ABSTRACT
Readmissions of patients with chronic obstructive pulmonary disease (COPD) to hospitals cast a heavy burden to health care systems. This meta-analysis was aimed to assess the efficacy of continuity of care as interventions, which reduced readmission and mortality rates of such patients. PubMed, Cochrane Library and Embase were searched for articles published before July 2015. A total of 31 reports with randomized controlled trials (RCTs) were finally included in this meta-analysis. The results showed that health education reduced all-cause readmission at 3 months. In addition, health education, comprehensive nursing intervention (CNI) and telemonitoring reduced all-cause readmissions over 6–12 months, and the effect of CNI was best because CNI also reduced COPD-specific readmissions. Home visits also reduced COPD-specific readmissions (the quality more than moderate), but it did not reduce the risk for all-cause readmissions (risk ratios (RRs), 0.92 [95% CI, 0.82–1.04]; moderate quality). There was no statistically significant difference in reducing mortality and quality of life (QOL) among various continued cares. In conclusion, CNI, telemonitoring, health education and home visits should receive more consideration than other interventions by caregivers seeking to implement continued care interventions for patients with COPD.
Introduction
COPD is a major global chronic progressive disease affecting millions of people worldwide Citation(1). This disease causes considerable morbidity, hospital readmission and mortality worldwide Citation(2–8). According to the World Health Organization, more than 67 million people had COPD and over 3 million people died of COPD in 2005, which represented 5% of all deaths globally in 2011, and total deaths from COPD are projected to increase by more than 30% in the following 10 years Citation(9). By 2030, COPD is predicted to be the third leading cause of death worldwide Citation(10). As COPD mainly affects old people, the associated disease burden is expected to increase in the coming decades due to the ageing global population. Potentially affecting one in four adults by the age of 80 years Citation(11), COPD is already a leading cause of death in developed and developing countries Citation(12,13).
COPD casts a heavy burden on healthcare systems worldwide Citation(4,14) and on individuals as a result of impaired QOL Citation(15,16). The majority of COPD healthcare costs are accounted for by hospital admissions Citation(17). An audit of COPD in England found that nearly one-third of patients had a readmission within 90 days Citation(18). One study reported that 18% of COPD patients were readmitted once and 14% twice within 1 year Citation(19). Successful reduction of COPD rehospitalisation rates would yield important economic benefits. Therefore, COPD has been regarded as a major public health concern Citation(20,21), requiring management strategies after discharges to prevent early rehospitalisations and reduce healthcare costs Citation(22).
Continuity of care is recognised as a core prevention of readmissions and a potential improvement of health outcomes for patients with COPD Citation(23,24). Continuity of care means that the care is provided after a patient is discharged from a hospital. Hereafter, it is interchangeably referred to as continued care in this paper. Although there are no clear compositions of continued care, they often include health education, medication reconciliation, self-management project and adjustment of the nursing measures according to the patient condition such as telemonitoring. The telemonitoring interventions used equipment installed in discharged patients' homes to remotely monitor the patients' biophysical data to assess their health condition Citation(25). Unlike other chronic conditions Citation(26,27), the efficacy of continued care to prevent COPD from rehospitalisation has been controversial Citation(28–31). Two RCTs did not show positive effects Citation(30,31), and a systematic review showed that insufficient evidence supported self-management interventions Citation(32). A study that reported a continuum of self-management in a comprehensive patient education program could significantly reduce the utilization of health care services and improve health status Citation(28), which contained weekly visits over a 2-month period and monthly telephone follow-ups. Rees Citation(33) claimed that the self-management program (1-hour weekly home self-management program lasted 7–8 weeks, a customized action plan, an exercise program and telephone case manager) might be the major reason for readmission reductions and health improvement. Gallefoss argued that an education of mild-to-moderate COPD patients could improve their outcomes and reduce costs in a 1-year follow-up Citation(29). Other researchers also reported that health education interventions could reduce hospital readmission Citation(28,34–36). One comprehensive systematic review suggested that disease management programs for patients with COPD reduced hospitalisation and slightly improved QOF Citation(37). However, other systematic reviews have reached different conclusions about the overall value of disease management for COPD, but all reviews have recognized the potential value of such interventions and the need for more detailed studies Citation(38,39).
Most of these studies only evaluated the efficacy of a single intervention. In addition to inconsistent findings, few studies in the literature were comprehensive evaluations of the efficacy of multiple continuous care interventions. Therefore, the aims of this study were to update and synthesize findings on continued care interventions for patients with COPD and evaluate which intervention was the most effective in improving patients' outcomes.
