ABSTRACT
The study aimed to assess the preferences of expert physicians about the requirements for inhalation devices for patients with chronic obstructive pulmonary disease (COPD) and to identify the most relevant advantages and disadvantages to their prescription. In a two-round Delphi survey, 96 Spanish COPD-expert pulmonologists completed an internet-based questionnaire to evaluate the degree of importance of the characteristics of the inhaler devices in their choice for COPD. The requirements needed for use in COPD were that the device permits a high pulmonary deposit of the drug, allowed its dispensation at low inspiratory flows, did not require hand-mouth coordination, generated an exact and reproducible dose, its operation was easy to teach, provided the perception of a correct inhalation, had an intuitive use mechanism and security mechanisms to prevent overdosing and generates a reduced oropharyngeal deposit (very good consensus). Modulite®, Respimat® and NEXThaler® were associated with high pulmonary deposit, and Respimat® showed correct dispensation at low inspiratory flows. All dry-powder inhaler devices were associated with the advantage of not requiring coordination, and Respimat® was the only device considered as difficult to teach by more than 50% of the experts. Breezhaler® and Genuair® were positively associated with patients' awareness of correct inhalation, whereas Spiromax® stood out for its intuitive use mechanism. In conclusion, our study contributes to defining the inhaler device properties required for their use in patients with COPD, and to identify the devices that, in the opinion of experts, best meet each requirement.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality and represents a substantial economic and social burden throughout the world Citation(1). It is currently the fourth-leading cause of death worldwide, and further increases in its prevalence and mortality are expected in the coming decades Citation(2). Current guidelines for the management of COPD recommend the regular use of inhaled drugs to relieve symptoms and prevent exacerbations Citation(3,4).
A variety of inhaler devices are available for the treatment of patients with COPD, including metered dose inhalers (MDIs), soft mist inhalers (SMIs) and single-dose or multidose dry powder inhalers (DPIs) Citation(5,6). Each of these devices has several advantages and inconveniences, which are widely recognized Citation(6–8); thus there is a broad therapeutic arsenal with the potential to be adapted to the characteristics and needs of various types of patients. However, decades after the introduction of inhaler devices, their correct use remains an obstacle to achieving optimal disease outcomes Citation(9). Moreover, and despite evidence that adherence is strongly associated with a reduced risk of death and fewer hospital admissions Citation(10), poor adherence to inhaled medication is common among patients with COPD Citation(11), and it has been reported that an average of 60% of patients with COPD do not adhere to prescribed therapy and that up to 85% use their inhaler ineffectively Citation(12).
The choice of inhaler device for a particular patient is an important issue in COPD because it can influence the patient's adherence to treatment Citation(13), and thus potentially affect the drug effectiveness and long-term outcomes. This decision typically requires careful consideration of the patient's specific needs, preferences and abilities Citation(14). To understand how to improve the choice of inhaler devices, a number of studies have been conducted with the objectives of comparing the characteristics and requirements of several inhalers Citation(15,16), examining the number of critical errors with each device Citation(17–19) and eliciting patient preferences for various inhalation devices Citation(5,20,21). To our knowledge, however, there is little information regarding how the COPD-treating physicians integrate these data into their daily clinical practice, or about the prescribing physician's real preferences in terms of the most appropriate device for patients with COPD.
The aim of this study was to establish a consensus, among Spanish pulmonologists with preferential dedication to COPD, regarding the most important characteristics that an inhalation device must meet for use in COPD patients. Moreover, we try to identify the advantages and disadvantages of each available model of MDI, SMI and DPI considered as most relevant to prescription decisions.
Methods
Study design
A cross-sectional Internet-based survey was conducted between March and August 2016 among Spanish COPD-expert pulmonologists using a Delphi procedure with two rounds. The study involved the remittance of consecutive surveys to a group of previously selected experts, with the goal of achieving a consensus about the required characteristics of an inhaler device for to be used in COPD patients while ensuring complete independence of the participants Citation(22). First, we asked which general and specific characteristics of the various currently available inhaler devices they considered the most important when making the decision for their patients with COPD. The consensus was obtained through a process that involved aggregating individual opinions Citation(23). Once the first round ended, each panel member received a summary of the responses to the first survey. Second, each expert reviewed their previous responses, comparing them with those of all the other panel members, and then completed the questionnaire again Citation(24).
