ABSTRACT
There is growing evidence that emphysema on thoracic computed tomography (CT) is associated with poor exercise tolerance in COPD patients with only mild-to-moderate airflow obstruction. We hypothesized that an excessive ventilatory response to exercise (ventilatory inefficiency) would underlie these abnormalities. In a prospective study, 19 patients (FEV1 = 82 ± 13%, 12 Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 1) and 26 controls underwent an incremental exercise test. Ventilatory inefficiency was assessed by the ventilation (E)/CO2 output (
CO2) nadir. Pulmonary blood flow (PBF) in a submaximal test was calculated by inert gas rebreathing. Emphysema was quantified as % of attenuation areas below 950 HU. Patients typically presented with centrilobular emphysema (76.8 ± 10.1% of total emphysema) in the upper lobes (upper/total lung ratio = 0.82 ± 0.04). They had lower peak oxygen uptake (
O2), higher
E/
CO2 nadir, and greater dyspnea scores than controls (p < 0.05). Lower peak
O2 and worse dyspnea were found in patients with higher
E/
CO2 nadirs (≥30). Patients had blunted increases in PBF from rest to iso-
O2 exercise (p < 0.05). Higher
E/
CO2 nadir in COPD was associated with emphysema severity (r = 0.63) which, in turn, was related to reduced lung diffusing capacity (r = −0.72) and blunted changes in PBF from rest to exercise (r = −0.69) (p < 0.01). Ventilation “wasted” in emphysematous areas is associated with impaired exercise ventilatory efficiency in mild-to-moderate COPD. Exercise ventilatory inefficiency links structure (emphysema) and function (DLCO) to a key clinical outcome (poor exercise tolerance) in COPD patients with only modest spirometric abnormalities.
Introduction
There is mounting evidence that COPD patients with largely preserved FEV1 may present with substantial burden of emphysema on thoracic computed tomography (CT) Citation(1–5). These abnormalities have been associated with meaningful patient-centered outcomes, including breathlessness Citation(1,3) and poor exercise tolerance Citation(1,3,6–8). Elucidation of the mechanisms underlying these associations might provide novel insights into the function–structure relationship prior to the development of the severe mechanical constraints that characterize patients with advanced COPD Citation(9).
In this context, it is noteworthy that both exertional breathlessness and exercise intolerance have been consistently associated with an excessive ventilatory response to exercise in COPD patients (or even healthy smokers) with largely preserved FEV1 Citation(10–15). The physiological bases of the so-called “ventilatory inefficiency” stem from an enlarged physiological dead space, i.e., a high dead space (VD)/tidal volume (VT) ratio reflecting increased “wasted” ventilation Citation(15). Of note, (Enghoff's) VD/VT is sensitive to any pathophysiological mechanism leading to increased arterial–alveolar CO2 difference, including alveolar ventilation/perfusion (VA/Q′) heterogeneity Citation(16). Emphysema, in particular, is associated with enlarged airspaces, loss of alveolar attachments to small airways, and disturbed microvascular perfusion Citation(17,18), which jointly induce marked VA/Q′ mismatching, i.e., either low or high VA/Q′ ratios Citation(16,19–22). It is therefore conceivable that under the magnifying effects of increased ventilation and cardiac output (exercise), patients with greater burden of emphysema and more disturbed pulmonary hemodynamics would present with particularly poor ventilatory efficiency. Confirmation of these premises would support the notion that early emphysema provides the structural bases for the increased “wasted” ventilation previously found in patients at the beginning of the spectrum of COPD severity Citation(10–15).
The present study, therefore, aimed to determine the structural correlates of exercise ventilatory inefficiency in COPD patients with mild-to-moderate airflow obstruction. We specifically hypothesized that CT measurements of emphysema would be associated with blunted increases in PBF from rest to exercise, worse ventilatory inefficiency, greater activity-related dyspnea and lower exercise tolerance.
Methods
Subjects
Clinical and physiological data from 54 patients with mild-to-moderate COPD (smoking history of at least 10 pack-years, post-bronchodilator FEV1/FVC < 0.7 and FEV1 ≥ 60% predicted) were reviewed for potential study inclusion. All patients had established clinical diagnosis of COPD, being followed by respirologists. Disease severity was classified according to the latest Global Initiative for Chronic Obstructive Lung Disease (GOLD)'s recommendation Citation(23). Twenty-six sedentary volunteers were recruited by advertisement from our hospital workforce. They were free from pulmonary, cardiac, or metabolic conditions as established by their medical records that could contribute to dyspnea or exercise limitation. All participants had preserved left ventricular ejection fraction (> 40%) as assessed by echocardiography, and they were considered “physically inactive” according to the Baecke's questionnaire Citation(24). Co-morbidity burden was determined by the combined 19-disease Charlson index Citation(25). Dyspnea was assessed by the modified Medical Research Council (mMRC) questionnaire. All participants signed an informed consent, which had been previously approved by the Queen's University and Affiliated Teaching Hospitals Research Ethics Board (DMED-1701–14).
