ABSTRACT
Inhaled indacaterol/glycopyrronium fixed-dose combination (IND/GLY) is approved in over 80 countries, including the EU, Japan, Australia and Switzerland and the US. The LANTERN study evaluated the efficacy of IND/GLY compared with inhaled long-acting β2-agonist (LABA)/inhaled corticosteroid (ICS) or salmeterol/fluticasone (SFC) in patients with moderate-to-severe COPD with a history of ≤1 exacerbation in the previous year. Here we present the efficacy and safety of IND/GLY versus SFC in the Chinese cohort from the LANTERN study. LANTERN was a 26-week, multicenter, randomized, double-blind, double-dummy, parallel-group study conducted in patients with moderate-to-severe COPD with a history of ≤1 exacerbation in the previous year. The patients were randomized (1:1) to once-daily IND/GLY (110/50 μg) or twice-daily SFC (50/500 μg). The primary endpoint was non-inferiority of IND/GLY versus SFC in terms of trough FEV1. Of the total 744 patients randomized in the LANTERN study, 598 (80.4%) were from Mainland China and randomized to IND/GLY (n = 298) or SFC (n = 300), and 553 (92.5%) completed the study. IND/GLY showed superiority over SFC with a statistically significant and clinically meaningful improvement in trough FEV1, FEV1 AUC0–4h, peak FEV1 and trough forced vital capacity (FVC) change from the baseline. Annualized rate of moderate or severe COPD exacerbations was significantly lower (43%) with IND/GLY compared with SFC (rate ratio: 0.57, p = 0.015). Overall, adverse events were lower for IND/GLY (34.6%) versus SFC (43.1%). IND/GLY was superior in achieving bronchodilation versus SFC in a Chinese subgroup of patients from this study.
Clinicaltrials.gov identifier: NCT01709903
Introduction
Chronic obstructive pulmonary disease (COPD) is a respiratory disease characterized by moderate-to-severe partially reversible airflow limitation, resulting in breathlessness that worsens over time in most patients Citation(1). In China, COPD is increasingly becoming a public health hazard which is indicated by the increase in the overall prevalence rate of COPD from 3.2% in 1999 Citation(2) to 8.2% in 2011 Citation(3). The burden of COPD in China is currently greater compared with developed countries, probably because of greater exposure to epidemiological risk factors and high smoking prevalence Citation(4). Underuse of spirometry in primary care facilities in China may cause the disease to remain undiagnosed until later stages Citation(5). A majority of COPD patients (60.3%) diagnosed in China were categorized under the Global Initiative for Chronic Obstructive Lung Disease (GOLD) group D Citation(6). The combination of high prevalence, low diagnosis rate and suboptimal access to treatment results in poor quality of life and restricted daily activities for a significant number of Chinese patients with COPD Citation(7).
As per the current GOLD strategy, the management of COPD is based on patients' airflow limitation, severity of symptoms and history of COPD exacerbations Citation(1). The national strategy for the management of COPD in China encourages physicians to adopt the GOLD strategy for diagnosis and treatment of COPD Citation(8). As per the strategy, combinations of long-acting bronchodilators are preferred as an alternate treatment option if symptoms are not adequately controlled by a single bronchodilator. Indacaterol/glycopyrronium (IND/GLY), a fixed-dose combination of two long-acting bronchodilators, IND 110 µg (a long-acting β2-agonist [LABA]) and GLY 50 µg (a long-acting muscarinic antagonist [LAMA]) is indicated for maintenance treatment of adult patients with moderate-to-severe COPD. IND/GLY showed additional therapeutic benefits over its monocomponents (IND and GLY) Citation(9) and the LABA/inhaled corticosteroids (ICSs) fixed-dose combination, salmeterol/fluticasone (SFC) Citation(10). In a post hoc analysis, IND/GLY showed comparable reduction in the risk of COPD exacerbations, versus SFC in patients with a history of ≤1 COPD exacerbation in the previous year Citation(11). Ethnic differences among patient populations may affect the therapeutic intervention because of differences in pharmacological factors, including pharmacokinetics and pharmacodynamics Citation(12). Previous studies of IND/GLY have predominantly, but not exclusively, Citation(13) involved Caucasian populations (Citation9, 10, 14).
The LANTERN was the first study to evaluate the efficacy and safety of a dual bronchodilator, IND/GLY versus SFC, predominantly in Chinese patients with moderate-to-severe COPD, with or without a COPD exacerbation in the previous year Citation(15). Here we report the results of the Chinese cohort from this study.
