure) (Citation[1]). GOLD and the ATS/ERS guideline criteria for obstruction conflict with those of ATS/ERS task force for the standardization of lung function testing statement (Citation[6]), which characterizes obstruction as an FEV1/FVC% below the statistically defined fifth percentile of normal. GOLD clinical practice guidelines, however, still use the FEV1/FVC ratio of < 70% as indicative of airflow limitation and stratify severity by FEV1 as a percent of predicted.
Results from the third National Health and Nutrition Examination Survey (NHANES III) created a strong rationale for early identification and intervention in COPD by documenting the ability of spirometry to detect mild airflow problems in many asymptomatic smokers (Citation[7]). NHANES III, conducted from 1988 to 1994, collected spirometry data on 20,627 survey participants, which included an oversampling of African-American and Mexican-American populations, allowed for valid comparisons among different race/ethnic groups. As a result, NHANES III generated reference equations to describe normal pulmonary function for three major race/ethnic groups: Caucasians, African-Americans, and Mexican-Americans (Citation[8]). Many experts agree that NHANES III reference equations are much better suited to COPD practice than most other predicted value standards.
Additionally, NHANES III generated a new awareness of the scope and impact of COPD on a national level, which ultimately led to the formation of the National Lung Health Education Program (NHLEP). This collaboration of government, medical, and other health profession organizations is designed to advance COPD awareness and to promote use of spirometry testing in the primary care setting. NHLEP's overall goals are to the earlier identification and management of COPD – perhaps before patients acknowledge clinical symptoms and thus potentially slowing disease progression. Both the NLHEP and ATS support the spirometry reference equations generated by NHANES III as outlined next.
HISTORY OF SPIROMETRY
The history of the spirometer dates back to 1846 when an English surgeon, John Hutchinson, invented the device and coined the term “vital capacity.” Dr. Hutchinson reported measurements on 2130 individuals and showed that vital capacity was directly proportional to height and inversely proportional to age (Citation[9]). These data were deemed a monumental advancement by actuaries who used the information to predict patient longevity and mortality. Nonetheless, the spirometer was not widely appreciated nor implemented into day-to-day practice, and Dr. Hutchinson retired to Fiji at age 40 and then died without appreciating the eventual impact his invention would have on medical practice worldwide.
More than a century later, another surgeon, Edward A. Gaensler, further demonstrated the value of vital capacity and added a timing element to the measure (Citation[10]). Thus, the concept of “forced expiratory volume as a function of time”—FEV1 and FVC—was created in 1951. Nearly 3 decades later, the Framingham Study corroborated Hutchinson's observations by reporting that FEV1 is a powerful prognostic indicator for both pulmonary disease and cardiac failure (Citation[11]), suggesting that it may have utility for primary care practitioners, pulmonologists and cardiologists alike. Despite its ability to document airflow obstruction, an essential component to diagnosing COPD, spirometry must be complemented by a thorough history and physical exam in order to direct clinicians to etiological diagnosis(es) of COPD (Citation[6]). By itself, spirometry is inadequate to follow the course of COPD or to monitor the impact of pharmacologic and non-pharmacologic COPD treatments. Rather, it is used in conjunction with other measures of activity, exercise, exacerbation rate, quality of life, health status etc. to assess disease progression and the impact(s) of therapies. This approach is utilized currently to assess where in the disease continuum of COPD patients fall.
Prevailing myths notwithstanding—that spirometry is of little benefit, costly, and difficult to perform and interpret—FEV1 and its ratio to the FVC (or FEV6) are, in fact, fairly simple to assess when spirometry is performed by trained staff. A major goal of the NLHEP is to establish an earlier diagnosis of COPD in those patients at risk for the disease (Citation[12]). The NLHEP does not advocate “screening” for COPD, but rather “selected early detection” or case finding. That is, spirometry should be performed on individuals at risk for COPD – those 45 years of age or older who are current smokers, former smokers, or exposed chronically to environmental/occupational exposures, or those regardless of age who have the early warning(s) of COPD – chronic cough ± mucus/sputum production, dyspnea on mild exertion out of proportion to age or the activity being performed, or wheeze (Citation[7]).