Measurements
Lung function
Spirometry, lung diffusing capacity for carbon monoxide (DLCO), and static lung volumes were evaluated according to current guidelines (1085 ELITE DTM, Medical Graphics). Controls performed only spirometry.
CT imaging
Thoracic CT scans were acquired only in the patient group, i.e., for ethical reasons, controls did not undergo imaging assessment. Scans were obtained at suspended inspiration without intravenous contrast and reconstructed using a spatial contrast algorithm with 1.25-mm slice thickness. Study scans were acquired on Siemens and GE 64-slice scanners (GE Healthcare, Waukesha, Wisconsin) using a single spiral acquisition from apex to base (64 × 0.625 mm collimation, 120 kVp, 100 mA). Quantitative measures of emphysema and airway wall thickness were generated with VIDA software (VIDA Diagnostics, Iowa City, IA). Threshold-based measures of % low attenuation areas (LAA)-950 were calculated for each lung CT scan by quantifying the percentage of the overall lung density histogram below the −950 Hounsfield unit threshold (emphysema index, %) Citation(26). The radiologist used an electronic score sheet to record the extent of each emphysema subtype assessed visually on CT according to the following definitions Citation(27): a) centrilobular emphysema: focal regions of low attenuation, surrounded by normal lung attenuation, located within the central portion of secondary pulmonary lobules; b) panlobular emphysema: diffuse regions of low attenuation involving entire secondary pulmonary lobules; and c) paraseptal emphysema: regions of low attenuation adjacent to visceral pleura (including fissures). Upper lobe (including right middle)/total lung ratio was calculated for each lung and averaged for reporting. Airway wall thickness of airways with an internal perimeter of 10 mm (Pi10) were also obtained Citation(26).
Incremental cardiopulmonary exercise tests
Patients performed a symptom-limited incremental cardiopulmonary exercise test (CPET) on an electronically braked cycle ergometer using the Vmax229d System (SensorMedics). The rate of work rate (WR) increment was individually selected according to reported exercise tolerance (typically 10–15 W). Oxygen uptake (O2, L/min), carbon dioxide output (
CO2, L/min), respiratory exchange ratio (RER,
CO2/
O2), minute ventilation (
E, L/min), end-tidal partial pressure for carbon dioxide (PETCO2, mmHg), tidal volume (VT, L), and breathing frequency (f, cycles/min) were averaged at 20-second intervals. Peak
E was also expressed relative to the estimated maximal voluntary ventilation (MVV (L/min) = FEV1 × 35).
E/
CO2 nadir was the lowest test data point. Slope and intercept of the linear
E-
CO2 relationship were determined by linear regression Citation(9). Gas exchange threshold (GET) was estimated by the gas exchange method (V-slope). Arterial oxygen saturation was measured non-invasively by pulse oximetry (SpO2, %). Breathlessness and leg effort scores were rated according to the 10-point Borg category-ratio scale.
Constant work rate exercise tests
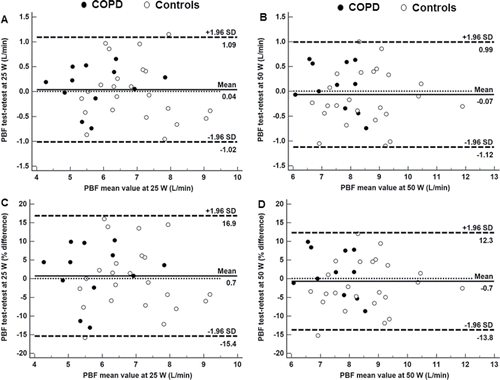
On a different day (at least 48 hours apart), subjects performed 5-minute 25 and 50 W exercise bouts separated by 5-minute resting periods. At rest and after 3 minutes of exercise, patients were switched to breathe from a rebreathing bag filled with a gas mixture consisting of 5% blood soluble nitrous oxide (N2O), 1% blood insoluble hexafluoride (SF6), and 94% O2 (InnocorTM; Innovision, Odense/Denmark). The bag volume and rebreathing frequency were adjusted in accordance with the subjects' VT and f at each WR. Assuming that pulmonary uptake of a blood soluble gas is proportional to PBF (L/min) Citation(28), pulmonary N2O uptake was assessed as the decrease in N2O over three expirations after a stable SF6 concentration was established Citation(29–31). Only measurements in which the SF6 curve indicated complete mixing of gases were included in the analysis. In order to assess the method's limits of agreement (LoA) between test and retest, 12 patients and 21 controls repeated 25 and 50 W bouts, respectively. This analysis revealed LoA values for PBF in the range of ∼±1 L/min at both 20 and 50 W for patients and controls (∼±15% of mean value). Test–retest variability did not change as a function of the measured value, i.e., there was no significant data heteroscedasticity ().
