ABSTRACT
There is little long-term follow-up data concerning the association between past pulmonary tuberculosis (TB), airway obstruction and mortality. We aimed to analyse a national health examination survey data from 6701 adult Finns undergoing spirometry between 1978 and 1980 (follow-up through 2013). We identified TB either through a disease history or by a TB-indicative scar on a chest x-ray. We specified obstruction using the lower limit of normal (LLN) and classified severity using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages 1–4. After adjusting for smoking and other confounders, past TB associated with obstruction. Compared to non-TB patients, the adjusted odds ratio (OR; 95% CI) of obstruction reached 2.21 (1.52–3.21) among patients with a scar recorded by one radiologist, 2.48 (1.63–3.78) when recorded by both radiologists and 4.59 (2.86–7.37) among patients with a disease history. Among those with neither past TB nor obstruction, with past TB only, with an obstruction only and with both, we found hazard ratios (HRs; 95% CIs) for subsequent mortality of 1.00 (reference), 1.11 (1.03–1.20), 1.62 (1.31–2.00) and 1.77 (1.45–2.16), adjusted for age, gender, smoking, body mass index (BMI), physical activity, education and general health. In conclusion, past TB strongly determines obstruction, although on its own quite weakly predicts premature death. TB and obstruction combined predict an additive mortality pattern.
Introduction
Tuberculosis (TB) and chronic obstructive pulmonary disease (COPD) represent worldwide problems causing unnecessary deaths and resulting in high costs when left untreated. Therefore, the World Health Organization (WHO) set similar goals for both diseases: to prevent them, to improve treatment outcomes and to reduce mortalityCitation(1,2). COPD manifests as a slowly progressive inflammatory disease, while TB is an infectious disease afflicting one-third of the world´s population and affecting roughly 9 million annually via active infectionsCitation(1,2). Similar risk factors predispose individuals to TB and COPD: low socioeconomic status, being male, smoking, co-morbid diseases and advancing ageCitation(1–4). In addition, both COPD and active TB cause early deathsCitation(2,5–7).
On the one hand, COPD and smoking predispose individuals to active TB and possibly to scars indicating TB on x-raysCitation(4,7–9). On the other hand, a history of active TB or a scar indicating TB on a chest x-ray represent independent risk factors for airway obstructionCitation(10–17). The level of active TB or scarring affects the degree of obstructionCitation(10–12,18).
However, the long-term follow-up data concerning the association between TB, chronic obstruction and all-cause mortality is scarce. In our study, we aimed to assess these rarely reported associations during a 35-year follow-up time period. Thus, we evaluated how a history of pulmonary TB or a scar indicating TB on a chest x-ray associated with obstruction and mortality among Finnish adults. When our material was collected in the 1970s, TB incidence stood at a high level (50–80 cases per 100 000 population), numbers comparable with those currently found in developing countriesCitation(1,3,19).
Materials and methods
Study population and baseline examination
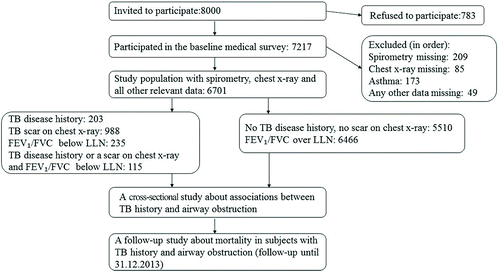
The Mini-Finland Health Survey was a population study based on a stratified two-stage cluster sample running between 1978 and 1980 (https://www.thl.fi/en/web/thlfi-en/research-and-expertwork/population-studies/finnish-mobile-clinic/mini-finland-health-survey). In the first stage, 40 nationally representative clusters were selected, whereby a selected cluster represented a specific area. Areas were, then, stratified according to the degree of urbanisation and occupation of the residents. The second stage consisted of selecting a representative sample (3637 men and 4363 women) of Finnish adults aged 30 or older from the Social Insurance Institution population register from these 40 areas, whereby each individual in the population had an equal probability of selection. After excluding subjects with asthma, our study included 3125 men and 3576 women who participated in a comprehensive health examination, underwent spirometry and a chest x-ray and for whom all relevant health information was collected through clinical examinations, interviews and questionnaires ()Citation(20,21).