Another NLHEP goal is to have currently available small, accurate, reliable, and inexpensive spirometers routinely used in the office setting of all primary care clinicians and other healthcare workers in order to establish a diagnosis of COPD in those patients at risk (Citation[7], Citation[12], Citation[13]). In addition, the NLHEP is collaborating with the American Association for Respiratory Care to create instructional videos and DVDs to facilitate staff training. With properly trained staff, the variability of spirometry results can be diminished, the measurement accuracy improved, and the range of normal values adjusted for specific populations (Citation[6]).
Diagnosis
Spirometry predictive reference values have been established and useful for decades. A limitation that has recently received some criticism is that these values are based upon studies of healthy people, as opposed to the health-compromised patients (e.g. COPD) typically seen in practice. Predicted values, like the % of predicted FEV1, normalize airflow for height, age, gender, and, in some cases, race, but generally are not accurate for African-American and Hispanic populations. Further, weight is not a factor in predicted value, even though extreme overweight can at times reduce lung function.
Applying GOLD and ATS guidelines to NHANES III data, Hansen and colleagues analyzed individual values of the FEV1/FVC ratio in relation to age in nearly 10,000 individuals (Citation[14]). In the never-smoking population, the lower limits of normal set by established guidelines correlated “reasonably well” with the NHANES III statistically defined fifth percentile guidelines. But the investigators found that almost half of younger adults with FEV1/FVC ratios below the NHANES III fifth percentile of normal were misidentified as normal because their FEV1/FVC ratio was > 70%. In contrast, approximately one fifth of older adults with FEV1/FVC ratios above the NHANES III fifth percentile had FEV1/FVC ratios < 70%, but were misidentified as having abnormal ratios. These findings indicate that the widely used GOLD criteria may inappropriately classify many younger adults who actually have airflow obstruction as ‘normal’, and approximately one-fifth of older adults who actually have normal lung function as “abnormal,” potentially leading to widespread misdiagnosis of COPD and mis-directed therapies in clinical practice (Citation[14]).
Recent and current research further points to the need for more precise values for diagnosing obstruction in non-Whites. Fulambarker and colleagues (Citation[15]), for example, found that healthy adult Asian Indians in the U.S. showed FVC to be 20% to 24% lower in men and 25% to 28% lower in women as compared to Whites. FEV1 was 16 to 23% lower in men and 20% to 26% lower in women. Comparisons with African Americans yielded smaller differences (Citation[15]). Although some race-specific equations for pulmonary function have been developed, they are not universally used. Additional equations for various ethnic groups are currently being developed.
Despite the shortcomings of established diagnostic predicted values, spirometry remains the best available tool for early and accurate diagnosis of COPD (Citation[7], Citation[16]), usually detecting the disease well before patients admit or acknowledge their symptoms, and certainly long before abnormalities in arterial blood gas, chest X-ray, and electrocardiogram findings become abnormal. The most common symptom which prompts patients to seek medical advice for COPD is dyspnea; however, they usually do not present and acknowledge this symptom until half their lung function is gone (FEV1 < 50% predicted). Individuals with COPD slowly modify their lifestyle over years to decades to take part in activities that do not cause shortness of breath. Therefore the disease is often far advanced before dyspnea on mild exertion is acknowledged. The overall utility of spirometry for an earlier and accurate diagnosis of COPD and asthma has led the National Committee for Quality Assurance (NCQA) to establish a HEDIS (Healthcare Effectiveness Data and Information Set) measure especially for spirometry use to document airflow limitation when a diagnosis of COPD is considered (Citation[17]).
Spirometry has also been incorporated as a standard parameter in the pay-for-performance, or physician-reporting program of the Centers for Medicare and Medicaid Services (CMS). The Joint Commission, formerly known as the Joint Commission on Accreditation of Healthcare Organizations (JCAHO), has created an accreditation program for COPD, which will likely establish additional standards for use of spirometry in inpatient, outpatient, and emergency department settings. These developments suggest that use of spirometry for definitive diagnosis of COPD will soon be inexorably related to provider reimbursement in virtually every practice setting.