Statistical analysis
Values are reported as means ± standard deviation unless otherwise specified (IBM® SPPS® Statistics version 22.0.0.0). Based on previous studies that contrasted ventilatory efficiency in COPD patients with similar disease severity versus controls Citation(9–15), we estimated that a sample size of 20 subjects in each group would be required. According to variable distribution (Kolmogorov–Smirnov), controls and COPD patients were contrasted by non-paired t or Mann–Whitney's test. A chi-square test assessed differences in proportions. Pearson's r assessed linear association between continuous variables. The accepted risk for a type I error was less than 5% (p < 0.05).
Results
Subject characteristics
Twenty patients were excluded due to major co-morbidity, which could potentially interfere with patients' response to exercise (neoplasms = 4, heart failure = 4, active coronary disease = 3, recent myocardial infarction = 3, advanced liver disease = 2, orthopedic limitation = 2, morbid obesity = 2). Fifteen patients either recused participation or failed to attend the initial screening visit. All but 5 of the 19 enrolled patients were receiving short-acting bronchodilators as needed, and 10 were under long-acting bronchodilator treatment (7 of them with associated inhaled steroids).
Patients and controls were well matched by key demographic and anthropometric variables. Moreover, there were no between-group differences in the regular physical activity scores or co-morbidity burden as indicated by the Charlson index (). As expected, patients presented with higher mMRC dyspnea scores and greater impairment on resting lung function compared to controls (). Most patients presented with COPD GOLD stage 1. Most noticeable abnormalities on lung volumes included mild absolute and relative air trapping, increased airway resistance, and mild decrements in DLCO and KCO ().
Table 1. Subject characteristics.
Evidence of emphysema was found in all patients with 14/19 (73.7%) presenting with more than 5% LAA Citation(26). As shown in , there was a predominance of centrilobular over panlobular (18.4 ± 6.3%) and paraseptal (6.5 ± 3.1%) emphysema involving the upper lobes. In contrast, evidence of airway disease was less extensive compared to emphysematous changes.
Symptom-limited incremental CPET
Patients with COPD presented with significantly lower symptom-limited peak exercise capacity than healthy controls (p < 0.05; ). There were no significant between-group differences in cardiovascular and arterial oxygenation variables. Variables typically related to deconditioning (e.g., low O2GET and low peak O2 pulse) did not differ between patients and controls (p > 0.05). However, a greater symptom burden (both in relation to dyspnea and leg discomfort scores) was found in the patient group ().
Table 2. Measurements during symptom-limited incremental CPET.
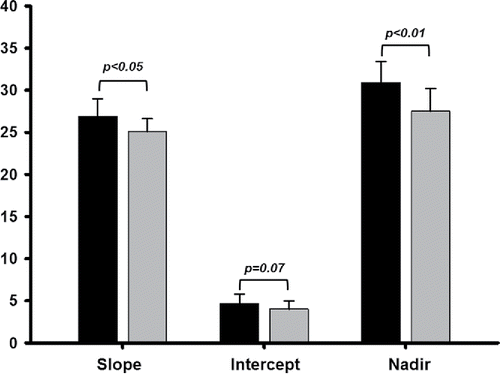
As shown in , significantly steeper E–
CO2 slope and, marginally, higher
E–
CO2 intercept led to greater
E/
CO2 nadir in patients compared to controls, i.e., poorer ventilatory efficiency Citation(9). Particularly low peak
O2 and high dyspnea scores were found in a subgroup of patients with
E/
CO2 nadir ≥ 30 (N = 12) compared to their counterparts with lower nadirs (peak
O2 = 76 ± 16% pred vs. 97 ± 12% pred and dyspnea = 6.1 ± 1.6 vs. 4.4 ± 1.9, respectively; p < 0.05).
Figure 2. Parameters of the ventilation (E)–carbon dioxide output (
CO2) relationship obtained in the symptom-limited incremental CPET in patients with mild-to-moderate COPD (N = 19) and controls (N = 26). Values are mean + SD.