Methods
Patients
A total of 931 patients with moderate-to-severe stable COPD (stages II and III as defined by the GOLD 2010 criteria) were screened, of whom 598 were randomized in the Chinese centers as part of the global multicenter study. Adult men and women aged ≥40 years with a post-bronchodilator forced expiratory volume in 1 second (FEV1) ≥30% and <80% of the predicted normal and a post-bronchodilator FEV1/forced vital capacity (FVC) <0.7 were included in the study. All patients had a modified Medical Research Council (mMRC) grade ≥2 at screening. Patients who had experienced two or more COPD exacerbations that required treatment with antibiotics and/or oral corticosteroids and/or hospitalization in the year before the screening visit or during the run-in period were excluded from this study. Detailed inclusion and exclusion criteria were delineated in the LANTERN primary manuscript Citation(15).
Study design
This is a subgroup analysis of the 26-week, multicenter, randomized, double-blind, double-dummy parallel-group LANTERN study Citation(15). Following a screening and run-in period, patients were randomized (1:1) to receive either once-daily IND/GLY 110/50 μg (via the Breezhaler® device; Novartis Pharma AG, Stein, Switzerland) or twice-daily SFC 50/500 μg (via the Accuhaler® device; Glaxo Operations UK, Ware, UK) and matching placebos for 26 weeks. The study was approved by the institutional review board and ethics committee at each participating center and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Efficacy assessments
The primary endpoint was non-inferiority of IND/GLY versus SFC after 26 weeks of treatment in terms of post-dose trough FEV1 (mean of 23 hours 15 minutes and 23 hours 45 minutes post dose). The non-inferiority margin was defined as −60 mL as per previous findings Citation(16). If non-inferiority of IND/GLY versus SFC was met, IND/GLY would be tested for superiority, controlled by the pre-specified hierarchical procedure.
The key secondary endpoint was superiority of IND/GLY versus SFC in terms of standardized area under the curve (AUC) from 0 to 4 hours post dose for FEV1 (FEV1 AUC0–4h) at Week 26. The other secondary endpoints included peak FEV1; trough FVC; St. George's Respiratory Questionnaire (SGRQ) total score and Transitional Dyspnea Index (TDI) focal score. Rescue medication use, COPD exacerbations and other symptoms experienced by patients were captured via an electronic diary (e-diary).
Exploratory objectives included health-related quality of life measured by COPD assessment test (CAT) scores and the number of COPD exacerbations (mild, moderate and severe) that occurred over 26 weeks. COPD exacerbations were defined as worsening of the symptoms captured via the e-diary. Moderate and severe COPD exacerbations were also captured using case report forms (CRFs).
Safety assessments
Safety was assessed by monitoring treatment-emergent adverse events (AEs) and serious AEs (SAEs) during the study. Vital signs (electrocardiogram, pulse rate, and systolic and diastolic blood pressure) and laboratory analyses (hematology, clinical chemistry and urinalysis) were evaluated as a part of the safety assessment. All incidences of serious cardiovascular and cerebrovascular (CCV) events, atrial fibrillation events and deaths during the treatment and follow-up period were independently adjudicated by an Adjudication Committee. CCV events were adjudicated as major adverse cardiovascular events (MACE) or non-MACE.
Statistical analysis
Full analysis set (FAS; included all randomized patients who received at least one dose of study drug) was used to analyze all efficacy endpoints, unless otherwise stated. Per-protocol set (PPS; included all patients in FAS without any major protocol deviations) was used for the primary analysis of non-inferiority and the supportive analyses of the key secondary variables. The safety set (included patients who received at least one dose of study drug) was used to analyze safety endpoints. The primary endpoint, mean trough FEV1 after 26 weeks, was analyzed using a mixed-effect model with treatment, smoking status), COPD exacerbation history, baseline ICS use and region included as fixed effects; baseline FEV1 measurement and FEV1 reversibility as covariates; and center nested within region as a random effect. Non-inferiority of IND/GLY to SFC was demonstrated if the lower bound of the two-sided 95% confidence interval (CI) was greater than −60 mL for PPS. Superiority testing on trough FEV1 was performed on FAS after the primary objective was met. Secondary endpoints were evaluated using the same mixed model as the primary endpoint in FAS, but the respective baseline values replaced FEV1 as a covariate.