In some cases, the differential diagnosis between asthma and COPD can be supported with spirometry testing. The key measurements necessary for the early detection and monitoring of obstructive lung diseases are FEV1, FVC, and in asthma but not in COPD, the peak expiratory flow (PEF) (Citation[18]). Whereas most asthmatic patients will achieve normal airflow following bronchodilator therapy, assessed by spirometry, COPD patients will never show improvement to normal airflow in response to bronchodilators, as they have a fixed component of their airflow obstruction (Citation[18]), but they can show significant improvement in FEV1, or diminished air-trapping, after bronchodilators compared to their baseline lung function. Differences between the clinical manifestation of asthma and COPD are shown in . Making the differential diagnosis can be clinically challenging due to similarities in clinical presentation (symptoms) and the coexistence of both diseases in 10% to 15% of COPD patients (Citation[19]).
Table 1 Clinical variations between COPD and asthma
Prognosis
The association between FEV1 measures and prognosis was established more than 15 years ago when Burrows and colleagues revealed that 10-year mortality in COPD patients was directly related to the degree of airflow obstruction (Citation[20]). A comprehensive review of the medical literature to date by Hodgkin and colleagues in 1990 (Citation[21]), concluded that mortality in patients with a baseline postbronchodilator FEV1 greater than or equal to 50% of predicted is only a slightly greater than that of a group of healthy smokers. Marked reversibility of FEV1 has shown to be a favorable prognostic factor in COPD, suggesting that elevation of FEV1 may prompt other physiological changes to improve prognostic outlook (Citation[22]).
Based on this medical evidence, both ATS/ERS and GOLD guidelines recommend that post-bronchodilator FEV1 readings be used to establish the severity of COPD to determine the patient's prognosis (Citation[2], Citation[23], Citation[24]). The ATS/ERS guidelines stipulate, however, that patient activity level is a more significant prognostic factor than the FEV1 measure. This suggests that a more active patient will have a better prognosis despite the level of FEV1 and also suggests that spirometry alone is a suboptimal tool for assessing patient outcomes.
A recent study by O'Donnell and colleagues demonstrates both premises to be true (Citation[25]). The study was designed to determine which form of spirometry measurements provided the best representation of improvements in exercise capacity in patients with COPD. Following baseline spirometry testing, patients were given either nebulized ipratropium bromide (500 μ g) or placebo. After 1 hour, spirometry testing was performed again. The investigators found that a change in FEV1 was not a good predictor of overall patient improvement, and specifically, that spirometric measurements did not always correlate to patients' improved exercise performance. These findings demonstrate that FEV1 is a measurement that does not always accurately reflect outcomes due to the underlying disease mechanisms, but nevertheless is important for establishing the correct pulmonary diagnosis.
Studies in other disease states have shown FEV1 to be useful for predicting morbidity and mortality for cancer (Citation[26]), heart disease (Citation[27]), stroke (Citation[28]), and all-cause mortality (Citation[11], Citation[29]). Within the broader framework of utilization of health-care resources, spirometry classification has proven useful in predicting health status (Citation[30]), utilization of health-care resources (Citation[31]), development of acute exacerbations (Citation[32], Citation[33]), and mortality (Citation[34]). Thus, an abnormal FEV1 reading is a highly useful clinical tool as it provides a signal for physicians to focus the clinical history and examination to probe not just for COPD, but also for a broad spectrum of diseases associated with tobacco use. Baseline and follow-up spirometry is also now recommended for patients being considered for, and placed on, inhaled medications such as inhaled insulin (Citation[35]).
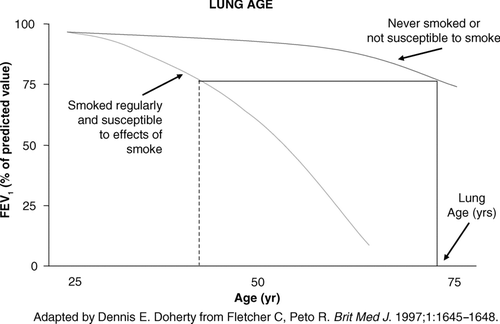
Even under normal circumstances, lung function declines continuously after the age of 21 (approximately 10–15 ml per year), and among smokers the decline is usually accelerated and more profound (up to 80–150 ml per year) (Citation[36]). A landmark study by Fletcher et al which suggested that the rate in decline of lung function (FEV1) in susceptible smokers could be slowed and revert toward the rate of loss in non-smokers has long been used to encourage smokers to quit in order to slow the onset of symptoms, disability, and even morbidity verses those individuals who continue to smoke. To the best of our knowledge, only “susceptible smokers” will develop accelerated decline in lung function. shows patterns of FEV1 decline with age and smoking status, illustrating that smokers who are susceptible to tobacco smoke and eventually die with COPD have already suffered significant FEV1 loss in their 40s (Citation[36]). “Most smokers whose FEV1 is already below the normal range for nonsmokers by early middle age are thus at grave risk of later death from airflow obstruction unless they stop smoking immediately, while smokers whose FEV1 is still above average in middle age will probably not get serious obstruction,” the authors report, with one caveat: Men with above normal lung capacity in their youth may still lie within the normal range by middle age, even with considerable FEV1 loss (Citation[36]).