Measurements and definition of determinants
Specially trained laboratory technicians followed standard guidelines and instructions for performing spirometries using a Vitalograph spirometer (Vitalograph Ltd., Buckingham, United Kingdom). The intention was that at least two spirometry curves that were as consistent as possible would be recorded for each participant. Spirometry was performed without bronchodilation. The quotient FEV1/FVC was calculated using the highest readings for FEV1 and FVC from the technically accepted measurements for the body temperature and pressure saturated with water vapour (BTPS) valuesCitation(21–23).
Individual results were computed using the SAPALDIA reference values for the subject's corresponding age, gender and height Citation(24). SAPALDIA reference values were determined for Swiss adults aged 18–60 years and can be extrapolated up to 75 years. In subjects older than 75 years, the statistical formulae may cause a bias in the valuesCitation(24,25).
Those with FEV1/FVC below the lower limit of normal (LLN) were identified as having an obstruction Citation(22), while others had no obstruction. The obstructed subjects were staged by severity using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages 1–4 Citation(2).
Height and weight were measured using standard methodologies. Body mass index [BMI, weight (kg)/height² (m²)] was computed as a measure of relative weight. The basic questionnaire elicited the general health status, leisure physical activity and education. General health was categorised as: good, moderate and poor. Questions about the duration, intensity and frequency of physical activity assessed leisure physical activity. This activity was categorised as inactive (little physical exercise), occasionally active (exercise in connection with some hobbies or irregularly) or regularly active (regular exercise). The level of education was categorised based on the years of schooling, consisting of basic (<8 years), intermediate (8–12 years) and higher (>12 years). A standard interview elicited smoking habits, categorised as non-smokers, former smokers and current smokers of either 1–19 or ≥20 cigarettes/day. Former smokers had quit smoking at least 1 month prior to the survey. Current smokers smoked at least one cigarette, cigar or pipe daily or almost daily during the year preceding the surveyCitation(20,21,23).
The basic questionnaire included questions about chronic disease symptoms, history of any chronic disease diagnosed by a physician, overall health status and lifestyle. Those with any abnormal findings in the examination or questionnaires were asked to participate in a standardised physical examination by a specially trained physician. We also analysed prescription medications taken by subjectsCitation(20,21,23).
Diagnosis and classification of TB
The basic questionnaire elicited TB history using the following questions: ‘Have you had, according to a physician's diagnosis, pulmonary (lung) TB? Have you ever been hospitalised for it? Have you ever received medications for it Citation(20)?’
Chest radiographs with anteroposterior and lateral views were taken in a mobile unit at a constant 120 kV power, using automatic exposure control and a 135-cm film focus. If the technical quality was low, the radiography was later repeated Citation(21). Two experienced radiologists working on the study analysed all chest x-rays and independently assessed the scars or local fibrosis representing earlier TB. We computed the kappa coefficient for their diagnostic agreement among subjects without a TB history () Citation(26).
Table 1. Agreement between two radiologists in detecting a local scar or fibrosis on a chest x-ray indicative of past TB.
Based on this analysis, we classified past TB hierarchically as follows: a disease history (TB treated in hospital or with TB medications), a TB-indicative scar on a chest x-ray identified by one or both radiologists (no TB treated in hospital and no history of TB medications), past TB (either treated TB or a scar identified by chest x-ray) and no TB. The category of no TB included subjects without any TB disease history or radiological scar.
Follow-up
We followed mortality in the cohort from Statistics Finland using the subjects´ individual identification numbers to track participants from baseline examination through 31 December 2013 Citation(27). All-cause mortality included all deaths in the study population during the follow-up time period. The outcome of respiratory deaths included all main causes of deaths registered for that disease category according to the eighth, ninth and tenth revisions of the International Classification of Diseases (ICD-8, ICD-9 and ICD-10, respectively). The category of natural deaths included all deaths other than those resulting from trauma and poisoning according to ICD-8, ICD-9 and ICD-10.
Statistical methods
We analysed the cross-sectional associations between baseline characteristics, past TB and obstruction using logistic regression, expressing results as adjusted odds ratios (ORs) with 95% confidence intervals (CIs). We used Cox's proportional hazards regression model and estimated adjusted hazard ratios (HRs) using 95% CIs. The effect modification was analysed by entering the first-degree interaction terms one-by-one into the models. We tested the statistical significance of the interactions using likelihood ratio statistics expressed with exact p-values. The formula for analysing the SAPALDIA reference values may create a bias among subjects older than 75; therefore, we assumed all such subjects were 75 years old in the statistical formulae. We performed all analyses using the SAS System for Windows, version 9.1 (SAS Institute, Inc., Cary, NC).