Methods
Data sources and searches
Reports published before July 2015 that involved only humans were searched in the Embase, Cochrane Library and PubMed (including MEDLINE). References of the selected studies were also manually checked to identify additional relevant studies.
Study selection
Inclusion of each study was determined independently by two reviewers, and inclusion criteria were evaluated. Each study had to meet four criteria. First, it had to be based on an RCT design and reported in full text with a title and abstract. Second, it had to include adults with a clinical diagnosis of COPD and compare a continued care with other eligible interventions or usual care, (namely, routine or standard care, as defined by the primary studies). Third, it had to have the interventions with one or more of the following components: health education before or after discharge, discharge action plans, planned or scheduled home visits, frequent telephone contact, self-management, telemonitoring, CNI (health education, self-management, action plan and home visits/follow-up telephone), home-based rehabilitation (HBR), or interventions to increase provider continuity. Fourth, the primary outcome for patients had to include a readmission or mortality rate, or a composite outcome (all-cause readmission or mortality). Secondary outcomes included QOF, hospital days of subsequent readmissions, subjective health status and caregiver or self-care cost. Studies that examined only health care service expenditures and costs were excluded.
Data extraction and quality assessment
Each article that met these inclusion criteria was independently scrutinized by two reviewers to extract a description of objectives, design, participants, interventions and follow-up time. Any disagreement was resolved by discussion among the reviewers, and a final decision was made by the third reviewer.
The Cochrane Group's predesigned table Citation(40) was used to ensure standardized scoring. Methodological quality was assessed in terms of selection, confounding, performance, attrition and detection biases. The two reviewers independently assessed risks of biases for each study. Disagreements were resolved by consensus from discussions. Studies were given a score of one point for each fulfilled criterion. Based on the scores, the quality of the studies was divided into three levels: low (≤3 points), moderate (4–6 points) and high (7–9 points). All individual patients' data were checked for consistency internally and with published reports.
Data synthesis and analysis
A meta-analysis was conducted with DerSimonian–Laird random effects models. The outcomes reported by similar multiple studies were combined for the analysis. Also the meta-analyzed RCTs were reported the number of deaths or number of persons readmitted in each group. If the number of persons readmitted could not be obtained, the articles were excluded.
Table 1. Quality assessments of the included studies.
Risk ratios (RRs) were calculated for readmission and mortality rates. Statistical heterogeneity was measured with the chi-square and I2 statistics Citation(41). Begg's plot was used to test the publication bias. The meta-analyses were carried out using Stata (version 12.0). Subgroup analyses were planned on the type of intervention and the time of follow-up. Health-related QOF was assessed using a standardized self-completed questionnaire, namely, the St. George's Respiratory Questionnaire (SGRQ) Citation(42). Weighted mean differences were estimated using a random-effects model.
Results
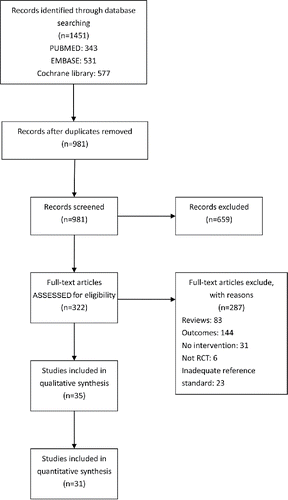
The literature flow diagram () summarizes the search and selection of articles. From the 1,451 potentially relevant citations initially identified, 1,129 were excluded. The remaining 322 retrieved reports were selected for in-depth screening and 287 studies for detailed evaluation. A total of 31 RCTs were finalized as eligible for the analysis. Seven RCTs from four countries (New Zealand, Spain, England, Slovenia) both reported the primary outcome of COPD readmission and mortality Citation(43–49). All studies reported readmission, but some did other outcomes. Begg's plot was used to examine publication bias, and the results showed that there was no evident publication bias (p = 1.000).
Methodological quality
Overall methodological quality of the studies () was relatively high, with scores ranging from 3 to 9. Three articles scored 3, 15 did less than or equal to 6 and 13 did no more than 9. All the studies were randomized, with inclusion/exclusion criteria and similarity at baselines. The most common reason for lower scores was the absence of double-blind procedure, which was impossible due to the nature of the intervention. The assessors were not blinded to outcome in 26 studies (83.9%), and researchers/participants were not blinded in 23 studies (74.2%). Eleven studies (35.5%) did not report the characteristics of participants lost to follow-ups. Only eight studies (25.8%) involved an intervention group of more than 100 participants and concealed allocation.