Constant work rate exercise tests
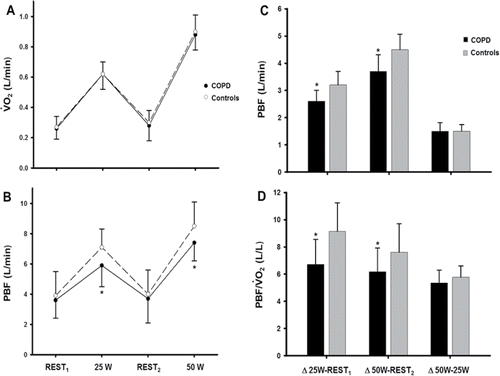
Constant WR tests were performed by 16 (25 W) and 13 (50 W) patients and 26 controls. Technically acceptable PBF measurements were obtained in 14 (25 W) and 12 (50 W) patients and 21 controls, respectively. As expected from similar WRs in subjects with comparable body dimensions (), metabolic demand (expressed as O2) () was equivalent between patients and controls. We found that despite similar resting values, patients presented with lower PBF at both exercise intensities (). Thus, PBF increased to a lesser extent from rest to exercise in patients compared to controls (p < 0.05) (). However, there were no between-group differences in PBF changes from 25 to 50 W (p > 0.05). As expected from similar metabolic demands,
O2-corrected PBF values closely followed this pattern of abnormalities ().
Figure 3. (A) Absolute metabolic (oxygen uptake, O2) and (B) pulmonary hemodynamic responses (pulmonary blood flow, PBF) to two constant work rate exercise tests in patients with mild-to-moderate COPD and controls. Exercise-induced changes in PBF either in absolute or
O2-corrected values are depicted in (C) and (D), respectively. Values are mean ± SD. *p < 0.05 for patients vs. controls at a given testing condition.
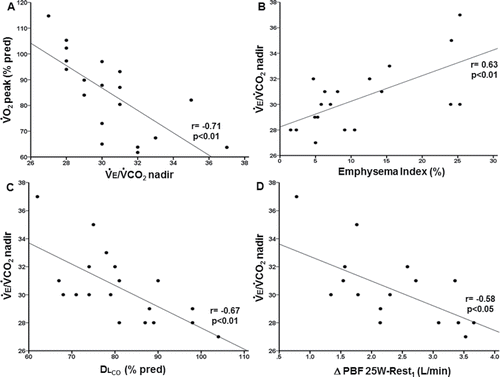
Structural and functional correlates of ventilatory inefficiency
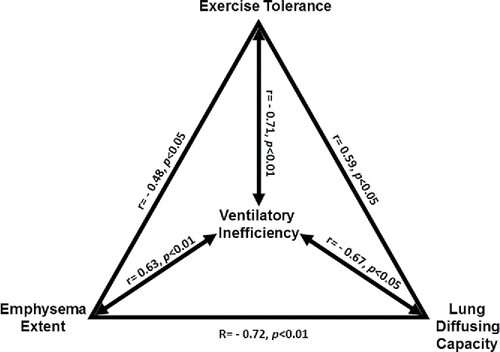
Patients with low peak O2 presented with greater ventilatory inefficiency (). Emphysema severity was positively related to
E/
CO2 nadir (); in contrast, we did not find a significant correlation between airway wall thickness (Pi10) with
E/
CO2 nadir or any of the resting physiological variables (p > 0.05). Low DLCO (but not low FEV1 or high RV; p > 0.05) was associated with higher
E/
CO2 nadir () and more impaired PBF from rest to 25 W (r = 0.60; p < 0.05). Of note, lower exercise-induced changes in PBF from rest to 25 W were associated with greater emphysema extent (r = −0.69, p < 0.01) and higher
E/
CO2 nadir (). Moreover,
E/
CO2 nadir was marginally related to changes in PBF from rest to 50 W (r = 0.44; p = 0.07). An integrative overview of the key inter-measurement correlations is shown in .
Figure 4. Correlates of ventilatory inefficiency (higher ventilation (E)/carbon dioxide output (
CO2) nadir) in patients with mild-to-moderate COPD (N = 19 with exception on panel D (N = 16): (A) lower peak oxygen uptake (
O2), (B) higher emphysema extent by thoracic CT, (C) lower lung diffusing capacity for carbon monoxide (DLCO), and (D) blunted increase in pulmonary blood flow (PBF) from rest to mild exercise (25 W).