Moderate or severe COPD exacerbations were analyzed as rate of exacerbations and time to first exacerbation. The rate of COPD exacerbations was analyzed using a negative binomial model, and the time to first COPD exacerbation using a Cox proportional hazard model. These two models included treatment, baseline ICS use, baseline total symptom score, baseline COPD exacerbation history, FEV1 reversibility components, smoking history and region.
Results
Patients
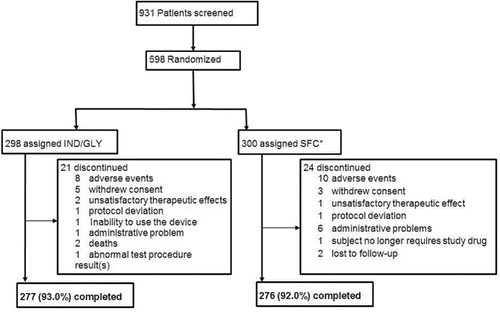
Of the 598 patients from China who were randomized in the LANTERN study (IND/GLY, n = 298; SFC, n = 300), 553 (92.5%) completed the study (). Among these, a total of 595 patients were included in the safety set and FAS as 3 patients were randomized to SFC group in error and did not receive the study drug. The rate of discontinuation was similar in both treatment groups (IND/GLY, 7.0% vs. SFC, 8.0%). Overall, the most common reason for discontinuation in both groups was AEs (IND/GLY, 2.7% and SFC, 3.3%).
Patient demographics and baseline characteristics
Patient demographics and baseline characteristics are summarized in . In the Chinese cohort, most of the patients were men (95.1%) with moderate (50.3%) or severe (48.6%) COPD, as defined by the GOLD 2010 guidelines. Most patients (80.7%) had no COPD exacerbation in the year prior to randomization and approximately half of the patients (49.7%) were using ICS at baseline.
Table 1. Patient demographics and baseline characteristics.
Lung function
In terms of trough FEV1, IND/GLY was found to be non-inferior to SFC after 26 weeks of treatment as the lower bound 95% CI (0.033 L) in PPS was greater than the protocol-defined non-inferiority margin of −0.060 L. In addition, IND/GLY demonstrated significant improvement in trough FEV1 compared with SFC in the FAS at Week 26 with a least squares mean (LSM) treatment difference of 0.073 L (95% CI, 0.039, 0.107; p < 0.001; ). A significant improvement in trough FEV1 was observed on Day 1 and at Week 12 with IND/GLY versus SFC. Improvement in trough FEV1 at Week 26 with IND/GLY versus SFC was consistent across different subgroups based on age, smoking history, baseline ICS use, COPD severity and COPD exacerbation history (). IND/GLY showed a significant (p < 0.001) improvement in FEV1 AUC0–4h versus SFC on Day 1 (LSM treatment difference, 0.067 L) and at Week 12 (LSM treatment difference, 0.131 L) and Week 26 (LSM treatment difference, 0.122 L; ). The improvement in FEV1 at each time point (5 minutes, 4 hours, 23 hours 15 minutes and 23 hours 45 minutes post-dose) on Day 1 and at Weeks 12 and 26 was significantly in favor of IND/GLY versus SFC. Other lung function parameters such as peak FEV1 during the first 4 hours and trough FVC improvements were significant (p < 0.001) with IND/GLY versus SFC on Day 1, Week 12 and Week 26 ().
Figure 2. Improvement in trough FEV1 on Day 1 and at Weeks 12 and 26 in the Chinese cohort of the LANTERN study (full analysis set) Data are least squares mean (standard error); ***p < 0.001, *p = 0.011 FEV1, forced expiratory volume in one second; IND/GLY, indacaterol/glycopyrronium; SFC, salmeterol/fluticasone.
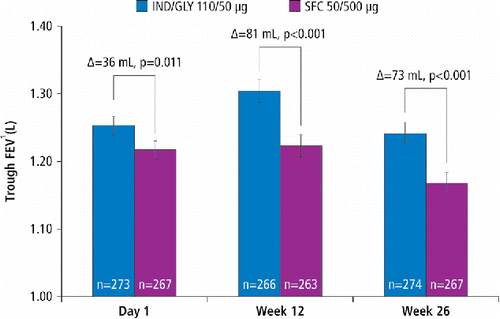
Figure 3. Forest plot of the treatment difference in trough FEV1 (L) at Week 26 across different subgroups of the LANTERN study N1, number of patients analyzed in the QVA149 group; N2, number of patients analyzed in the SFC group; CI, confidence interval; IND/GLY, indacaterol/glycopyrronium; LSM, least square means; SFC, salmeterol/fluticasone.