Basic characteristics of included studies
RCT characteristics are shown in . Most RCTs compared a continued care with a usual care. Twenty studies were single-centre RCTs, and eleven studies were conducted in multicentres. Twenty-two RCTs were conducted in European and American countries, nine were in Australia and one was in China. The sample sizes of the included studies ranged from 14 to 554 participants for the intervention groups and from 12 to 532 patients for the control groups. The mean age of study participants varied from 63 to 74 and was under 70 years in 20 studies and 70 years or above in eleven studies. Only seven studies reported the patients' smoking history. Follow-up was conducted for a range of 3–12 months and a mean of 8 months. The presence of lung dysfunction was indicated by a mean FEV1 /FVC or FEV1 (%Predicted) of less than 70% in 20 RCTs and non-reported in 11 RCTs.
Table 2. Description of the included trails.
Interventions characteristics of included studies
Five RCTs compared home visits with the usual care Citation(28,45,49,50). Four studies compared an early discharge home visiting program with the conventional hospital care Citation(48,51–53). Most interventions involved home visits and health education; three RCT interventions added regulated monthly telephone calls Citation(28,45,51). In most RCTs, community nurses conducted the home visits, most of which began within 7 days after discharge. Two RCTs included visits within 1–3 months of discharge Citation(49,51), and only one RCT specified that visits were done within 14 days of discharge Citation(50).
Seven RCTs compared home telemonitoring with usual care Citation(25,43,46,53–56), and one RCT compared home telemonitoring with home visits Citation(44). Five evaluated remote clinical data monitoring using equipment installed in patients' homes after discharge, which transmitted data to a central site Citation(43,44,53,55,56). Most telemonitoring systems needed the patients to input the clinical data to assess their health condition. One RCT used a wristband that could get the data such as heart rate and couple with cellular telephone via a Bluetooth, and then, the telephone sent the data to the monitoring system Citation(54). One study used cell phone call connected to the chronic care telemedicine system Citation(46).
Five RCTs evaluated CNI Citation(37,47,57–59). Most of the measures contained health education, disease self-management, action plan and home visits or telephone follow-up. One assessed enhanced access to individual practice plan on a network platform, such as smoking cessation, drug adherence and physiotherapeutic reactivation Citation(57). RCTs that described disease management interventions emphasized more patients' participation and also had a multidisciplinary care team (nurses, physiotherapists and pharmacists).
Three RCTs evaluated a health education intervention. One compared the effects of 1-hour duration standardized self-management plan education from a practice nurse or respiratory educator in association with their general practitioner Citation(60). One RCT investigated individualised education (20–30 minutes per session, 5–6 sessions) in a structured fashion step by step during the clinic visit and a series of 10-minute telephone counselling Citation(61). One RCT featured education on inhalation technique and adherence to maintenance therapy at the start of the RCT by pharmacists Citation(62).
Three RCTs evaluated action plan interventions Citation(63–65). Two studies assessed patients' discharge needs and developed discharge action plans. One of them updated the action plan when it is necessary after discharge Citation(65); the other one visited by the nurse at the study start and thereafter at 3, 6 and 12 months to provide routine support Citation(64). One trail investigated a written self-management plan, which was developed in consultation with their treating general practitioner and patients Citation(63).
Three RCTs evaluated HBR Citation(66–68). Two RCTs used structured hospital-based supervised training such as walking and home-based training after discharged Citation(67,68). One RCT investigated outpatients participated in standard pulmonary rehabilitation classes consisting of twice-weekly exercise, which was a mixture of limb strengthening and aerobic activities tailored to individual baseline function and exercised after discharge Citation(66).
Readmission rates
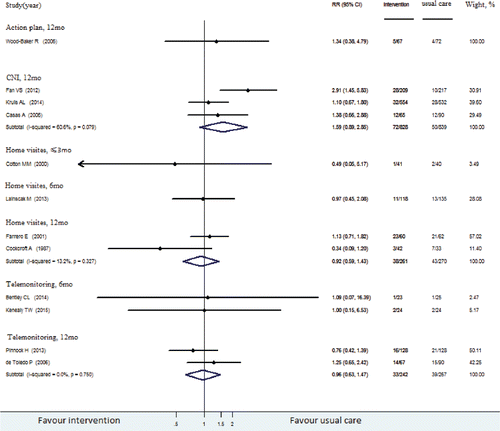
presented our meta-analyses and RR calculations of RCTs reported all-cause readmission and COPD readmission, respectively. Results in both figures are stratified by types of post-discharge intervention and follow-up time.