Figure 1A Source (Citation[36]), permission to reproduce required. “Risks” for various men if they smoke: differences between these lines illustrate effects that smoking, and stopping smoking, can have on FEV1 in those men who are liable to develop chronic obstructive lung disease if they smoke. “Death” indicates deaths whose underlying cause is irreversible chronic obstructive lung disease, whether the immediate cause of death is respiratory failure, pneumonia, cor pulmonale, or aggravation of other heart disease by respiratory insufficiency. [These data are the composite of several individuals measured at various times (cross-sectional) and not following patients over several years (not longitudinal)]. Although this shows rate of loss of FEV, for one particular susceptible smoker, other susceptible smokers will have different rates of loss, thus reaching ‘disability’ at different ages” (Citation[36]).
![Figure 1A Source (Citation[36]), permission to reproduce required. “Risks” for various men if they smoke: differences between these lines illustrate effects that smoking, and stopping smoking, can have on FEV1 in those men who are liable to develop chronic obstructive lung disease if they smoke. “Death” indicates deaths whose underlying cause is irreversible chronic obstructive lung disease, whether the immediate cause of death is respiratory failure, pneumonia, cor pulmonale, or aggravation of other heart disease by respiratory insufficiency. [These data are the composite of several individuals measured at various times (cross-sectional) and not following patients over several years (not longitudinal)]. Although this shows rate of loss of FEV, for one particular susceptible smoker, other susceptible smokers will have different rates of loss, thus reaching ‘disability’ at different ages” (Citation[36]).](/cms/asset/fcbc5e92-fbba-41de-ae26-bcde8af576b1/icop_a_336505_uf0001_b.gif)
The positive impact of smoking cessation has been conclusively demonstrated by the Lung Health Study, which revealed that sustained quitters slow the progression of their loss of lung function significantly more than do intermittent quitters or continuous smokers (Citation[34]). At 11 years, 38% of continuing smokers had an FEV1 less than 60% of the predicted normal value compared with 10% of sustained quitters (Citation[34]). These findings support other studies that linked smoking cessation to FEV1 declines comparable to those of never smokers (Citation[36], Citation[37], Citation[38], Citation[39], Citation[40], Citation[41]). Communicating this information to patients can be accomplished best by putting it in the context of their Lung Age, a value derived by determining the age of a ‘normal’ individual with respect to FEV1 who has the same absolute value of FEV1 as this patient of a younger age with an abnormal FEV1 (see ). “You're 45, but you have the lung function of a 78-year-old; you need to stop smoking.” A low FEV1 combined with such smoking cessation advice has been shown by some reports to increase smoking cessation rates (Citation[42], Citation[43], Citation[44], Citation[45], Citation[46]), but another study was unable to demonstrate any positive impact (Citation[47]).
Figure 1B The upper dotted line represents the normal decline in those who have not smoked, or in smokers who are not susceptible to the effects of tobacco smoke on lung function decline. The lower dashed line represents an accelerated loss of lung function over time in those ‘susceptible’ smokers. The FEV1 a susceptible 40 year old smoker was measured at 75% predicted (dotted vertical line). If one extrapolates that level of lung function of this susceptible individual (draw a horizontal line to the curve of a never smoker or one not susceptible to smoking), their ‘Lung Age’ is approximately 74 – equivalent lung function to that of a never smoker (or non-susceptible smoker) when they are 74.
Other prognostic indicators
The Inspiratory Capacity/Total Lung Capacity Ratio (IC/TLC) can also be used as an independent prognostic factor to predict mortality. Although it is a more sensitive predictor than FEV1, dyspnea, the 6-minute walk, body mass index, and hypoxemia (Citation[48]), IC/TLC may be more difficult to obtain in community practice. The threshold of significance for the IC/TLC ratio is 25% (Citation[48]).