The study was conducted in four phases: (a) development of the survey questionnaire by the scientific committee; (b) selection of the expert panel; (c) administration of the online survey in two rounds; and (d) analysis of the results and discussion of conclusions in an on-site meeting with the scientific committee.
Development of the questionnaire
A nonsystematic literature review was conducted to identify the variables associated with the characteristics of inhalation devices for the treatment of patients with COPD. A questionnaire was designed that included the most relevant aspects found, as well as additional contributions based on the experience of the scientific committee.
The final version of the survey consisted of 18 items comprising several aspects of an inhaler device, mainly related to its technical characteristics, mechanism of use, handling and maintenance, pulmonary and oropharyngeal deposit, fraction of fine particles and facility for teaching its operation. Two aspects were evaluated in the questionnaire: Citation(1) the degree of importance given by each expert to the various characteristics of an inhaler device, and Citation(2) the three prioritized advantages and disadvantages of each device available in Spain to be used in clinical practice for the management of patients with COPD. The following 16 devices were evaluated: conventional MDI, Modulite®, Respimat®, Aerolizer®, Breezhaler®, Handihaler®, Spinhaler®, Accuhaler®, Easyhaler®, Ellipta®, Genuair®, NEXThaler®, Novolizer®, Spiromax®, Turbuhaler® and Twisthaler®.
Recruitment of experts
The expert panel was comprised of COPD-expert pulmonologists from the Spanish health care system proposed by the scientific committee with criteria of representativeness by geographic distribution. The inclusion criteria were as follows: more than 4 years of professional experience, preferential dedication to COPD (treating an average of 20 patients with COPD or more per month) and contrasted scientific activity related to COPD and/or inhaled therapy. This resulted in a panel of 96 experts from all the autonomous regions in Spain who ultimately agreed to participate in the study.
Questionnaire administration
All the selected experts received an email with an invitation letter and a link to the Internet-based questionnaire for the first and second round. Email reminders were sent after 4 weeks. Participation in the study was voluntary, and experts participated anonymously. The respondent's sociodemographic data, such as age, sex, years of work experience and number of patients with COPD attended monthly, were also collected.
The first round of the survey was conducted between March and May 2016. The experts were required to respond to 18 questions about general characteristics, according to a 9-point, Likert-type ordinal scale (where 1 = not important and 9 = very important). From the 18 general characteristics, the experts were also asked to rank the three advantages and limitations of each evaluated inhaler device (1 = most important advantage/limitation; 2 = second-most important advantage/limitation; 3 = third-most important advantage/limitation), to identify its strengths and weaknesses.
In the second round, conducted between June and August 2016, only those items for which there was no clear consensus in the preceding round were included.
Data analysis
The questionnaire responses were grouped into 3 categories taken from the 9-point, Likert-type scale, depending on the assigned level of importance: not important Citation(1–3), neutral Citation(4–6), and important Citation(7–9) Citation(25); the response frequency was calculated for each level. In the first round of the study, an agreement/consensus was considered to be reached when ≥ 80% of the panelists scored a characteristic within the same interval; in the second round, for the purposes of strengthening the consensus, an agreement/consensus was considered to be reached when ≥ 85% of the panelists scored a characteristic within the same bracket. The purpose of this increase was the need for a more robust final consensus.
For the questions related to the advantages/limitations of the devices, the number of times a characteristic was chosen was calculated, regardless of whether it was the 1st, 2nd or 3rd option. The percentage was calculated based only on responses from those experts who had reported clinical experience with the device. Each selection was weighted according to the following criteria: most important advantage/limitation, 3 points; second-most important advantage/limitation, 2 points; third-most important advantage/limitation, 1 point. Based on this, the weighted average and standard deviation of each characteristic selected for each inhaler was calculated. Given the low number of panelists who had clinical experience with Spinhaler® (25 experts), this device was excluded from the reported results.
The data were comprehensively analyzed in a descriptive manner. For the analysis between both rounds, the McNemar-Bowker test Citation(26), an adaptation of the McNemar test, was used to compare paired variables with more than two categories. A two-sided significance level α of 0.05 was defined. The data were analyzed using the statistics software package SAS v9.2. (SAS Institute Inc., Cary, NC, USA).