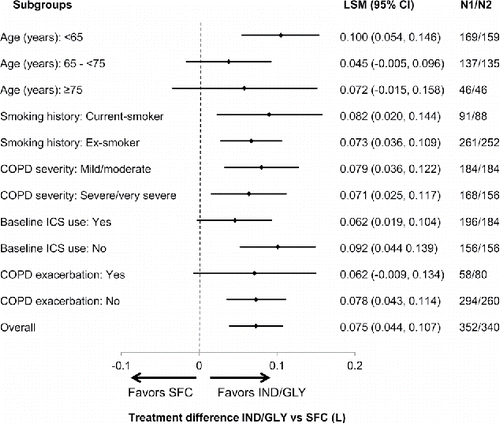
Table 2. Primary and secondary efficacy outcomes in the Chinese cohort of the LANTERN study (full analysis set).
Dyspnea, health status and rescue medication
Improvement in TDI focal score with IND/GLY and SFC at Week 12 (LSM treatment difference, 0.33) and Week 26 (LSM treatment difference, 0.11) was comparable (). Detailed results are available in online supplementary appendix.
Exacerbations
IND/GLY significantly lowered the annualized rate of moderate or severe COPD exacerbations compared with SFC (rate ratio: 0.57; p = 0.015), indicating a risk reduction of 43% (). In addition, in comparison with SFC, IND/GLY significantly prolonged the time to first moderate or severe COPD exacerbation and reduced the risk of such exacerbations by 46% (p = 0.011; ). In patients with a history of COPD exacerbations, the annualized rate of moderate or severe COPD exacerbations (rate ratio: 0.43; p = 0.057) was lower in the IND/GLY group patients compared with the SFC group patients. Conversely, in patients without a history of COPD exacerbation, the reduction in the rate of moderate or severe COPD exacerbations (rate ratio: 0.64; p = 0.124) with IND/GLY and SFC was comparable ().
Table 3. Summary and analysis of COPD exacerbations over 26 weeks in the Chinese cohort of the LANTERN study (full analysis set).
Figure 4. Kaplan–Meier plot of the time to first moderate or severe COPD exacerbation over 26 weeks of treatment (full analysis set; Chinese cohort) CI, confidence interval; HR, hazard ratio; SFC, salmeterol/fluticasone; IND/GLY, indacaterol/glycopyrronium; Subjects who withdraw from the study and do not experience a COPD exacerbation are censored at the date of withdrawal defined as the maximum of the last visit date and date of last study medication; Subjects who complete the study and do not experience a COPD exacerbation are censored at the completion visit date; Rate of exacerbations per year = total number of exacerbations/total number of treatment years; Total number of treatment years = sum of exposure to the study drug expressed in years (days/365.25) exacerbation.
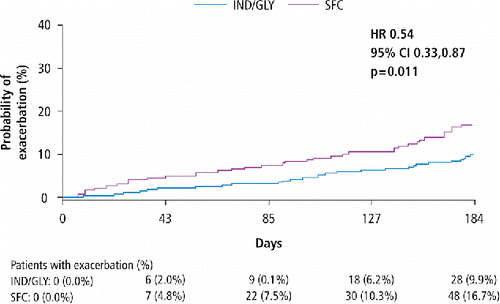
Table 4. Annualized rate of moderate or severe COPD exacerbation by baseline COPD exacerbation history.
Safety
The proportion of patients with any incidence of AEs was lower with IND/GLY (34.6%) than with SFC (43.1%). COPD worsening was the most common AE, reported by 12.8% of patients in the IND/GLY group and 20.5% of those in the SFC group. The incidence of pneumonia (IND/GLY, 0.3%; SFC, 2.0%), upper respiratory tract infection (IND/GLY, 3.0%; SFC, 8.4%) and nasopharyngitis (IND/GLY, 8.1%; SFC, 13.8%) was lower with IND/GLY than with SFC (). Detailed results are available in supplementary appendix.
Table 5. Number (%) of adverse events, serious adverse events and deaths in the Chinese cohort of the LANTERN study (safety set).