While one parameter may predict an outcome, often the combination of two or more parameters will better predict that outcome verses any one parameter alone. The BODE index combines factors to predict mortality in COPD: Body mass index (BMI), Obstruction (Airflow-FEV1), Dyspnea (MRC scale), and Exercise limitation (6-minute walk) (Citation[49]). This index is used to capture systemic manifestations that are not reflected by the FEV1 alone in order to better categorize and predict the outcome of mortality. Variables and point values used for the computation of the BODE index are shown in . In a validation study of the BODE index, analysis of survival showed that each quartile increase in the BODE score was associated with increased mortality (p < 0.001). At 52 months, the highest quartile (a BODE score of 7 to 10) was associated with a mortality rate of 80% at 52 months. Using the staging system of the American Thoracic Society in place in 2004 to stratify the severity of COPD, the ability of the BODE index to predict the risk of death was substantially higher that that of the FEV1 alone (Citation[49]). Adding the frequency of acute exacerbations to the BODE index assessment may help clinicians to better prognosticate, but in this study, the addition of this parameter to the other four did not increase the predictive value for mortality.
Table 2 Variables and point values used for the computation of the BODE index
FEV1 as clinical endpoint
Change in FEV1 is frequently a primary endpoint in clinical trials evaluating the efficacy of bronchodilators. Improvement in FEV1 is seen as a positive result in studies of patients with asthma, but in studies of COPD, patients who at baseline achieve FEV1 changes > 12% in response to a short-acting bronchodilator such as albuterol, are typically excluded from clinical trials. Although the rationale may be to separate asthmatics from COPD study subjects, such exclusionary criteria create a research anomaly: Studies of response to bronchodilators in COPD exclude patient responders, yet set patient response measured by FEV1 as a primary study endpoint. This contradiction clearly points to the need to revisit study inclusion/exclusion criteria and to adopt a broader approach to study endpoints that include measures beyond FEV1. Although spirometric assessment is important for the characterization of airflow impairment and improvements following therapy, other consequences of chronic disease relate to a patient's disability, health status, and quality of life.
Recent clinical studies have added measures to assess these outcomes. A study of once-daily inhaled tiotropium in COPD set multiple endpoints: trough FEV1 (i.e., FEV1 prior to dosing); changes in dyspnea measured by the Baseline and Transition Dyspnea Index (BDI-TDI); and health status, measured with the disease-specific St. George's Respiratory Questionnaire (SGRQ) and the generic Short Form 36 (Citation[50]). The study also captured exacerbation and hospitalization data.
Tiotropium produced rapid bronchodilation upon initial dosing. With continued once daily maintenance therapy, trough FEV1 was elevated 110 ± 10-130 ± 10mL (11 ± 1-13 ± 1%) over baseline, superior to placebo by 120 ± 10-150 ± 20 mL (p < 0.01). The study showed continuous bronchodilation above baseline once a steady-state of inhaled tiotropium was reached, which appears to be a bronchodilator class effect. In a separate study comparing tiotropium with ipratropium, only tiotropium increased trough FEV1 at the end of each medication's dosing period – 6 hrs after the last dose of ipratropium, a q.i.d. medication, and 23 hrs after the last dose of tiotropium, a once a day medication (Citation[51]).
Disease modification in COPD
To date, the only successful intervention shown to conclusively attenuate the loss of lung function over time is smoking cessation. However, emerging evidence is suggesting that bronchodilators may slow the progressive loss of lung function in COPD. In the study of tiotropium vs. placebo referenced earlier, patients who received treatment experienced a 10 mL reduction in FEV1 from baseline to study end (one year) as compared to a 40 mL loss in the placebo group (Citation[50]). These changes were statistically significant, but small, indicating a need for further study, the primary endpoint for the Understanding the Potential Long-Term Impacts on Function with Tiotropium (UPLIFT) trial.