Results
All 96 expert pulmonologists from the 17 Spanish regions completed both rounds. Thirty-six were women (37.5%), and their mean age was 46 ± 9 years (range: 32 to 63 years). Half the experts had more than 15 years of work experience treating patients with COPD, and the majority of the selected experts (80%) treated an average of more than 40 patients with COPD per month. Their professional categories were head of service (13.5%), attending physician or hospitalist (26.0%) and area specialist physician (60.4%).
Characteristics of the ideal inhaler device for COPD
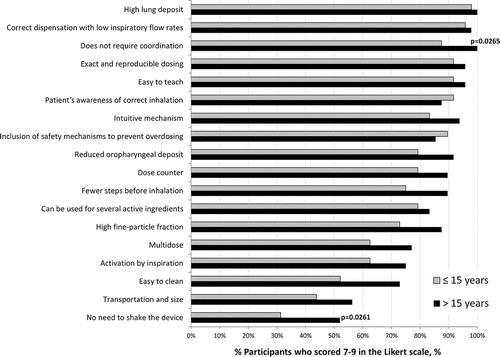
shows the importance rating of the 18 evaluated characteristics of an optimum inhaler device for patients with COPD; nine were rated as important (scored between 7 and 9 on the Likert scale of importance) by ≥85% of the experts. This led to the establishment of a typology of characteristics for the ideal device, which would include Citation(1) “high pulmonary deposit”; Citation(2) “correct dispensation with low inspiratory flow rates”; Citation(3) “does not require coordination”; Citation(4) “exact and reproducible dosing”; Citation(5) operation is “easy to teach”; Citation(6) “patient's awareness of correct inhalation”; Citation(7) “intuitive use mechanism”; Citation(8) “inclusion of safety mechanisms to prevent overdosing”; and Citation(9) “reduced oropharyngeal deposit”. The feature that was rated the highest was “high pulmonary deposit,” with 99% of the experts placing it in the “important” category and obtaining an average score of 8.4 out of 9. The next most important characteristic was “correct dispensation with low inspiratory flow rates” (97%). The characteristics “does not require coordination,” “exact and reproducible dosing” and “easy to teach” were all considered equally “important” by the experts (94%). The other ideal device characteristics obtained percentages of between 85% and 90%. The remaining evaluated characteristics were considered as important by less than 85% of the experts ().
Table 1. Most important characteristics to consider in the choice of an inhaler device in patients with COPD.
Interestingly, we found differences in the importance attributed to the characteristics of inhaler devices for their prescription according to the years of professional practice. Compared with the most experienced experts, those with less than 15 years of professional experience rated 7–9 less frequently on the Likert scale for “does not require coordination” (87.5% vs. 100%; p = 0.027) and “no need to shake the device” (31.3% vs. 52.1%, p = 0.026) (). No other differences were detected between these subgroups. There were also no differences between the importance attributed to the characteristics of the inhaler devices according to age, sex or number of patients with COPD attended monthly.
Main advantages and limitations of the available inhaler devices for patients with COPD
shows the three main advantages and disadvantages of the MDIs, SMIs and single-dose and multidose dry powder inhalers (sdDPIs and mdDPIs, respectively).
Table 2. Advantages and disadvantages considered most important for the election of an inhaler device in patients with COPD.
In the case of the conventional MDI, the main advantage indicated by the experts was that “it can be used with a spacer” (86%); however, 66% of the experts considered the fact that the device “requires coordination” as a limitation. With regard to Modulite®, the main advantage, according to 54% of experts, was the “high fine-particle fraction,” whereas its main limitation was also that “it requires coordination” (49%). The main advantage of Respimat® was “the correct dispensation with low inspiratory flow rates” (71%), and its most cited limitation was that “it is not easy to teach” (64%).
With respect to the sdDPIs, their main advantage was that they “[do] not require coordination” (56.8% of the experts for Handihaler®, 53.7% for Breezhaler®, 52.1% for Aerolizer® and 40.0% for Spinhaler®), whereas their main limitations were the “high number of steps before inhalation” for Aerolizer® (43.8% of the experts), Breezhaler® (38.9%) and Handihaler® (38.9%), as well as the “high oropharyngeal deposit” for Spinhaler® (40.0% of the experts).