Ethical aspects
The Mini-Finland Health Survey predated current legislation on ethics in medical research. However, all participants were fully informed about the study, they participated in the study voluntarily and the use of their information for medical research was explained to them. Agreeing to participate in the baseline health examination was taken to indicate informed consent. The register authority Statistics Finland approved the linkage of national mortality data to the survey data used here.
According to a statement from the Medical Ethics Committee of the Hospital District of Helsinki and Uusimaa in Finland (June 2013), this study does not fall under the purview of laws regarding medical research. Thus, the study protocol does not violate any ethical considerations or standards.
Results
describes the prevalence of past TB by age and gender. We found that the proportion of TB cases increased with age. Out of the 203 subjects with a disease history of TB, 180 received treatment in hospital. Seven subjects were taking TB medications at the time of the survey.
Table 2. The prevalence of past TB by age and gender in the study population between 1978 and 1980.
Age, male gender, smoking history, poor general health and less than 8 years of education closely associated with both past TB and obstruction (). After adjusting for these factors, leisure time physical activity and BMI, a close association remained between past TB and obstruction. None of the baseline characteristics appeared to modify the association between past TB and obstruction. For example, among never-smokers, former smokers and current smokers, we found adjusted ORs (95% CIs) for the associations between past TB and obstruction of 2.89 (1.65–5.08), 3.44 (1.84–6.41) and 2.18 (1.36–3.50) respectively (for the interaction term of TB and smoking, p = 0.66).
Table 3. Odds ratio (OR) for the presence of past TB and airway obstruction (FEV1/FVC below the limit of LLN, obstructed subjects categorised further as GOLD stages 1–4) in the study population in a multivariate model.
By the end of 2013, 3824 (57%) subjects had died (). Obstruction and scars indicating TB predicted mortality independently of each other and the potential confounding factors. However, there was no association between a disease history of TB and mortality. Yet, the interaction term of past TB and obstruction (p = 0.80) remained far from statistically significant, indicating that past TB and obstruction did not affect one another. Among those with neither past TB nor obstruction, with past TB only, with obstruction only and with both, we found HRs (95% CIs) of 1.00 (reference), 1.11 (1.03–1.20), 1.62 (1.31–2.00) and 1.77 (1.45–2.16), respectively, adjusted for age, gender, smoking, BMI, physical activity, education and general health.
Table 4. Association between past TB, airway obstruction (FEV1/FVC below the limit of LLN, obstructed subjects categorised further as GOLD stages 1–4) and all-cause mortality in a multivariate model.
Respiratory diseases were a cause of death in 265 cases (6.9% of all 3824 deaths). After adjusting for all of the baseline characteristics, we found HRs (95% CIs) for respiratory deaths in subjects with neither TB nor obstruction, with TB only, with obstruction only and with both TB and obstruction of 1.00 (reference), 1.49 (1.11–1.98), 5.38 (3.28–8.81) and 5.05 (3.11–8.22), respectively. The corresponding HRs (95% CIs) for natural deaths were 1.00 (reference), 1.11 (1.03–1.21), 1.62 (1.30–2.01) and 1.82 (1.49–2.23), respectively; 101 deaths (2.6% of all-cause mortality) resulted from traumatic injuries and poisonings.
Discussion
In this population-based study, we analysed the associations between past TB, obstruction and mortality in a Finnish sample followed for 35 years. We observed that past TB, either a previously treated disease or a scar indicating TB on a chest radiograph, emerged as a strong risk factor for obstruction. Previously treated TB did not predict premature deaths, although a scar on a chest x-ray diagnosed by two radiologists did. Past TB and obstruction predicted mortality with an additive pattern.
A self-reported history of TB and earlier treated TB both associated with obstructionCitation(7,10–13,16,17), including among never-smokers Citation(14). After active TB, the increased loss of lung function continues for years and depends on the degree and the time of presentation with TB Citation(18), as well as the level of obstruction associated with the grade of changes observed on the chest x-rayCitation(10,12). In addition, an association exists between the elevated risk for obstruction and earlier TB presented as inactive scars on a chest x-rayCitation(14,15) and latent TB Citation(11), which we take as comparable to scars observed on a chest x-ray. However, active treated TB caused significantly more obstruction than latent TB Citation(11), while an association exists between the extent of TB on the original chest x-ray and obstruction Citation(10). We showed that a scar indicating TB on a chest x-ray associates with obstruction, yet the association appeared stronger after a history of disease. Smoking history, however, was not a confounding factor nor did it interact additively. Presumably, a scar on a chest x-ray indicates less active TB than treated disease, thus, leading to less obstruction.