Figure 2. All-cause readmission for transitional care compared with usual care. Weights are from the random-effects analysis.
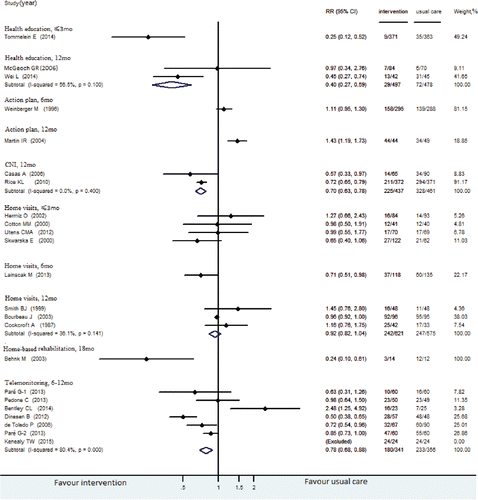
Figure 3. COPD readmission for transitional care compared with usual care. Weights are from the random-effects analysis.
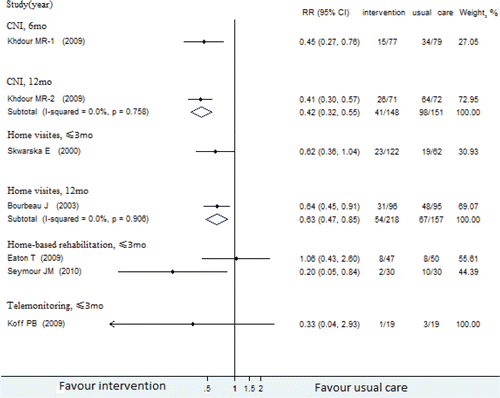
Readmission rates within 3 months after discharge
One RCT evaluating health education found a lower risk for readmission among patients receiving health education than that of the usual care group (RR, 0.25 [95% CI, 0.12–0.52]) Citation(62). The intervention placed emphasis on educating inhalation technique, self-management and adherence to maintenance therapy at the start of the RCT by pharmacists, which had a high methodological quality.
Four RCTs about home visits did not reduce all-cause readmission rates, with quality scores ranging from 4 to 7. One of them found no difference in the risk for COPD-specific readmissions between persons receiving home visits at intervals of 2–3 days and those receiving inpatient care Citation(53). The other RCTs found that patients with acute exacerbations of COPD discharged early, and supported home treatment by nurse did not reduce all-cause readmission rates Citation(30,48,52).
Three other RCTs across different intervention categories reported no more than 3-month COPD-specific readmission: 1 telemonitoring RCT Citation(69), 2 HBR RCTs Citation(66,67). None of these interventions found a statistically significant reduction in COPD readmissions.
Readmission rates in 6–12 months after discharge
The interventions of health education (RR, 0.40 [95% CI, 0.27–0.59]), CNI (RR, 0.70 [95% CI, 0.63–0.48]) and telemonitoring (RR, 0.78 [95% CI, 0.58–0.88]) reduced all-cause readmissions over 6–12 months after discharge. Both CNI (RR, 0.42 [95% CI, 0.32–0.55]) and home visits (RR, 0.63 [95% CI, 0.47–0.85]) interventions reduced COPD readmissions in 6–12 months after discharge, with methodogical quality above moderate. The effects of CNI and home visit interventions were better than usual care. However, home visits did not reduce the risk for all-cause readmissions (RR, 0.92 [95% CI, 0.82–1.04]; moderate quality).
Readmission rates more than 12 months after discharge
One RCT found that patients with COPD who had an individually defined, 18-month home-based, long-term walking program initiated by a short hospital-based training, had a lower risk for all-cause readmission than those in the control group (RR, 0.24 [95% CI, 0.10–0.61]; low quality) Citation(68). However, because of limited evidence from a single RCT and small sample (n = 26), we graded the evidence on increasing access to HBR as insufficient. Evidence was insufficient to determine whether HBR interventions were effective in reducing all-cause readmission.