Evaluation of the mdDPIs showed greater differences between the devices. The main advantages attributed to Accuhaler® were “the dose counter” (56%) and that it “does not require coordination” (53.7%), whereas the fact that they “did not require coordination” was considered the main advantage of the rest of the mdDPIs (from 57.4% of the experts for Ellipta® to 37.9% for Genuair®). The “lower number of steps before inhalation” was also considered as a relevant advantage of Ellipta® (45.7%), Spiromax® (40.4%), Easyhaler® (34.6%) and Twisthaler® (22.3%). In contrast, the “high oropharyngeal deposit” was considered the main limitation of Novolizer® (40.1%), Accuhaler® (38.9%) and Easyhaler® (33.3%); the “patient's unawareness of correct inhalation” was the main limitation of Ellipta® (51.1%) and Twisthaler® (46.5%); the “incorrect dispensation with low inspiratory flow rate” was the main limitation of Genuair® (40.0%) and Turbuhaler® (38.9%); and that “it cannot be used for several active ingredients/drugs” was the main limitation of NEXThaler® (57.4%) and Spiromax® (46.6%).
Characteristics of the ideal inhaler for COPD in the currently available devices
shows the nine characteristics of the ideal inhaler device and the extent to which each device is associated with one of these, both positively (a characteristic that, according to the experts, is an advantage of the device) and negatively (a characteristic that, according to the experts, is a limitation of the device).
Table 3. Main advantages and disadvantages to prescribing each inhaler device in patients with COPD in relation to the ideal device.Table Footnote*
The most valued characteristic, “high pulmonary deposit,” is an advantage that the experts only associated with Modulite®, Respimat® and NEXThaler®. In the case of “correct dispensation with low inspiratory flow rate,” Respimat® was considered by the experts to be the only device that had this characteristic as an advantage, whereas this requirement was considered as a disadvantage for Aerolizer®, Breezhaler®, Handihaler®, Accuhaler®, Genuair®, Novolizer®, Turbuhaler® and Twisthaler®.
All the DPI devices, both single-dose and multidose, were associated with the advantage of “not requiring coordination,” whereas in the case of the conventional MDIs and Modulite®, this characteristic was considered a specific limitation. Respimat® is the only device whose use was considered “difficult to teach” by more than 50% of the experts, whereas the absence of this aspect was considered an advantage of Aerolizer®, Breezhaler®, Handihaler®, Easyhaler®, Novolizer® and Twisthaler®.
In addition, Breezhaler® and Genuair® were the only devices that the experts positively associated with “patient's awareness of correct inhalation,” whereas Spiromax® was the only device for which the advantage of having an “intuitive mechanism” was identified.
Interestingly, the experts did not consider any of the devices to fulfill the ideal characteristics of “exact and reproducible dosing,” “inclusion of safety mechanisms to avoid overdosing” or “reduced oropharyngeal deposit.” Furthermore, this last characteristic was identified as a limitation for all the devices except Modulite®, Respimat®, Handihaler® and NEXThaler®.
Discussion
The main findings of the present study are the identification of features that COPD-expert pneumologists consider essential to choosing the inhaler device to prescribe to their patients. In addition, the advantages and disadvantages of the various available devices perceived as being more relevant to clinical practice are identified, as well as the devices which, in the opinion of expert prescribers, better meet the requirements considered essential for treating patients with COPD.
According to the results obtained, the two most important characteristics that an inhaler must meet for use in patients with COPD is that it permits a high pulmonary deposit of the drug and allows its delivery at low inspiratory flows. Those characteristics were followed by not requiring hand-mouth coordination, generating an exact and reproducible dose and ease of teaching its use to the patient. Although with a slightly lower level of agreement, it is also important that it provides the perception of a correct inhalation, has an intuitive mechanism of use, has security mechanisms to prevent overdosage and that it generates a reduced oropharyngeal deposit. However, the participating experts did not consider other features that have previously been proposed as relevant for the choice of an inhaler device, such as not being necessary to shake the device before use, its suitable size and portability, its ease of cleaning, its activation with inspiration, a multidose feature, its generation of a high fraction of ultrafine particles, its possibility of use with different active principles, the desire for a smaller number of steps before inhalation and having a dose counter Citation(8,14,18,27–30). From the data obtained, it is not possible to discriminate whether the nonselection of these properties is due to the fact that the interviewees consider that these properties are already satisfactorily fulfilled in most of the available inhalers or because they appear less relevant for establishing a prescription in patients with COPD.