Few COPD clinical trials have confirmed that therapies lead to disease modification. A recent 3-year, multi-center, randomized, double-blind, parallel group, placebo-controlled study of more than 6000 patients with moderate to severe COPD examined the impact of treatment on mortality over a 3-year period (Citation[52]). Towards a Revolution in COPD Health (TORCH) (Citation[53]) had a primary endpoint of determining the impact of salmeterol/ fluticasone propionate (50/500) combination (SFC) vs each component alone (S, F) vs placebo on mortality in the setting of COPD. TORCH also evaluated a secondary endpoint: the impact of treatment on slowing of the decline of FEV1. The study's primary endpoint of reduced mortality failed to achieve statistical significance. Spirometric measurements (an increase in FEV1 of 0.092 liter), albeit a secondary endpoint, in the combination-therapy group were significantly better than in the groups receiving placebo, salmeterol alone, or fluticasone propionate alone (p ≤ 0.001). However, both the F and SFC arms had significant increases in pneumonia vs. placebo (p ≤ 0.001).
In the Understanding the Potential Long-Term Impacts on Function with Tiotropium (UPLIFT) trial, the yearly rate of decline of FEV1 (trough and post-dose) following treatment with once-daily tiotropium has been established as a primary study endpoint (Citation[54]). In this 4-year, randomized, double-blind, placebo-controlled, parallel-group trial, patients will be requested to return for a final visit 30 days after the completion of 4 years of study medication.
This last visit will document outcomes of baseline verses 4 year post treatment-placebo FEV1 after a 1-month treatment washout period. Secondary study endpoints include the yearly rate of decline in FEV1 (trough and post-dose) from steady state until after 1 month off treatment; rate of decline in FEV1 after 1 month; rate of decline of FVC and SVC; health-related quality of life (HRQoL) measured by St. George's Respiratory Questionnaire; exacerbations; hospitalizations due to exacerbations; and mortality (respiratory and all-cause). The UPLIFT trial will furnish important results that will help to increase understanding of the long-term natural history of COPD and to elucidate tiotropium's potential impact on disease progression.
CONCLUSIONS
Ample evidence exists to support the use of FEV1 for establishing the diagnosis and prognosis of COPD and, in conjunction with other measures, to monitor treatment outcomes. Emergent evidence, based on the parameter of FEV1 (spirometry testing), suggests a potential role for long-acting bronchodilators to slow disease progression in certain COPD patients, and analyses of the outcomes from these studies are eagerly awaited.
Given the multiple applications and benefits of spirometry testing, payers are moving toward widespread requirements for its use within the primary care and hospital settings to establish the diagnosis, or lack thereof, in those at risk for COPD—current or former smokers, those chronically exposed to environmental-occupational risk factors, or those with the cardinal signs-symptoms of COPD as outlined above. However, a recent Canadian survey suggests that the majority of primary care physicians still do not use spirometry to diagnose COPD, even when it is readily available (Citation[55]). There is a clear need for education within the lay and medical communities to raise awareness of COPD, and also to promote appropriate routine use in the office of spirometry in the context of case findings in both subclinical “at risk” and symptomatic patients.
This article was prepared with the editorial assistance of Genevieve Belfiglio, a medical writer working with Advanced Studies in Medicine. This paper is based on a presentation at a meeting, titled “Influencing the Spiral of Decline in COPD,” which took place in Atlanta, Georgia, on May 2–4, 2007. The author is responsible for the content of the article but gratefully acknowledges assistance from Dick Briggs Jr., MD, University of Alabama at Birmingham, Birmingham, AL. This article was funded by Boehringer-Ingelheim Pharmaceuticals, Inc and Pfizer Inc. Dr. Doherty reports receiving clinical study grant funding as a Principal Investigator in multi-centered trials from (in the past 3 years) Boehringer-Ingelheim, GlaxoSmithKline, Pfizer Inc, Schering-Plough, and Novartis; serving on the advisory boards (in the last 3 years) for CSL Behring, Novartis, Pfizer Inc, and Schering-Plough; and serving on the speakers' bureau for Boehringer-Ingelheim, Pfizer Inc, and Schering-Plough.