Discussion
This study investigated the structural (emphysema and airway wall thickness by thoracic CT) and resting functional correlates of exercise ventilatory inefficiency in COPD patients with mild-to-moderate airflow obstruction. Our main results indicate that, compared to controls, patients with COPD presented with impaired lung diffusing capacity (DLCO), lower maximal exercise capacity (peak O2), greater ventilatory inefficiency (
E/
CO2), and blunted changes in PBF from rest to exercise. Emphysema severity (but not airway disease) was associated with DLCO and PBF impairments and greater ventilatory inefficiency. These data provide evidence that ventilation “wasted” in emphysematous areas has an important role in disturbing pulmonary gas exchange at rest and ventilation and lung hemodynamics during exercise in COPD patients with only modest spirometric abnormalities. Considering its association with meaningful patient-centered outcomes (poor exercise tolerance and exertional dyspnea), emphysema on thoracic CT should be clinically valued in COPD patients with largely preserved FEV1.
Our population of patients with mild-to-moderate COPD was typically younger than that evaluated in most studies with more advanced COPD, a finding expected by the natural history of COPD Citation(32). Most patients were overweight or had only mild obesity; thus, we avoided the complex effects of obesity on exercise responses in patients with COPD Citation(33). We excluded patients with major cardiovascular co-morbidities and impaired left ventricular systolic function. Moreover, patients and controls were well matched by regular physical activity and the burden of co-morbidities as assessed by the Charlson index Citation(25). Thus, we avoided major confounding factors regarding the determinants of peak exercise capacity and ventilatory efficiency in COPD, e.g., heart failure Citation(34) and coronary artery disease Citation(35). The patient population presented with a pattern of resting physiological abnormalities which closely resembles that found in previous studies involving mild-to-moderate COPD Citation(11,14,15).
The key novel finding of the present study was the significant cross-correlations between emphysema severity, DLCO impairment, and worse ventilatory inefficiency (). These data therefore provide compelling evidence that increased alveolar dead space due to emphysema was a relevant mediator of impaired ventilatory efficiency in our patients. Increased “wasted” ventilation represents a major challenge for the lungs as an efficient gas exchanger Citation(16). Enghoff's physiological dead space is strongly influenced by A/
mismatching Citation(16). Structure–function correlation studies in patients with mild-to-moderate COPD have shown that centrilobular emphysema results in a wide spectrum of
A/
heterogeneity, including: a) areas of low
A/
ratio, due to reduced ventilation secondary to airway narrowing and distortion Citation(36); and b) areas of high
A/
ratio due to more extensive microvascular destruction than loss of alveolar units Citation(37) and mechanical compression by over-distended air spaces in the distal acinus Citation(38–40). Thus, emphysema may have not only contributed to decreased DLCO but also impaired exercise ventilatory efficiency. Considering that ventilation delivered to non-perfused alveolar spaces has an even greater impact on alveolar dead space, microvascular destruction in non-emphysematous areas may have also contributed to poor ventilatory efficiency Citation(37). Thus, inflammatory/hyperoxidative processes involving the genesis of emphysema may also have damaged the lung microvasculature in adjacent non-emphysematous areas, thereby contributing to further increase in “wasted” ventilation Citation(37).
In line with our main premises, we found poorer ventilatory efficiency in the patient group. Thus, higher nadirs in patients were consequence of steeper slopes and, secondarily, higher intercepts (). These data corroborate the notion that all parameters of the E-
CO2 relationship are able to reflect the presence of ventilatory inefficiency in mild-to-moderate COPD Citation(9). Considering, however, that the nadir represents the combined effects of increases in slope and intercept Citation(9), the nadir might constitute a more sensitive index of ventilatory inefficiency in these patients. Greater dyspnea scores were found in patients with higher nadirs. This finding largely stems from the combined effects of higher neural drive and greater erosion of the mechanical reserves induced by an excessive ventilatory response to exertion Citation(9–15).
Our study confirms previous findings that despite the presence of only modest spirometric abnormalities, emphysema on CT and low DLCO carries are associated with poor tolerance to exertion Citation(1–5). Of note, most of our patients presented with > 5% LAA on chest CT scan, a widely used threshold to suggest “clinically-significant” emphysema Citation(26). There is evidence that patients with mild-to-moderate COPD presenting with an emphysema-dominant phenotype—as in the present study—had increased lung volumes and greater impairment of gas exchange in comparison with an airways disease-dominant phenotype Citation(41). In the MESA study, smokers with centrilobular emphysema had greater dyspnea, reduced walk distance, greater hyperinflation, and low DLCO than their counterparts without emphysema Citation(42). Lung volume reduction surgery, targeting areas with more extensive emphysema, has been associated with improved dead space ventilation Citation(43) and better ventilatory efficiency Citation(44). Collectively, these data (and ours) indicate that emphysema is more likely to be associated with negative physiological outcomes pertinent to exercise tolerance than airway disease in patients with mild-to-moderate disease.