Some of the features selected as necessary in the ideal inhaler device had already been highlighted in previous surveys among health professionals, such as the ease of use and teaching Citation(16,31,32). However, some discrepancies were also identified. In a web-based questionnaire performed among Danish nurses who treat patients with COPD, it was indicated that having a dose meter was one of the two most important characteristics of an inhaler Citation(32), a circumstance that did not appear so highly valued by the participants in our study. Our study also allows the identification of some features that might be considered more specific for COPD patients. In a previous Delphi survey, 50 Belgian pulmonologists performed a global evaluation of the criteria to choose an inhaler device when prescribing inhalation treatment for obstructive lung disease (asthma or COPD) Citation(31). In addition to features related to the patient (preferences and ability for use the device correctly), the prescriber (acquaintance with the system) and the cost, the only selected features of the inhaler device were the ease to teach and use and that it can be used for any active substance Citation(31). In contrast, the present study identifies some COPD-specific features of inhaler devices particularly relevant for several aspects of these patients, such as the severity of airflow limitation (allows its delivery at low inspiratory flows) or the advanced age (not requiring hand-mouth coordination). Probably, these features are less relevant in patients with other obstructive pulmonary diseases, such asthma or cystic fibrosis, because they usually are younger and do not present such a marked impairment of lung function.
With regard to the influence of the characteristics of the expert prescribers, the only variable that is associated with a difference in their preferences is the length of professional activity. Thus, a lower percentage of pulmonologists with less than 15 years of professional experience considered important that the inhaler does not require coordination or that it has to be shaken before use, probably because during the period of development of their professional activity they have always had alternatives to the MDI, for which such a requirement is essential.
Interestingly, the characteristics considered essential by expert pulmonologists do not completely coincide with patients' preferences. In an international survey of patients with COPD, the most important attributes of inhalers were their ease of use, dose counter, dose capacity (multidose) and requiring a smaller number of steps before inhalation Citation(16). This finding reveals that although patient preference should be a factor to consider when choosing an inhaler device Citation(33), the prescriber also considers another set of aspects. In this sense, the controversy around whether the choice of the inhaler preferred by the patient guarantees a lower number of critical errors and greater compliance remains unresolved Citation(19,20).
With respect to the perception of the strengths and weaknesses of the various types of inhaler devices, the experts do not equally value all the factors described in the literature. For the MDIs, they select commonly recognized advantages, such as the possibility of adapting to space chambers, the portability or the possibility of administering different active principles, as well as classic disadvantages, such as the need for hand-mouth coordination, the high oropharyngeal deposit and the absence of dose counter Citation(9,27,29,30). However, the experts do not rate other advantages indicated in the literature, such as the low dependence of the inspiratory flow and the good reproducibility of the emitted dose, or some disadvantages, such as the difficulty of teaching its use, the need for a propellant and the dose sensitive to temperature of the canister Citation(27,30).
In the case of SMIs, the experts recognize previously reported advantages and disadvantages Citation(8), such as the good reproducibility of the emitted dose as well as its difficulty of use, the need for high inspiratory flow, and reduced pulmonary deposition. However, and unlike what has been described by other authors Citation(30), they do not consider that the lower oropharyngeal deposit or the dose meter implies an advantage, nor does the need for coordination appear to be a limitation. Finally, the experts agree with previous studies that indicate, as advantages of DPIs, that coordination is not needed Citation(15,27,30) and the existence of a dose counter Citation(27,30); whereas, high oropharyngeal deposition is interpreted as a limitation Citation(14,27,29). However, the experts attach no particular value to the fact that different active principles might be administered or to the speed of administration Citation(27). Contrary to what has been described by other authors Citation(30), they also do not consider that the difficulty of loading or the variability in the inhaled dose constitute very relevant limitations.