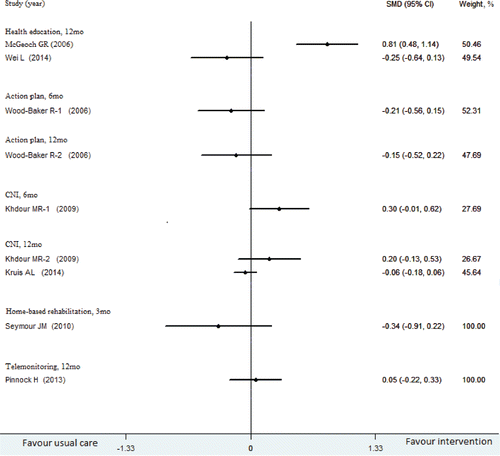
Mortality rates
presents the mortality rates stratified by intervention category and follow-up time. There was no statistical significance in reducing mortality of various interventions and usual care. Telemonitoring, action plan and home visit interventions did not reduce mortality (high quality). Evidence for a reduction in mortality was insufficient for CNI interventions. Two studies demonstrated that there was no statistical significance in reducing mortality, but one study showed that the mortality of the patients in the intervention group was even higher than that of the usual care group (RR, 2.91 [95% CI, 1.54–5.83]; high quality).
QOF
As shown in , it was possible that the interventions of action plan and home-based rehabilitation could improve patients' QOF. CNI and telemonitoring interventions found no statistically significant reduction in quality of life (moderate-to-high quality). Evidence was insufficient to determine whether health education could improve patients' QOF.
Sensitivity analysis
Sensitivity analysis comparing fixed and random-effects statistical models yielded relatively same results (RR = 0.82; p = 0.000). The effect of sequentially omitting a low-quality study Citation(68) and recalculating the pooled estimates for the remaining studies did not significantly alter the effect on all-cause readmission (RR, 0.82 vs. 0.80; p = 0.000).
Discussion
Our meta-analysis showed that CNI and telemonitoring interventions currently have the best evidence for reducing all-cause readmissions up to 6 months for patients with COPD. The effect of CNI was better than that of telemonitoring because CNI also reduced COPD-specific readmissions during 1-year follow-up. Sensitivity analysis showed that excluding small studies (n < 100) altered the overall estimates for readmission insignificantly. Despite multiple components of continued care and study heterogeneity, the beneficial effect of continued care in reducing readmission rates remained stable across the range of patients, post-discharge interventions, follow-up periods and countries.
Such positive effects could have stemmed from the patient characteristics and additive and/or interaction effects of multiple components of continued care. Most of the patients in CNI group were outpatients with a confirmed diagnosis of COPD without serious complications, and they received a plethora of measures, which mainly included self-management of the disease, individual health education, action plan for self-treatment of exacerbations, scheduled telephone calls and a 12-month follow-up. This was similar with two systematic reviews, which showed that simple self-management education without support was not effective Citation(70), but supported self-management interventions could be effective in reducing respiratory admissions among stable COPD patients Citation(71). This explanation was also supported by our result that telemonitoring was effective for only stable COPD patients. There was no statistical significance among various continued cares in reducing mortality of various continue nursing interventions, which was consistent with the reports of Bergner et al. and Cockcroft et al. Citation(49,72). One RCT of CNI Citation(58) was even stopped because the mortality of intervention group became worse than that of the control group, and the author could not explain this phenomenon. We attributed such failures to the severity and complications of the patients' COPD and the simplicity of continued cares. For instance, the study Citation(58) was based on 416 COPD patients recruited from 20 hospital-based outpatient clinics of Veterans Affairs. The intervention included COPD education during four 90-minute weekly individual and one group sessions, an action plan for identification and treatment of exacerbations and scheduled proactive telephone calls for case management. The number of reinforcement telephone calls was once per month for 3 months and every 3 months thereafter, as was fewer than other studies Citation(28,59). In contrast, our meta-analysis selected clearly defined COPD patients with an average age of 69 or older and excluded patients with complex diseases. Thus, our result showed no statistical differences among various continued cares in reducing mortality rates.
Several limitations of this review should be mentioned here. First, most RCTs compared an intervention with “usual care” the details of which were not reported. Second, we only selected studies that reported the proportion of readmitted patients but ignored information about secondary outcomes such as costs. Third, we were unable to obtain individual patient-level data. Information on specific components of the intervention was limited. Some RCTs did not clearly describe the methods used for assessing readmissions. Methods for handling missing data varied across studies.