The proportion of patients with any SAE was lower with IND/GLY (5.7%) compared with SFC (9.8%). The incidence of CCV SAEs was similar between both treatment groups (both IND/GLY and SFC, 1.3%). All CCV SAEs were adjudicated by an external committee. MACE was reported in 2 patients on IND/GLY versus 1 patient on SFC. Non-MACE was similar between the treatment arms (IND/GLY, 0.7% vs SFC, 1.0%). One case (0.3%) of adjudicated new-onset atrial fibrillation on IND/GLY and 2 cases (0.7%) on SFC were reported during the study. As would be expected in an elderly patient population with co-morbidities, two patients in the IND/GLY treated group died during the treatment period. These deaths were due to cardiovascular causes and were adjudicated as a sudden death and abdominal aortic aneurysm rupture. Based on investigator assessments, sudden death was related to the study medication.
Discussion
The current GOLD strategy focuses on minimizing the magnitude of airflow obstruction, symptoms and risk of exacerbations in the management of patients with COPD Citation(1). For managing the COPD symptoms and risk of exacerbations, the strategy recommends a combination of bronchodilators to achieve maximum therapeutic benefits in the GOLD B–D patient groups. This was the first study to report the efficacy and safety of a LABA/LAMA dual bronchodilator, IND/GLY, versus a LABA/ICS, SFC, in COPD patients from China with a history ≤1 COPD exacerbation in the previous year. The results of the Chinese cohort of the LANTERN study are in agreement with those of the overall study population Citation(15). In this analysis, IND/GLY has been shown to improve lung function and also decrease the risk of exacerbations in patients with moderate to severe COPD from China. Improvement in health status was comparable between both treatment groups. The primary endpoint of non-inferiority in terms of trough FEV1 with IND/GLY versus SFC was met, and superiority after 26 weeks of treatment was also demonstrated. IND/GLY also showed improvements in other lung function outcomes such as FEV1 AUC0–4h, trough FVC and peak FEV1 compared with SFC at Week 26. Moreover, there was a greater improvement in FVC compared to trough FEV1 suggesting that dual bronchodilation may have additional benefits over the single bronchodilator and ICS combination in reducing lung hyperinflation in patients with moderate-to-severe COPD.
Improvements in lung function with IND/GLY in the Chinese patient cohort with moderate-to-severe COPD were in agreement with earlier studies conducted in predominantly Caucasian populations (Citation9, 10).
The Chinese Medical Association first published a national strategy for the management of COPD in 2002 and updates it annually, which encourages physicians to adopt the GOLD strategy for management of COPD Citation(8). As per the strategy, ICS is only recommended for high-risk COPD patients with severe-to-very-severe airflow limitation and/or frequent exacerbations (≥2 exacerbations/year) not sufficiently controlled with long-acting bronchodilators Citation(17). Regardless of this recommendation, ICS is commonly prescribed inappropriately to COPD patients with a moderate risk of exacerbations (Citation18–20). In China, interim results of the INTACT study showed that patients with mild-to-moderate COPD (GOLD 2007) or those belonging to GOLD 2011 Group A (47.6%) and Group B (48.5%) were inappropriately prescribed LABA/ICS Citation(6). Numerous reports have concluded an additional risk (Citation21–25) with long-term use of ICS in patients with COPD. A current report demonstrates lack of effect of ICS therapy in the early systemic inflammatory response to and clinical presentation of COPD exacerbation Citation(26). Moreover, it has been shown that in patients with a low risk of COPD exacerbation, ICS can be withdrawn safely without affecting the efficacy and safety of the long-acting maintenance bronchodilator therapy (Citation27–29). Interim analysis of the INTACT study in China highlighted that LABA/ICS is the most preferred treatment option and that the prescribing pattern of bronchodilators as first-line therapy in low-risk patients is relatively low Citation(6). Hence, physicians need to play an important role in managing patients with COPD by providing the most appropriate bronchodilator therapy, either alone or in combination, for each patient on the basis of disease severity.
COPD exacerbation is the major factor determining healthcare resource utilization and disease-associated morbidity and mortality Citation(30). Although the present study was not powered primarily to evaluate COPD exacerbations, and with only 20 percent of the patients having a history of exacerbations, it is important to know that the results on exacerbations were consistent with improvement in lung function; however this was limited by number of events. Previously, a post hoc analysis of the ILLUMINATE study showed that IND/GLY delayed the time to first COPD exacerbation compared with SFC in a population wherein 19.8% of patients had severe COPD(11). IND/GLY was also found to be cost-effective compared to free combination of LABA and LAMA and LABA/ICS (SFC) in moderate-to-severe COPD patients with low risk of exacerbations (Citation31, 32).