When identifying the models of inhaler devices best suited to the essential requirements for use in COPD, the experts agree that the need for a high inspiratory flow is a limitation of all sdDPIs and of most of the mdDPIs except Easyhaler®, Ellipta® and Spiromax®. Among the sdDPIs, a greater percentage of the experts consider that this aspect is more limiting for Handihaler® (35.8%) than for Breezhaler® (28.4%), coinciding with the description of a lower internal resistance of the latter device with respect to the former Citation(15). Although some mdDPIs, such as Spiromax®, have been reported to require less inspiratory flow than Turbuhaler® Citation(34), experts only recognize this as a relevant advantage of Respimat® versus most DPIs. Regarding the intuitive mechanism of use, although multiple previous studies have pointed out differences between various inhaler devices in both the percentage of critical errors and in the correct performance of the first administration Citation(17–19,35–37), with some supposed advantage of the DPIs against the MDIs Citation(9,19,35–38), experts only recognize this advantage in Spiromax® compared with other devices. This aspect may be particularly relevant, since a more intuitive inhaler device will be easier to teach and will generate fewer errors in its daily management. In addition, they note that this requirement constitutes a limitation of Respimat®, coinciding with the results of a real-life study of 2935 patients with COPD, who identified a higher percentage of errors with this device compared with other inhalers Citation(39).
Our study has several limitations. First, it reflects the opinion of a select sample of expert pulmonologists who do not represent all the health professionals involved in COPD treatment, although these experts could be considered to have a certain role of leadership and influence over the whole. Second, it is a study of national scope and is limited to the devices currently available in Spain. Third, the Delphi model provides a view of opinions, but we have no objective data to confirm that these preferences correspond to the experts' actual prescribing patterns. Fourth, the state of opinion collected has a temporal validity, given it might fluctuate over time. Finally, the price of inhalers has not been considered in the survey because it is not an intrinsic feature of the device, although it is surely an element that can condition the prescription.
In conclusion, our study contributes to the definition of the required features of an inhaler device to be used in daily clinical practice for patients with COPD and to identify the devices that, according to experts, best meet each requirement. In this sense, it is important to emphasize that the experts participating in the study consider that the administration of an exact and reproducible dose, the inclusion of safety mechanisms to avoid overdosing and the generation of a reduced oropharyngeal deposit are three essential aspects for the use in patients with COPD, for which there is no clear predominance of any of the currently available inhaler devices.
Declaration of interest
The authors report no conflicts of interest.
Acknowledgements
Funding was provided by an unrestricted educational grant from TEVA Pharmaceutical SLU. The EPOCA Steering Committee, comprising five academics, developed the design and concept, approved the statistical analysis plan, had full access to and interpreted the data, wrote the article, and was responsible for decisions with regard to publication.
References
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2013;380(9859):2163–2196.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PloS Med. 2006;3(11):e442.
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582.
- Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, et al. Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Arch Bronconeumol. 2017;53(6):324–335.
- Hodder R, Price D. Patient preferences for inhaler devices in chronic obstructive pulmonary disease: experience with respimat soft mist inhaler. Int J Chron Obstruct Pulmon Dis. 2009;4:381–390.
- Sanchis J, Corrigan C, Levy ML, Viejo JL, ADMIT Group. Inhaler devices – from theory to practice. Respir Med. 2013;107(4):495–502.
- Pritchard JN. Industry guidance for the selection of a delivery system for the development of novel respiratory products. Expert Opin Drug Deliv. 2015;12(11):1755–1765.
- Rogliani P, Calzetta L, Coppola A, Cavalli F, Ora J, Puxeddu E, et al. Optimizing drug delivery in COPD: The role of inhaler devices. Respir Med. 2017;124(3):6–14.
- Melani AS, Bonavia M, Cilenti V, Cinti C, Lodi M, Martucci P, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930–938.
- Vestbo J, Anderson JA, Calverley PMA, Celli B, Ferguson GT, Jenkins C, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64(11):939–943.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831–838.
- Restrepo RD, Alvarez MT, Wittnebel LD, Sorenson H, Wettstein R, Vines DL, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(3):371–384.
- Bateman ED. Improving inhaler use in COPD and the role of patient preference. Eur Respir Rev. 2005;14:85–88.
- Dekhuijzen PNR, Vincken W, Virchow JC, Roche N, Agusti A, Lavorini F, et al. Prescription of inhalers in asthma and COPD: towards a rational, rapid and effective approach. Respir Med. 2013;107(12):1817–1821.
- Chapman KR, Fogarty CM, Peckitt C, Lassen C, Jadayel D, Dederichs J, et al. Delivery characteristics and patients' handling of two single-dose dry-powder inhalers used in COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:353–363.
- Molimard M, Colthorpe P. Inhaler devices for chronic obstructive pulmonary disease: insights from patients and healthcare practitioners. J Aerosol Med Pulm Drug Deliv. 2015;28(3):219–228.