We assume that regardless of other factors, such as a history of smoking or a particular TB treatment, mycobacterium TB causes inflammation-inducing obstruction. The more severe the TB disease is, the greater the inflammation and obstruction it causes. The process generating chronic obstruction after TB is presumably multifactorial, even though the mechanism remains unclearCitation(7,28). TB´s endobronchial involvement may produce local obstruction and fibrosis, parenchymal lung destruction might decrease compliance in the lungs and cause peripheral airway collapse and ventilation–perfusion relationships may become abnormalCitation(7,13,28). In addition, some activities resembling active TB persist in inactive TB Citation(29), potentially causing tissue changes.
COPD and active TB both cause early deathsCitation(2,8,23)—TB in the short term Citation(5) and up to decades after treatmentCitation(6,30), while COPD primarily results in long-term mortality Citation(2). However, the interaction of TB and chronic obstruction related to early death remains relatively unstudied. Subjects with COPD died more frequently than those without COPD in the first year after a diagnosis of active TB in Sweden Citation(8), despite researchers considering the role of other COPD-related diseases on mortality. We found that past TB and obstruction increased mortality rates, resulting in an additive effect on premature death.
Few long-term follow-up studies on TB mortality exist. In particular, little data exist on mortality after inactive disease and among those with scars on an x-ray indicating a history of TB. Two studies with follow-up periods as long as 30 years found elevated all-cause mortality rates: one study after active TB in Denmark Citation(6) and one examining a history of TB diagnosed from scars on x-rays in Norway Citation(30). Both studies proved comparable to ours. In a Danish study, they detected an increased risk for respiratory mortality in a cause-specific analysis Citation(6), similar to our findings as well. In a multivariate model, we found slightly elevated all-cause death rates among subjects with a scar on a chest x-ray, but not among those with a TB disease history. Our study consisted of too few subjects with a disease history to achieve statistical significance. However, the increased mortality rate among those with a scar on an x-ray was statistically significant, which may be coincidental. Alternatively, a continuous process or chronic inflammation may have resulted in deaths associated with long-term inactive disease.
Among the Finnish population, x-ray-based screening for TB served as the norm from the 1940s until 1990 Citation(19). Both study radiologists who analysed chest x-rays in the Mini-Finland Health Survey participated in earlier mass screenings and, therefore, proved highly experienced in the x-ray-based diagnosis of TB. In previous studies, researchers found that two experienced doctors reliably diagnosed TB using chest x-rays Citation(31) when they diagnostically agreed Citation(32), and satisfactorily after expert analysis Citation(33). In subsequent studies, TB was a culture-controlled active disease. In addition, the diagnostic reliability of diagnosing TB from x-rays increased with a high TB incidence Citation(32), representative of the Finnish case during our study's data collectionCitation(1,3,19). In our study, the same radiologists analysed over 7000 chest x-rays independently of one another, which represents a strength to our study.
In earlier studies, the inter-reader kappa ranged between 0.50 and 0.80 when diagnosing any abnormality indicating TB on a chest x-rayCitation(15,31,32,34,35). The kappa was higher when the discordant findings were re-analysed Citation(15), when the tuberculin skin test or interferon-γ test was positive Citation(36) and when the diagnostic criteria for TB on a chest x-ray were agreed uponCitation(31,32,36). In our study, we considered a moderate kappa between radiologists indicative of an overestimation of TB diagnoses, and therefore, analysed separately the diagnoses made by only one or both radiologists. There was indeed a difference in the associations with past TB, obstruction and mortality depending on whether one or both radiologists identified the scars. Logically, the diagnosis of past TB seems more accurate when two radiologists are in agreement.
Our study sample featured a high participation rate representative of the adult Finnish population, and was examined by a well-educated and experienced team with a 35-year follow-up time period during which 48% of the sample diedCitation(20,21,23). While TB incidence currently remains low in Finland, our results carry continued relevance for countries with a high incidence. Only a few subjects presented with a history of TB and obstruction of GOLD stages 1 and 4. However, we followed a representative number of subjects with scars on chest x-rays and obstructions of GOLD stages 2 and 3 with reasonable results. In addition, our material constituted a relatively homogenous population with fewer confounding factors compared to more complex TB situations (e.g., TB among HIV-positive subjects, TB associated with immigration and drug-resistant TB)Citation(1,3,4,20).