It is also noteworthy that we found a predominance of centrilobular emphysema involving the upper lobes. Similarly, Pike et al. Citation(2) found that patients with milder COPD present with a higher probability to develop centrilobular emphysema in the upper lobes compared to that in the lower lobes in patients with equivalent degrees of airflow obstruction. Of note, Wang and co-workers also found that DLCO and air trapping were primarily affected by the % LAA of the upper lobes Citation(45). The upper lobes characteristically present with areas of high V/Q ratios, including during exercise Citation(46). Thus, it is conceivable that areas of centrilobular emphysema in the upper lobes are more prone to “waste” ventilation upon exertion than better perfused areas of the lower lobes.
We found a downward displacement of the PBF-O2 relationship in patients with COPD (). Thus, reduced absolute PBF in patients is unlikely to reflect lower cardiac output due to diminished metabolic demands. Our patients did not present with major cardiovascular co-morbidities, and, considering the presence of only mild resting air trapping, had a low probability of negative cardiopulmonary interactions Citation(32). In other words, it is also improbable that they had lower cardiac output/
O2 ratios than controls. The exact mechanism behind low exercise PBF in our patients, however, remains elusive. However, it is noteworthy that emphysema severity was associated with lower PBF/
O2 (). On one hand, this might reflect, at least partially, the inherent limitations of inert gas rebreathing in patients with ventilation distribution abnormalities (as discussed in the next paragraph) Citation(31). On the other hand, there is a morphological background to support the notion that emphysema-related vascular destruction/obliteration Citation(36) may have compromised PBF under the stress of exercise, i.e., when distension and recruitment of a healthy vasculature are particularly important Citation(47). Indirect compressive effects on lung microvasculature due to over-distension of expanded air spaces and less tethering effects due to loss of alveolar attachments may also have played a role Citation(36). Whether PBF was also impaired due to microvascular abnormalities beyond that induced by emphysema per se should be further investigated using advanced vascular imaging during exercise Citation(18).
As a small clinical physiology study, our investigation has some limitations. Functional assessment restricted to the conventional lung function tests and more sophisticated tests of small airways function and ventilation distribution abnormalities were not performed Citation(14). We were also unable to separate the membrane and vascular components of gas transfer, which precluded us to further advance on the seeds of a low DLCO in patients Citation(47). Technologically more advanced and accurate imaging techniques reflecting ventilation distribution abnormalities (such as hyperpolarized3 He magnetic resonance imaging) might have uncovered important abnormalities with a potential to increase “wasted” ventilation Citation(2,3). We did not measure operating lung volumes or arterial blood gases as the seeds and consequences of mechanical-ventilatory, and pulmonary gas exchange abnormalities have already been extensively investigated in patients with mild COPD Citation(10–15). At least in patients with moderate-to-severe COPD, inadequate gas mixing and gas exchange disturbances might lead to an underestimation of PBF Citation(31). Thus, we acknowledge that the observed association between emphysema extent and low exercise PBF likely represents the combined effects of poor lung perfusion in emphysematous areas with the undesired consequences of higher alveolar dead space on inert gas rebreathing measurements.
We conclude that centrilobular emphysema in the upper lobes is an important structural correlate of low DLCO and exertional ventilatory inefficiency in COPD patients with largely preserved FEV1. Low DLCO and ventilatory inefficiency, in turn, are associated with activity-related dyspnea and exercise intolerance. The present study shed new light on the mechanisms behind the previously reported correlations between emphysema extent and low DLCO with poor exercise capacity in the early stages of COPD Citation(1,3,6,8). Ventilatory inefficiency, therefore, is an important exercise-based biomarker that links structural abnormalities (emphysema) and resting physiological impairment (DLCO) to a key clinical outcome (exercise intolerance) in COPD patients with only modest spirometric abnormalities.
Acknowledgments
The authors would like to thank Kathy Webb, Kristin MacLeod, and Casey Ciavaglia for dedicating their valuable time in performing pulmonary function tests. They also thank Dr. Amany Elbehairy for patients' referral and Mrs. Ingrid Rafferty and Luiza Castanhas for their technical assistance. They are also grateful to Prof. John T Fisher (Department of Biomedical and Molecular Sciences, Queen's University) for his intellectual support. They are particularly indebted to patients and volunteers for their time and commitment to the study.
Funding
JH Jones was partially supported by the McLaughlin Fellowship during his MSc in Experimental Medicine at Queen's University. JA Neder was supported by the Southeastern Academic Medical Association (SEAMO)'s New Clinician Scientist Program. His laboratory was established owing to the Canadian Foundation for Innovation's Leader Operating Fund.
Declaration of interest
Each of the authors declare no conflict of interest regarding to this manuscript.