- Schulte M, Osseiran K, Betz R, Wencker M, Brand P, Meyer T, et al. Handling of and preferences for available dry powder inhaler systems by patients with asthma and COPD. J Aerosol Med Pulm Drug Deliv. 2008;21(4):321–328.
- Rootmensen GN, van Keimpema AR, Jansen HM, de Haan RJ. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. J Aerosol Med Pulm Drug Deliv. 2010;23(5):323–328.
- Chorão P, Pereira AM, Fonseca JA. Inhaler devices in asthma and COPD—an assessment of inhaler technique and patient preferences. Respir Med. 2014;108(7):968–975.
- Lenney J, Innes JA, Crompton GK. Inappropriate inhaler use: assessment of use and patient preference of seven inhalation devices. EDICI. Respir Med. 2000;94(5):496–500.
- Schürmann W, Schmidtmann S, Moroni P, Massey D, Qidan M. Respimat Soft Mist inhaler versus hydrofluoroalkane metered dose inhaler: patient preference and satisfaction. Treat Respir Med. 2005;4(1):53–61.
- Booberg AL, Morris-Khoo SA. The Delphi method: a review of methodology and an application in the evaluation of a higher education program. Can J Prog Evaluat. 1992;7(1):27–39.
- Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008–1015.
- Williams PL, Webb C. The Delphi technique: a methodological discussion. J Adv Nurs. 1994;19(1):180–186.
- Mattel M, Jacoby J. Is there an optimal number of alternatives for likert scale items – study – reliability and validity. Educ Psychological Meas. 1971;31:657.
- Bowker AH. A test for symmetry in contingency tables. J Am Stat Assoc. 1948;43(244):572–574.
- Fromer L, Goodwin E, Walsh J. Customizing inhaled therapy to meet the needs of COPD patients. Postgrad Med. 2010;122(2):83–93.
- Plaza V, Calle M, Molina J, Quirce S, Sanchis J, Viejo JL, et al. External validation of the recommendations of the multidisciplinary consensus about inhaled therapies. Arch Bronconeumol. 2012;48(6):189–196.
- Newman SP. Inhaler treatment options in COPD. Eur Resp Rev. 2005;14:102–108.
- Ferré A, Dres M, Roche N, Antignac M, Becquemin MH, Trosini V, et al. Inhalation devices: characteristics, modeling, regulation and use in routine practice. Rev Mal Respir. 2012;29(2):191–204.
- Ninane V, Brusselle GG, Louis R, Dupont L, Liistro G, De Backer W, et al. Usage of inhalation devices in asthma and chronic obstructive pulmonary disease: a Delphi consensus statement. Expert Opin Drug Deliv. 2014;11(3):313–323.
- Bøgelund M, Hagelund L, Asmussen MB. COPD-treating nurses' preferences for inhaler attributes – a discrete choice experiment. Curr Med Res Opin. 2017;33(1):71–75.
- Devillier P, Salvator H, Roche N. The choice of inhalation device: A medical act. Rev Mal Respir. 2015;32(6):599–607.
- Azouz W, Chetcuti P, Hosker H, Saralaya D, Chrystyn H. Inhalation characteristics of asthma patients, COPD patients and healthy volunteers with the Spiromax® and Turbuhaler® devices: a randomised, cross-over study. BMC Pulm Med. 2015;15:47.
- Molimard M, Raherison C, Lignot S, Depont F, Abouelfath A, Moore N. Assessment of handling of inhaler devices in real life: an observational study in 3811 patients in primary care. J Aerosol Med. 2003;16(3):249–254.
- Lee H, Boo S, Lim Y, Kim S, Kim IA. Accuracy of inhaler use in patients with chronic obstructive pulmonary disease. Clin Nurs Res. 2014;23(5):560–574.
- Aydemir Y. Assessment of the factors affecting the failure to use inhaler devices before and after training. Respir Med. 2015;109(4):451–458.
- Melzer AC, Ghassemieh BJ, Gillespie SE, Lindenauer PK, McBurnie MA, Mularski RA, et al. Patient characteristics associated with poor inhaler technique among a cohort of patients with COPD. Respir Med. 2017;123:124–130.
- Molimard M, Raherison C, Lignot S, Balestra A, Lamarque S, Chartier A, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017; 49: 1601794.