The Finnish Tuberculosis Agency oversaw further testing of TB cases diagnosed in the Mini-Finland Health Survey, but we were unable to access these results. Radiologists formulated together the diagnostic criteria for scarring indicating past TB, but these criteria were not available to us. Furthermore, the diagnosed scars were not categorised more specifically. Unfortunately, no bronchodilation was performed when measuring spirometry. Therefore, we may have misclassified some subjects with reversible obstruction as experiencing chronic obstruction. Moreover, the baseline characteristics, such as smoking habits and physical activity, may have changed during the follow-up period in the study population, possibly affecting some of our results.
Conclusions
In conclusion, we found that past TB and obstruction carried an additive effect on mortality. In addition, a history of TB disease and a scar on an x-ray associated with obstruction, including among never-smokers. Yet, the mechanism causing obstruction after TB remains unclear and warrants further research. In addition, the possibility of developing COPD should be kept in mind after TB treatment even when no history of smoking exists.
Funding
Support from the Foundation of the Finnish Anti-Tuberculosis Association, the Tuberculosis Foundation of Tampere and the Research Foundation of Pulmonary Diseases in Finland, as well as a Doctoral Candidate Position in the University of Helsinki/Hospital District of Helsinki and Uusimaa (from June 2016) awarded to the first author allowed for the write-up of our analysis.
Declaration of interest
The corresponding author Tiina Mattila completed this study through financial support from various sources between 2012 and 2016. Statistical work in this study was completed by the National Institute for Health and Welfare in Helsinki, Finland as a function of that agency's work. All other writers completed the work on this study as a function of their regular duties. The corresponding author and none of the other authors have any relevant conflicts of interest.
Acknowledgments
The authors wish to thank Vanessa Fuller for English-language revisions to this manuscript.
References
- World Health Organization (WHO). Global Tuberculosis report 2015. Feb. 29, 2016. Available from: http://www.who.int/tb/publications/global_report/en/.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Feb. 29, 2016. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html, http://www.goldcopd.org/guidelines-pocket-guide-to-copd-diagnosis.html (accessed December 2015).
- The National Tuberculosis Program of Finland from the year 2013 (summary in English, the whole text available only in Finnish). Feb. 29, 2016. Available from: http://urn.fi/URN:ISBN:978-952-00-3414-6.
- Leung CC, Yew WW, Chan CK, Tam CM, Lam CW, Chang KC, et al. Smoking and tuberculosis in Hong Kong. Int J Tuberc Lung Dis 2003; 7(10):980–986.
- Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis 2011; 15(7):871–885.
- Christensen AS, Roed C, Andersen PH, Andersen AB, Obel N. Long-term mortality in patients with pulmonary and extrapulmonary tuberculosis: a Danish nationwide cohort study. Clin Epidemiol 2014; 6:405–421.
- Chakrabarti B, Calverley PM, Davies PD. Tuberculosis and its incidence, special nature, and relationship with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2007; 2(3):263–272.
- Inghammar M, Ekbom A, Engström G, Ljungberg B, Romanus V, Löfdahl CG, et al. COPD and the risk of tuberculosis—a population-based cohort study. PLoS One 2010; 13; 5(4):e10138.
- Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med 2007; 167(4):335–342.
- Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir Med 1989; 83(3):195–198.
- Pasipanodya JG, Miller TL, Vecino M, Munguia G, Garmon R, Bae S, et al. Pulmonary impairment after tuberculosis. Chest 2007; 131(6):1817–1824.
- Rhee CK, Yoo KH, Lee JH, Park MJ, Kim WJ, Park YB, et al. Clinical characteristics of patients with tuberculosis-destroyed lung. Int J Tuberc Lung Dis 2013; 17(1):67–75.
- Snider GL, Doctor L, Demas TA, Shaw AR. Obstructive airways disease in patients with treated pulmonary tuberculosis. Am Rev Respir Dis 1971; 103(5):625–640.
- Lee SW, Kim YS, Kim D-S, Oh Y-M, Lee S-D. The risk of obstructive lung disease by previous pulmonary tuberculosis in a country with intermediate burden of tuberculosis. J Korean Med Sci 2011; 26(2):268–273.
- Lam KB, Jiang CQ, Jordan RE, Miller MR, Zhang WS, Cheng KK, et al. Prior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Chest 2010; 137(3):593–600.