Future interventions may unify standard specification requirements and compare them, respectively, to see which measure is the most effective. Future studies may also evaluate whether interventions that reduce readmission rates over 6–12 months also reduce readmission rates within 3 months, and could directly compare one intervention with another (for example, home visits vs. health education). The included studies in this review did not evaluate the cost-effectiveness of interventions. It is critical to study how the costs may be distributed to the care providers and patients to sustain any continued care. Finally, it should be considered to determine whether increased active management of psychological morbidity may improve outcomes and reduces the financial burden to achieve the optimal cost-effectiveness.
In conclusion, the best intervention was CNI, which reduced all-cause readmission and COPD-specific readmission in 6–12 months after hospital discharge; health education and telemonitoring reduced all-cause readmissions; home visits reduced COPD-specific readmission but not all-cause readmission. These interventions may be adopted differentially by systems or providers seeking to implement transitional care interventions for patients with COPD.
Declaration of interest
The authors are responsible for the content of the paper and have no conflicts of interest to disclose.
Funding
The study was funded by the Hubei Province Health and Family Planning Scientific Research Project (Grant No. WJ2015Z058) and the Hubei University of Chinese Medicine Scientific Research Project.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: updated 2014. GOLD 2014. Available from: www.goldcopd.org/uploads/users/files/GOLD_Report_2014_Jun11.pdf.
- Garcia-Aymerich J, Farrero E, Félez MA, Izquierdo J, Marrades RM, Antó JM, et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 2003; 58(2):100–105.
- Puhan MA, Scharplatz M, Troosters T, Steurer J. Respiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality—a systematic review. Respir Res 2005; 6(1):54.
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997, 349(9064):1498–1504.
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007; 370(9589):765–773.
- Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006; 27(2):397–412.
- Feinleib M, Rosenberg HM, Collins JG, Delozier JE, Pokras R, Chevarley FM. Trends in COPD morbidity arid mortality in the United States. Am Rev Respir Dis 1989; 140(3 Pt 2):S9–S18.
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007; 370(9589):741–750.
- World Health Organization. Chronic Obstructive Pulmonary Disease (COPD). Fact Sheet No. 315. Geneva: World Health Organization; 2011.
- World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization; 2008.
- Gershon AS, Warner L, Cascagnette P, Victor JC, To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011; 378(9795):991–996.
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380(9859):2095–2128.
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380(9859):2163–2196.
- Wouters EF. Economic analysis of the confronting COPD survey: an overview of results. Respir Med 2003; 97(Suppl C):S3–S14.
- Rabe KF. Guidelines for chronic obstructive pulmonary disease treatment and issues of implementation. Proc Am Thorac Soc 2006; 3(7):641–644.
- Gruffydd-Jones K, Langley-Johnson C, Dyer C, Badlan K, Ward S. What are the needs of patients following discharge from hospital after an acute exacerbation of chronic obstructive pulmonary disease (COPD)? Prim Care Respir J 2007, 16(6):363–368.
- Dahl R, Lofdahl CG. The economic impact of COPD in North America and Europe. Analysis of the Confronting COPD survey. Respir Med 2003; 97(Suppl C):S1–S2.
- Price LC, Lowe D, Hosker HS, Anstey K, Pearson MG, Roberts CM, et al. UK National COPD Audit 2003: impact of hospital resources and organisation of care on patient outcome following admission for acute COPD exacerbation. Thorax 2006; 61(10):837–842.
- Canadian Institutes for Health Information. Health indicators, 2008. Available from: http://secure.cihi.ca/freeproducts/Health Indicators 2008 ENG web.pdf (accessed August 15, 2012).
- Cosio BG, Agusti A. Update in chronic obstructive pulmonary disease 2009. Am J Respir Crit Care Med 2010; 181(7):655–660.
- Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, Thun MJ, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J 2006; 27(1):188–207.
- Ram FS, Wedzicha JA, Wright J, Greenstone M. Hospital at home for patients with acute exacerbations of chronic obstructive pulmonary disease: systematic review of evidence. BMJ 2004, 329(7461):315.
- Bindman AB, Blum JD, Kronick R. Medicare's transitional care paymentda step toward the medical home. N Engl J Med 2013; 368(8):692–694.
- Fairbrother P, Pinnock H, Hanley J, McCloughan L, Sheikh A, Pagliari C, et al. Continuity, but at what cost? The impact of telemonitoring COPD on continuities of care: a qualitative study. Prim Care Respir J 2012; 21(3):322–328.
- Paré G, Poba-Nzaou P, Sicotte C, Beaupré A, Lefrancois É, Nault D, et al. Comparing the costs of home telemonitoring and usual care of chronic obstructive pulmonary disease patients: a randomized controlled trial. European Research in Telemedicine 2013; 2(2):35—47.
- Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995; 333(18):1190–1195.
- Stewart S, Vandenbroek AJ, Pearson S, Horowitz JD. Prolonged beneficial effects of a home-based intervention on unplanned readmissions and mortality among patients with congestive heart failure. Arch Intern Med 1999; 159(3):257–261.
- Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupré A, Bégin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med 2003; 163(5):585–591.
- Gallefoss F. The effects of patient education in COPD in a 1-year follow-up randomised, controlled trial. Patient Educ Couns 2004; 52(3):259–266.
- Hermiz O, Comino E, Marks G, Daffurn K, Wilson S, Harris M. Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ 2002; 325(7370):938.
- Monninkhof E, van der Valk P, van der Palen J, van Herwaarden C, Zielhuis G. Effects of a comprehensive self-management programme in patients with chronic obstructive pulmonary disease. Eur Respir J 2003; 22(5):815–820.
- Majothi S, Jolly K, Heneghan NR, Price MJ, Riley RD, Turner AM, et al. Supported self-management for patients with COPD who have recently been discharged from hospital: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2015; 10:853–867.
- Rees PJ. A disease-specific self-management program reduced hospital utilization and improved health status in COPD. ACP J Club 2003; 139(3):65.
- Garcia-Aymerich J, Hernandez C, Alonso A, Casas A, Rodriguez-Roisin R, Anto JM, et al. Effects of an integrated care intervention on risk factors of COPD readmission. Respir Med 2007; 101(7):1462–1469.
- Jarab AS, Alqudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. Int J Clin Pharm 2012; 34(1):53–62.
- Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy-led disease and medicine management programme for patients with COPD. Br J Clin Pharmacol 2009; 68(4):588–598.
- Adams SG, Smith PK, Allan PF, Anzueto A, Pugh JA, Cornell JE. Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Arch Intern Med 2007; 167(6):551–561.
- Taylor SJ, Candy B, Bryar RM, Ramsay J, Vrijhoef HJ, Esmond G, et al. Effectiveness of innovations in nurse led chronic disease management for patients with chronic obstructive pulmonary disease: systematic review of evidence. BMJ 2005; 331(7515):485.
- Peytremann-Bridevaux I, Staeger P, Bridevaux PO, Ghali WA, Burnand B. Effectiveness of chronic obstructive pulmonary disease management programs: systematic review and meta analysis. Am J Med. Am J Med 2008; 121(5):433–443.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: Wiley, 2006.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414):557–560.
- Jones PW, Quick FH, Baveystock CM. The St George's respiratory questionnaire. Respir Med 1991; 85(Suppl B):25–31.
- Kenealy TW, Parsons MJ, Rouse AP, Doughty RN, Sheridan NF, Hindmarsh JK, et al. Telecare for diabetes, CHF or COPD: effect on quality of life, hospital use and costs. A randomised controlled trial and qualitative evaluation. PLoS One 2015; 10(3):e0116188.
- Bentley CL, Mountain GA, Thompson J, Fitzsimmons DA, Lowrie K, Parker SG, et al. A pilot randomised controlled trial of a Telehealth intervention in patients with chronic obstructive pulmonary disease: challenges of clinician-led data collection. Trials 2014; 15:313.
- Lainscak M, Kadivec S, Kosnik M, Benedik B, Bratkovic M, Jakhel T, et al. Discharge coordinator intervention prevents hospitalizations in patients with COPD: a randomized controlled trial. J Am Med Dir Assoc 2013; 14(6):450.e1–6.
- de Toledo P, Jiménez S, del Pozo F, Roca J, Alonso A, Hernandez C. Telemedicine experience for chronic care in COPD. IEEE Trans Inf Technol Biomed 2006; 10(3):567–573.
- Casas A, Troosters T, Garcia-Aymerich J, Roca J, Hernández C, Alonso A, et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur Respir J 2006; 28(1):123–130.
- Cotton MM, Bucknall CE, Dagg KD, Johnson MK, MacGregor G, Stewart C, et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomised controlled trial. Thorax 2000; 55(11):902–906.
- Cockcroft A, Bagnall P, Heslop A, Andersson N, Heaton R, Batstone J, et al. Controlled trial of respiratory health worker visiting patients with chronic respiratory disability. Br Med J (Clin Res Ed) 1987; 294(6566):225–228.