In this study, IND/GLY showed a lower incidence of AEs compared with SFC. The incidence of ICS-associated AEs such as pneumonia and upper respiratory tract infection was higher in the SFC group as has been previously reported in a meta-analysis of COPD patients with ICSs Citation(33). This is also supported by a study wherein a LABA/ICS combination, vilanterol/fluticasone, showed an increased risk of pneumonia than vilanterol alone in patients with a history of COPD exacerbation Citation(33). In addition, the number of AEs leading to hospitalization was higher in the SFC group compared with the IND/GLY group. Overall, the safety of IND/GLY in patients from China was similar to that observed in patients from a Caucasian population Citation(10).
This study had few limitations. First, patients with very severe COPD and a high risk of exacerbations were not included. Second, the relatively short duration of treatment limits the evaluation of certain endpoints such as exacerbations. Third, patient-reported outcomes (PROs) did not synchronize with improvements in lung function; although there was a numerical improvement in PROs with IND/GLY versus SFC, statistical significance was not achieved. This depends on how patients perceive their COPD symptoms impacting PROs (Citation34).
In conclusion, for symptomatic patients with COPD without frequent exacerbations, IND/GLY dual bronchodilation has the potential to be an effective and safe treatment option compared with the LABA/ICS combination in Chinese patients and therefore can be safely used at the same dose as predominantly Caucasian patients. IND/GLY showed a good safety profile and can reduce the healthcare resource utilization by decreasing the risk of COPD exacerbations.
Supplemental_materials_Zhong_et_al.docx
Download MS Word (24.1 KB)Acknowledgments
This study was sponsored by Novartis Pharma AG. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. They take full responsibility for the scope, direction, content of, and editorial decisions relating to, the manuscript, were involved at all stages of development, and have approved the submitted manuscript. The authors would like to thank the patients and staff at the participating centers in the study.
Declaration of interest statement
N Zhong, C Wang, X Zhou and N Zhang have no conflicts related to this manuscript. M Humphries, L Wang, F Patalano and D Banerji are the employees of the study sponsor and have no other conflicts. The authors also thank Santanu Sannigrahi and Kevin Roche of Novartis for assistance in writing this manuscript. Writing support was funded by the study sponsor.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2015. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Feb18.pdf
- Cheng XLJ, Zhang Z. The relationship between smoking and the incidence of COPD. Chin J Tuberc Respir Dis. 1999;22(5):290–292.
- Fang X, Wang X, Bai C. COPD in China: the burden and importance of proper management. Chest. 2011;139(4):920–929.
- Li Q, Hsia J, Yang G. Prevalence of smoking in China in 2010. N Engl J Med. 2011;364(25):2469–2470.
- Yu WC, Fu SN, Tai EL, Yeung YC, Kwong KC, Chang Y, et al. Spirometry is underused in the diagnosis and monitoring of patients with chronic obstructive pulmonary disease (COPD). Int J Chron Obstruct Pulmon Dis. 2013;8:389–395.
- Huang K, Wang C, Han J, Zheng J, Dai L, INTACT Study Investigators. Current maintenance treatment of chronic obstructive pulmonary disease in China. Eur Respir J. 2014;44(Suppl 58):P4108.
- Zhang L, Lou P, Zhu Y, Chen P, Zhang P, Yu J, et al. Impact of risk factors, activities and psychological disorders on the health of patients with chronic obstructive pulmonary disease in China: a cross-sectional study. BMC Public Health. 2013;13:627.
- Respiratory Diseases Branch of Chinese Medical Association. A national guideline for the diagnosis m, and prevention of chronic obstructive pulmonary disease in China (in Chinese). Chin J Tuberc Respir Dis. 2007;30:9–317.
- Bateman ED, Ferguson GT, Barnes N, Gallagher N, Green Y, Henley M, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42(6):1484–1494.
- Vogelmeier CF, Bateman ED, Pallante J, Alagappan VK, D'Andrea P, Chen H, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51–60.
- Banerji D, Fedele MJ, Chen H, Kim HJ. Dual bronchodilation with QVA149 reduces COPD exacerbations: results from the IGNITE program (OS36: COPD 3). Respirology. 2013;18:69–70.