REFERENCES
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2006 Executive Summary, Available from: www.goldcopd.com [cited 2007 May 16]
- Celli B R, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23: 932–946
- Centers for Disease Control and Prevention. Surveillance Summaries. U.S. Department of Health and Human Services. 2002
- Centers for Disease Control and Prevention. Surveillance Summaries. U.S. Department of Health and Human Services. 2002
- Mannino D, Homa D, Akinbami L, Ford E, Redd S. Chronic Obstructive Pulmonary Disease Surveillance—United States, 1971–2000. Morb Mortal Wkly Rep 2002; 51: 1–16
- Miller M R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten C P, Gustafsson P, Jensen R, Johnson D C, MacIntyre N, McKay R, Navajas D, Pedersen O F, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338
- Ferguson G T, Enright P L, Buist A S, Higgins M W. Office spirometry for lung health assessment in adults: A consensus statement from the National Lung Health Education Program. Chest 2000; 117: 1146–1161
- Hankinson J, Odencrantz J, Fedan K. Spirometric Reference Values from a Sample of the General U. S. Population. Am Rev Resp Crit Care Med 1999; 159: 179–187
- Hutchinson J. Med Chir Tr 1846; 29: 137
- Gaensler E A. Analysis of the ventilatory defect by timed capacity measurements. Am Rev Tuberc 1951; 64: 256–278
- Kannel W B, Lew E A, Hubert H B, Castelli W P. The value of measuring vital capacity for prognostic purposes. Trans Assoc Life Insur Med Dir Am 1980; 64: 66–83
- Petty T L, Doherty D E. The National Lung Health Education Program: roots, mission, future directions. Respir Care 2004; 49: 678–683
- Who we are. 2007, Available from: http://www.nlhep.org/who.html [cited 2007 June 7, 2007]
- Hansen J E, Sun X G, Wasserman K. Spirometric criteria for airway obstruction: Use percentage of FEV1/FVC ratio below the fifth percentile, not < 70%. Chest 2007; 131: 349–355
- Fulambarker A, Copur A S, Javeri A, Jere S, Cohen M E. Reference values for pulmonary function in Asian Indians living in the United States. Chest 2004; 126: 1225–1233
- Ferguson G T. Why does the lung hyperinflate?. Proc Am Thorac Soc 2006; 3: 176–179
- NCQA. NCQA Releases HEDIS®. 2006, Available from: http://www.ncqa.org/communications/news/hedis_2006.htm New measures address overuse, follow-up. [cited 2007 May 16]
- Doherty D E. The pathophysiology of airway dysfunction. Am J Med 2004; 117(Suppl 12A)11S–23S
- Barnes P J. Mechanisms in COPD: differences from asthma. Chest 2000; 117: 10S–14S
- Burrows B. Airways obstructive diseases: pathogenetic mechanisms and natural histories of the disorders. Med Clin North Am 1990; 74: 547–559
- Hodgkin J E. Prognosis in chronic obstructive pulmonary disease. Clin Chest Med 1990; 11: 555–569
- Traver G A, Cline M G, Burrows B. Predictors of mortality in chronic obstructive pulmonary disease. A 15-year follow-up study. Am Rev Respir Dis 1979; 119: 895–902
- Pauwels R A, Buist A S, Calverley P M, Jenkins C R, Hurd S S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001; 163: 1256–1276
- Global Initiative for Chronic Obstructive Lung Disease. Executive Summary: Global Strategy for the Diagnosis, Management, and Prevention of COPD. 2006, Available from: http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=996[cited 2007 May 5, 2007]
- O'Donnell D E, Lam M, Webb K A. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160: 542–549
- Van d en, Eeden S K, Friedman G D. Forced expiratory volume (1 second) and lung cancer incidence and mortality. Epidemiology 1992; 3: 253–257
- Buch P, Friberg J, Scharling H, Lange P, Prescott E. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur Respir J 2003; 21: 1012–1016
- Wannamethee S G, Shaper A G, Ebrahim S. Respiratory function and risk of stroke. Stroke 1995; 26: 2004–2010
- Mannino D M, Holguin F, Pavlin B I, Ferdinands J M. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis 2005; 9: 613–621
- Ferrer M, Alonso J, Morera J, Marrades R M, Khalaf A, Aguar M C, Plaza V, Prieto L, Anto J M. Chronic obstructive pulmonary disease stage and health-related quality of life. The Quality of Life of Chronic Obstructive Pulmonary Disease Study Group. Ann Intern Med 1997; 127: 1072–1079