- Smith BJ, Appleton SL, Bennett PW, Roberts GC, Del Fante P, Adams R, et al. The effect of a respiratory home nurse intervention in patients with chronic obstructive pulmonary disease (COPD). Aust N Z J Med 1999; 29(5):718–725.
- Farrero E, Escarrabill J, Prats E, Maderal M, Manresa F. Impact of a hospital-based home-care program on the management of COPD patients receiving long-term oxygen therapy. Chest 2001; 119(2):364–369.
- Utens CM, Goossens LM, Smeenk FW, Rutten-van Mölken MP, van Vliet M, Braken MW, et al. Early assisted discharge with generic community nursing for chronic obstructive pulmonary disease exacerbations: results of a randomized controlled trial. BMJ Open 2012; 2(5):e001684.
- Skwarska E, Cohen G, Skwarski KM, Lamb C, Bushell D, Parker S, et al. Randomized controlled trial of supported discharge in patients with exacerbations of chronic obstructive pulmonary disease. Thorax 2000; 55(11):907–912.
- Pedone C, Chiurco D, Scarlata S, Incalzi RA. Efficacy of multiparametric telemonitoring on respiratory outcomes in elderly people with COPD: a randomized controlled trial. BMC Health Serv Res 2013; 13:82.
- Pinnock H, Hanley J, McCloughan L, Todd A, Krishan A, Lewis S, et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial. BMJ 2013; 347:f6070.
- Dinesen B, Haesum LK, Soerensen N, Nielsen C, Grann O, Hejlesen O, et al. Using preventive home monitoring to reduce hospital admission rates and reduce costs: a case study of telehealth among chronic obstructive pulmonary disease patients. J Telemed Telecare 2012; 18(4):221–225.
- Kruis AL, Boland MR, Assendelft WJ, Gussekloo J, Tsiachristas A, Stijnen T, et al. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: results of cluster randomised trial. BMJ 2014; 349:g5392.
- Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med 2012; 156(10):673–683.
- Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2010; 182(7):890–896.
- McGeoch GR, Willsman KJ, Dowson CA, Town GI, Frampton CM, McCartin FJ, et al. Self-management plans in the primary care of patients with chronic obstructive pulmonary disease. Respirology 2006; 11(5):611–618.
- Wei L, Yang X, Li J, Liu L, Luo H, Zheng Z, et al. Effect of pharmaceutical care on medication adherence and hospital admission in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled study. J Thorac Dis 2014; 6(6):656–662.
- Tommelein E, Mehuys E, Van Hees T, Adriaens E, Van Bortel L, Christiaens T, et al. Effectiveness of pharmaceutical care for patients with COPD: translated review of the recently published PHARMACOP trial. J Pharm Belg 2014; Sep(3):4–14.
- Wood-Baker R, McGlone S, Venn A, Walters EH. Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology 2006; 11(5):619–626.
- Martin IR, McNamara D, Sutherland FR, Tilyard MW, Taylor DR. Care plans for acutely deteriorating COPD: a randomized controlled trial. Chron Respir Dis 2004; 1(4):191–195.
- Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions? Veterans affairs cooperative study group on primary care and hospital readmission. N Engl J Med 1996; 334(22):1441–1447.
- Seymour JM, Moore L, Jolley CJ, Ward K, Creasey J, Steier JS, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010; 65(5):423–428.
- Eaton T, Young P, Fergusson W, Moodie L, Zeng I, O'Kane F, et al. Does early pulmonary rehabilitation reduce acute health-care utilization in COPD patients admitted with an exacerbation? A randomized controlled study. Respirology 2009; 14(2):230–238.
- Behnke M, Jörres RA, Kirsten D, Magnussen H. Clinical benefits of a combined hospital and home-based exercise programme over 18 months in patients with severe COPD. Monaldi Arch Chest Dis 2003; 59(1):44–51.
- Koff PB, Jones RH, Cashman JM, Voelkel NF, Vandivier RW. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J 2009; 33(5):1031–1038.
- Walters JA, Turnock AC, Walters EH, Wood-Baker R. Action plans with limited patient education only for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2010; 12(5):CD005074.
- Zwerink M, Brusse-Keizer M, van der Valk PD, Zielhuis GA, Monninkhof EM, van der Palen J, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 19(3):CD002990.
- Bergner M, Hudson LD, Conrad DA, Patmont CM, McDonald GJ, Perrin EB, et al. The cost and efficacy of home care for patients with chronic lung disease. Med Care 1988; 26(6):566–579.