- Amaral AF, Coton S, Kato B, Tan WC, Studnicka M, Janson C, et al. Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J 2015; 46(4):1104–1112. doi: 10.1183/13993003.02325-2014. [ Published Online First June 25, 2015].
- Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration 2013; 86(1):76–85. doi: 10.1159/000350917. [ Published Online First May 3, 2013.
- Ross J, Ehrlich RI, Hnizdo E, White N, Churchyard GJ. Excess lung function decline in gold miners following pulmonary tuberculosis. Thorax 2010; 65(11):1010–1015.
- Tala-Heikkilä M. Tuberkuloosi Suomessa. Aikakauskirja Duodecim 2003; 119(17):1621–1628. ( in Finnish).
- Aromaa A, Heliövaara M, Impivaara O, Knekt P, Maatela J, Joukamaa M, et al. Health, functional limitations and need for care in Finland. Basic results from the Mini-Finland Health Survey (in Finnish with English Summary). Helsinki and Turku, Publications of the Social Insurance Institution 1989.
- Aromaa A, Heliövaara M, Knekt P, Reunanen A, Impivaara O, Maatela J. Cardiovascular and Respiratory Survey Methods. Part 2 (in Finnish with English summary). Helsinki and Turku: Publications of the Social Insurance Institution, 1985.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26(5):948–968.
- Mattila T, Vasankari T, Kanervisto M, Laitinen T, Impivaara O, Rissanen H, et al. Association between all-cause and cause-specific mortality and the GOLD stages 1–4: a 30-year follow-up among Finnish adults. Respir Med 2015; 109(8):1012–1018.
- Brändli O, Schindler C, Künzli N, Keller R, Perruchoud AP. Lung function in healthy never smoking adults: reference values and lower limits of normal of a Swiss population. Thorax 1996; 51(3):277–283.
- Kertsjens HA, Rijcken B, Schouten JP, Postma DS. Decline of FEV1 by age and smoking status: facts, figures, and fallacies. Thorax 1997; 52(9):820–827.
- Fleiss JL. Statistical Methods for Rates and Proportions. 2nd ed. New York: Wiley; 1981.
- Statistics Finland. 2008. The key between the short list in the time series of the causes of death since 1969 and the earlier classifications. Available from: http://www.stat.fi/index_en.html, http://www.stat.fi/til/ksyyt/2005/ksyyt_2005_2006-10-31_luo_002.html. (accessed July 30, 2016)
- Jordan TS, Spencer EM, Davies P. Tuberculosis, bronchiectasis and chronic airflow obstruction. Respirology 2010; 15(4):623–628.
- Barry CE 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 2009; 7(12):845–855. [ Published Online First October 26, 2009].
- Liestøl K, Tretli S, Tverdal A, Maehlen J. Tuberculin status, socioeconomic differences and differences in all-cause mortality: experience from Norwegian cohorts born 1910–49. Int J Epidemiol 2009; 38(2):427–434.
- Zellweger JP, Heinzer R, Touray M, Vidondo B, Altpeter E. Intra-observer and overall agreement in the radiological assessment of tuberculosis. Int J Tuberc Lung Dis 2006; 10(10):1123–1126.
- Abubakar I, Story A, Lipman M, Bothamley G, van Hest R, Andrews N, et al. Diagnostic accuracy of digital chest radiography for pulmonary tuberculosis in a UK urban population. Eur Respir J 2010; 35(3):689–692.
- Cohen R, Muzaffar S, Capellan J, Azar H, Chinikamwala M. The validity of classic symptoms and chest radiographic configuration in predicting pulmonary tuberculosis. Chest 1996; 109(2):420–423.
- Graham S, Das GK, Hidvegi RJ, Hanson R, Kosiuk J, Al ZK, et al. Chest radiograph abnormalities associated with tuberculosis: reproducibility and yield of active cases. Int J Tuberc Lung Dis 2002; 6(2):137–142.
- Balabanova Y, Coker R, Fedorin I, Zakharova S, Plavinskij S, Krukov N, et al. Variability in interpretation of chest radiographs among Russian clinicians and implications for screening programmes: observational study. BMJ 2005; 331(7513):379–382.
- Joshi R, Patil S, Kalantri S, Schwartzman K,Menzies D,Pai M. Prevalence of abnormal radiological findings in health care workers with latent tuberculosis infection and correlations with T cell immune response. PLoS ONE 2007; 2(8):e805. doi: 10.1371/journal.pone.0000805.