- Friedman M, Serby C W, Menjoge S S, Wilson J D, Hilleman D E, Witek T J, Jr. Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest 1999; 115: 635–641
- Burge P S, Calverley P M, Jones P W, Spencer S, Anderson J A, Maslen T K. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. Bmj 2000; 320: 1297–1303
- Dewan N A, Rafique S, Kanwar B, Satpathy H, Ryschon K, Tillotson G S, Niederman M S. Acute exacerbation of COPD: factors associated with poor treatment outcome. Chest 2000; 117: 662–671
- Anthonisen N R, Connett J E, Murray R P. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 2002; 166: 675–679
- Pfizer I. EXUBERA® Prescribing Information. New York 2007
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977; 1: 1645–1648
- Camilli A E, Burrows B, Knudson R J, Lyle S K, Lebowitz M D. Longitudinal changes in forced expiratory volume in one second in adults. Effects of smoking and smoking cessation. Am Rev Respir Dis 1987; 135: 794–799
- Lange P, Groth S, Nyboe G J, Mortensen J, Appleyard M, Jensen G, Schnohr P. Effects of smoking and changes in smoking habits on the decline of FEV1. Eur Respir J 1989; 2: 811–816
- Peat J K, Woolcock A J, Cullen K. Decline of lung function and development of chronic airflow limitation: a longitudinal study of non-smokers and smokers in Busselton, Western Australia. Thorax 1990; 45: 32–37
- Wilhelmsen L, Orha I, Tibblin G. Decrease in ventilatory capacity between ages of 50 and 54 in representative sample of Swedish men. Br Med J 1969; 3: 553–556
- Xu X, Dockery D W, Ware J H, Speizer F E, Ferris B G, Jr. Effects of cigarette smoking on rate of loss of pulmonary function in adults: a longitudinal assessment. Am Rev Respir Dis 1992; 146: 1345–1348
- Gorecka D, Bednarek M, Nowinski A, Puscinska E, Goljan-Geremek A, Zielinski J. Diagnosis of airflow limitation combined with smoking cessation advice increases stop-smoking rate. Chest 2003; 123: 1916–1923
- Hepper N G, Drage C W, Davies S F, Rupp W M, LaMothe J, Schoenfelder P G, Munson P. Chronic obstructive pulmonary disease: a community-oriented program including professional education and screening by a voluntary health agency. Am Rev Respir Dis 1980; 121: 97–104
- Petty T L, Pierson D J, Dick N P, Hudson L D, Walker S H. Follow-up evaluation of a prevalence study for chronic bronchitis and chronic airway obstruction. Am Rev Respir Dis 1976; 114: 881–890
- Zielinski J, Bednarek M. Early detection of COPD in a high-risk population using spirometric screening. Chest 2001; 119: 731–736
- Bednarek M, Gorecka D, Wielgomas J, Czajkowska-Malinowska M, Regula J, Mieszko-Filipczyk G, Jasionowicz M, Bijata-Bronisz R, Lempicka-Jastrzebska M, Czajkowski M, Przybylski G, Zielinski J. Smokers with airway obstruction are more likely to quit smoking. Thorax 2006; 61: 869–873
- Buffels J, Degryse J, Decramer M, Heyrman J. Spirometry and smoking cessation advice in general practice: a randomised clinical trial. Respir Med 2006; 100: 2012–2017
- Casanova C, Cote C, de Torres J P, Aguirre-Jaime A, Marin J M, Pinto-Plata V, Celli B R. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171: 591–597
- Celli B R, Cote C G, Marin J M, Casanova C, Montes de Oca M, Mendez R A, Pinto Plata V, Cabral H J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005–1012
- Casaburi R, Mahler D A, Jones P W, Wanner A, San P G, ZuWallack R L, Menjoge S S, Serby C W, Witek T, Jr. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002; 19: 217–224
- Vincken W, van Noord J A, Greefhorst A P, Bantje T A, Kesten S, Korducki L, Cornelissen P J. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J 2002; 19: 209–216
- Vestbo J. The TORCH (towards a revolution in COPD health) survival study protocol. Eur Respir J 2004; 24: 206–210
- Calverley P M, Anderson J A, Celli B, Ferguson G T, Jenkins C, Jones P W, Yates J C, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356: 775–789
- Decramer M, Celli B, Tashkin D P, Pauwels R A, Burkhart D, Cassino C, Kesten S. Clinical trial design considerations in assessing long-term functional impacts of tiotropium in COPD: the UPLIFT trial. Copd 2004; 1: 303–312
- Canadian Lung Association. Chronic obstructive pulmonary disease (COPD): A national report card. Canadian Lung Association and the Canadian Thoracic Society. 2005