- Martin A, Badrick E, Mathur R, Hull S. Effect of ethnicity on the prevalence, severity, and management of COPD in general practice. Br J Gen Pract. 2012;62(595):e76–81.
- Asai K, Minakata, Y., Hirata, K., Fukuchi, Y., Kitawaki, T., Ikeda, K., Banerji, D. QVA149 once-daily is safe and well tolerated and improves lung function and health status in Japanese patients with COPD: the ARISE study. Eur Respir J. 2013;42(57 Suppl):3392.
- Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandstrom T, Taylor AF, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209.
- Zhong N, Wang C, Zhou X, Zhang N, Humphries M, Wang L, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J COPD. 2015;10.
- Nannini LJ, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta-agonist in one inhaler versus long-acting beta-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007(4):CD006829.
- GOLD. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2014. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2014_Jun11.pdf
- Suissa S, Barnes PJ. Inhaled corticosteroids in COPD: the case against. Eur Respir J. 2009;34(1):13–16.
- de Miguel-Diez J, Carrasco-Garrido P, Rejas-Gutierrez J, Martin-Centeno A, Gobartt-Vazquez E, Hernandez-Barrera V, et al. Inappropriate overuse of inhaled corticosteroids for COPD patients: impact on health costs and health status. Lung. 2011;189(3):199–206.
- Rupert Jones NF, Marc Miravitlles, Guy Bruselle, Kevin Gruffydd-Jones, Mike Baldwin, Rebecca Stewart, Anna Rigazio, Annie Burden and David Price. Inappropriate prescriptions following initial COPD diagnosis. Eur Respir J. 2013;42(57 Suppl):P2391.
- DiSantostefano RL, Sampson T, Le HV, Hinds D, Davis KJ, Bakerly ND. Risk of pneumonia with inhaled corticosteroid versus long-acting bronchodilator regimens in chronic obstructive pulmonary disease: a new-user cohort study. PLoS One. 2014;9(5):e97149.
- Weatherall M, James K, Clay J, Perrin K, Masoli M, Wijesinghe M, et al. Dose-response relationship for risk of non-vertebral fracture with inhaled corticosteroids. Clin Exp Allergy. 2008;38(9):1451–1458.
- Herth FJ, Bramlage P, Muller-Wieland D. Current perspectives on the contribution of inhaled corticosteroids to an increased risk for diabetes onset and progression in patients with chronic obstructive pulmonary disease. Respiration 2015;89(1):66–75.
- Cumming RG, Mitchell P, Leeder SR. Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med. 1997;337(1):8–14.
- Kim JH, Park JS, Kim KH, Jeong HC, Kim EK, Lee JH. Inhaled corticosteroid is associated with an increased risk of TB in patients with COPD. Chest. 2013;143(4):1018–1024.
- Crisafulli E, Guerrero M, Menendez R, Huerta A, Martinez R, Gimeno A, et al. Inhaled corticosteroids do not influence the early inflammatory response and clinical presentation of hospitalized subjects with COPD exacerbation. Respir Care. 2014;59(10):1550–1559.
- Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294.
- Rossi A, Guerriero M, Corrado A, Group OAS. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15:77.
- Rossi A, van der Molen T, Olmo RD, Papi A, Wehbe L, Quinn M, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548–1556.
- Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19(116):113–118.
- Price D, Keininger D, Baldwin M, Mezzi K, Asukai Y, Ställberg B. Cost-effectiveness of the LABA/LAMA dual bronchodilator QVA149 in a Swedish setting. Eur Respir J. 2013;42(Suppl 57):P280.
- Geitona M, Kousoulakou H, Kalogerolpoulou M, Mistiki E, Steiropoulos P. Cost effectiveness of the fixed combination of indacaterol/glycopyrronium versus salmeterol/ fluticasone and tiotropium in the management of patients with COPD in Greece. J Health Policy Oucome Res. 2015;1:56–65.
- Crim C, Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, et al. Pneumonia Risk with Inhaled Fluticasone Furoate and Vilanterol Compared with Vilanterol Alone in Patients with COPD. Ann Am Thorac Soc. 2015;12(1):27–34.
- Weldam SW, Lammers JW, Heijmans MJ, Schuurmans MJ. Perceived quality of life in chronic obstructive pulmonary disease patients: a cross-sectional study in primary care on the role of illness perceptions. BMC Fam Pract. 2014;15:140.