References
- Kirby M, Pike D, Sin DD, Coxson HO, McCormack DG, Parraga G. COPD: Do imaging measurements of emphysema and airway disease explain symptoms and exercise capacity? Radiology 2015; 277:872–880.
- Pike D, Kirby M, Eddy RL, Guo F, Capaldi DPI, Ouriadov A, et al. Regional heterogeneity of chronic obstructive pulmonary disease phenotypes: pulmonary (3)He magnetic resonance imaging and computed tomography. COPD 2016; 13:601–609.
- Kirby M, Owrangi A, Svenningsen S, Wheatley A, Coxson HO, Paterson NAM, et al. On the role of abnormal DL(CO) in ex-smokers without airflow limitation: symptoms, exercise capacity and hyperpolarised helium-3 MRI. Thorax 2013; 68:752–759.
- Tan WC, Sin DD, Bourbeau J, Hernandez P, Chapman KR, Cowie R, et al. CanCOLD Collaborative Research Group. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax 2015; 70:822–829.
- Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, et al. Association between functional small airways disease and FEV1 decline in COPD. Am J Respir Crit Care Med 2016; 194:178–184.
- Díaz AA, Morales A, Díaz JC, Ramos C, Klaassen J, Saldías F, et al. CT and physiologic determinants of dyspnea and exercise capacity during the six-minute walk test in mild COPD. Respir Med 2013; 107:570–579.
- Yamasawa W, Tasaka S, Betsuyaku T, Yamaguchi K. Correlation of a decline in aerobic capacity with development of emphysema in patients with chronic obstructive pulmonary disease: a prospective observational study. PloS One 2015; 10:e0125053.
- Díaz AA, Pinto-Plata V, Hernández C, Peña J, Ramos C, Díaz JC, et al. Emphysema and DLCO predict a clinically important difference for 6MWD decline in COPD. Respir. Med. 2015; 109:882–889.
- Neder JA, Arbex FF, Alencar MCN, O'Donnell CDJ, Cory J, Webb KA, et al. Exercise ventilatory inefficiency in mild to end-stage COPD. Eur Respir J 2015; 45:377–387.
- Ofir D, Laveneziana P, Webb KA, Lam Y-M, O'Donnell DE. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177:622–629.
- Chin RC, Guenette JA, Cheng S, Raghavan N, Amornputtisathaporn N, Cortés-Télles A, et al. Does the respiratory system limit exercise in mild chronic obstructive pulmonary disease? Am J Respir Crit Care Med 2013; 187:1315–1323.
- Guenette JA, Chin RC, Cheng S, Dominelli PB, Raghavan N, Webb KA, et al. Mechanisms of exercise intolerance in global initiative for chronic obstructive lung disease grade 1 COPD. Eur Respir J 2014; 44:1177–1187.
- O'Donnell DE, Maltais F, Porszasz J, Webb KA, Albers FC, Deng Q, et al. The continuum of physiological impairment during treadmill walking in patients with mild-to-moderate COPD: patient characterization phase of a randomized clinical trial. PloS One 2014; 9:e96574.
- Elbehairy AF, Raghavan N, Cheng S, Yang L, Webb KA, Neder JA, et al. Physiologic characterization of the chronic bronchitis phenotype in GOLD grade IB COPD. Chest 2015; 147:1235–1245.
- Elbehairy AF, Ciavaglia CE, Webb KA, Guenette JA, Jensen D, Mourad SM, et al. Canadian respiratory research network. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med 2015; 191:1384–1394.
- Robertson HT. Dead space: the physiology of wasted ventilation. Eur Respir J 2015; 45:1704–1716.
- Barberà JA, Riverola A, Roca J, Ramirez J, Wagner PD, Ros D, et al. Pulmonary vascular abnormalities and ventilation-perfusion relationships in mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994; 149:423–429.
- Hueper K, Vogel-Claussen J, Parikh MA, Austin JHM, Bluemke DA, Carr J, et al. Pulmonary microvascular blood flow in mild chronic obstructive pulmonary disease and emphysema. The MESA COPD study. Am J Respir Crit Care Med 2015; 192:570–580.
- Wilschut FA, van der Grinten CP, Lamers RJ, Wouters EF, Luijendijk SC. Intrapulmonary gas mixing and the sloping alveolar plateau in COPD patients with macroscopic emphysema. Eur Respir J 1999; 14:166–171.
- Jögi J, Ekberg M, Jonson B, Bozovic G, Bajc M. Ventilation/perfusion SPECT in chronic obstructive pulmonary disease: an evaluation by reference to symptoms, spirometric lung function and emphysema, as assessed with HRCT. Eur J Nucl Med Mol Imaging 2011; 38:1344–1352.
- Rodríguez-Roisin R, Drakulovic M, Rodríguez DA, Roca J, Barberà JA, Wagner PD. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol 2009; 106:1902–1908.
- Schwaiblmair M, Beinert T, Seemann M, Behr J, Reiser M, Vogelmeier C. Relations between cardiopulmonary exercise testing and quantitative high-resolution computed tomography associated in patients with alpha-1-antitrypsin deficiency. Eur J Med Res 1998; 3:527–532.
- Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365.
- Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982; 36:936–942.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987; 40:373–383.
- Kirby M, Parraga G. COPD imaging: new tools to tackle an old problem? COPD 2014; 11:477–479.
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722.
- Laszlo G. Respiratory measurements of cardiac output: from elegant idea to useful test. J Appl Physiol 2004; 96:428–437.
- Saur J, Trinkmann F, Doesch C, Scherhag A, Brade J, Schoenberg SO, et al. The impact of pulmonary disease on noninvasive measurement of cardiac output by the inert gas rebreathing method. Lung 2010; 188:433–440.
- Fontana P, Boutellier U, Toigo M. Reliability of measurements with Innocor during exercise. Int J Sports Med 2009; 30:747–753.
- Perrault H, Richard R, Kapchinsky S, Baril J, Bourbeau J, Taivassalo T. Addressing assumptions for the use of non-invasive cardiac output measurement techniques during exercise in COPD. COPD 2016; 13:75–81.
- O'Donnell DE, Laveneziana P, Webb K, Neder JA. Chronic obstructive pulmonary disease: clinical integrative physiology. Clin Chest Med 2014; 35:51–69.
- Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA, O'Donnell DE. Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am J Respir Crit Care Med 2009; 180:964–971.
- Arbex FF, Alencar MC, Souza A, Mazzuco A, Sperandio PA, Rocha A, et al. Exercise ventilation in COPD: influence of systolic heart failure. COPD 2016; 12:1–8 [Epub ahead of print].
- Thirapatarapong W, Armstrong HF, Bartels MN. Comparison of cardiopulmonary exercise testing variables in COPD patients with and without coronary artery disease. Heart Lung J Crit Care 2014; 43:146–151.
- Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004; 364:709–721.
- Barr RG. The epidemiology of vascular dysfunction relating to chronic obstructive pulmonary disease and emphysema. Proc Am Thorac Soc 2011; 8:522–527.
- Barbera JA, Roca J, Ramirez J, Wagner PD, Ussetti P, Rodriguez-Roisin R. Gas exchange during exercise in mild chronic obstructive pulmonary disease. Correlation with lung structure. Am Rev Respir Dis 1991; 144:520–525.
- Barbera JA, Ramirez J, Roca J, Wagner PD, Sanchez-Lloret J, Rodriguez-Roisin R. Lung structure and gas exchange in mild chronic obstructive pulmonary disease. Am Rev Respir Dis 1990; 141:895–901.
- Barbera JA, Roca J, Ramirez J, Wagner PD, Ussetti P, Rodriguez-Roisin R. Gas exchange during exercise in mild chronic obstructive pulmonary disease. Correlation with lung structure. Am Rev Respir Dis 1991; 144:520–525.
- Subramanian DR, Gupta S, Burggraf D, Vom Silberberg SJ, Heimbeck I, Heiss-Neumann MS, et al. Emphysema- and airway-dominant COPD phenotypes defined by standardised quantitative computed tomography. Eur Respir J 2016; 48:92–103.
- Smith BM, Austin JHM, Newell JD, D'Souza BM, Rozenshtein A, Hoffman EA, et al. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med 2014; 127(94):e7–23.
- Criner GJ, Belt P, Sternberg AL, Mohsenifar Z, Make BJ, Utz JP, et al. National Emphysema Treatment Trial Research Group. Effects of lung volume reduction surgery on gas exchange and breathing pattern during maximum exercise. Chest 2009; 135:1268–1279.
- Kim V, Kretschman DM, Sternberg AL, DeCamp MM, Criner GJ, National Emphysema Treatment Trial Research Group. Weight gain after lung reduction surgery is related to improved lung function and ventilatory efficiency. Am J Respir Crit Care Med 2012; 186:1109–1116.
- Wang G, Wang L, Ma Z, Zhang C, Deng K. Quantitative emphysema assessment of pulmonary function impairment by computed tomography in chronic obstructive pulmonary disease. J Comput Assist Tomogr 2015; 39:171–175.
- Harf A, Pratt T, Hughes JM. Regional distribution of VA/Q in man at rest and with exercise measured with krypton-81m. J Appl Physiol 1978; 44:115–123.
- Tamhane RM, Johnson RL, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest 2001; 120:1850–1856.