Abstract
Acute exacerbations are associated with disease progression, hospital admission and death in people with chronic obstructive pulmonary disease (COPD). The detrimental outcomes associated with acute exacerbations highlights a need to understand the time course of recovery following acute exacerbation of COPD (AECOPD) so that effective and timely interventions can be provided. The aim of this narrative review was to describe the natural recovery in physiology, symptoms and function following AECOPD. Substantial recovery of lung function and airway inflammation occurs in the first week after onset of an AECOPD, whilst systemic inflammatory markers may take up to two weeks to recover. Symptoms generally improve over the first 14 days, however marked variation is evident between studies and individuals. There are limited data regarding the time course of recovery for functional capacity, quality of life and strength. In a small number of patients (<10%) recovery of lung function and symptoms has not occurred by three months. Features of patients at risk of a prolonged recovery following AECOPD include older age, more severe lung disease, presence of chronic bronchitis, lower body mass index and more chronic dyspnoea. Exacerbation features associated with prolonged recovery are symptoms of the common cold at exacerbation onset, evidence of viral infection, more severe dyspnoea during the exacerbation and persistent systemic inflammation. In clinical practice efforts should be made to recognise prolonged recovery, which puts patients at risk of poor outcomes, and to address the consequences of AECOPD including physical inactivity and skeletal muscle weakness. Whether delivery of specific interventions at distinct time points in the recovery process can enhance recovery remains to be determined.
Introduction
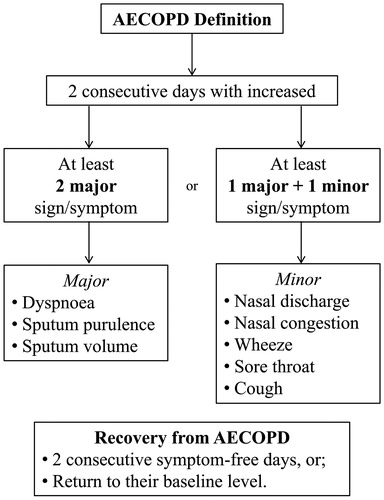
Chronic obstructive pulmonary disease (COPD) is characterised by significant functional limitation and high mortality (Citation1). One of the key factors associated with disease progression is an acute change in the condition, referred to as an acute exacerbation of COPD (AECOPD) (Citation2). There is no universally accepted definition for an AECOPD, however the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy for COPD management describes exacerbations as a worsening of symptoms compared with the patient’s baseline (Citation1). For research purposes (), an AECOPD has previously been defined as two consecutive days with an increase in at least two major signs and/or symptoms; or the presence of one major together with a minor sign and/or symptom (Citation3,Citation4). Recovery from an AECOPD has been defined as two consecutive days without symptoms; or, a return of symptoms to their baseline level (Citation2). The time course to achieve recovery of physiology, symptoms and function following AECOPD has not been comprehensively described.
Figure 1. Definition of acute exacerbation of COPD (AECOPD). Definition for AECOPD from Seemungal et al. (Citation3) and the definition of recovery for AECOPD from Donaldson et al. (Citation2).
Exacerbations of COPD reduce both quality of life and physical function, which may not spontaneously recover (Citation5,Citation6). Exacerbations can be considered both in terms of severity (e.g. the level of breathlessness) and frequency (recurrence of exacerbation, regardless of severity) (Citation7). During an AECOPD levels of breathlessness may become overwhelming, resulting in a need for changes in medication (signifying a moderate exacerbation) and/or hospitalisation for supportive therapy (a severe exacerbation) (Citation3). Exacerbation severity is meaningful to patients and measures of severity can be used to identify when recovery has occurred. However, frequency of AECOPD is also an important marker. Exacerbations are one of the leading causes of hospital admission and death in people with COPD (Citation5,Citation8). People with COPD who have frequent exacerbations over the course of a year often fail to return to their stable condition within the first month after exacerbation onset (Citation9). Frequent exacerbators are usually older, ex-smokers, with more severe disease and experience more chronic dyspnoea (Citation1,Citation10). Frequent exacerbators are more than twice as likely to have severe AECOPD (requiring hospitalisation) than infrequent exacerbators (15% vs 7%) (Citation11). Understanding the natural course of recovery from an AECOPD is important in order to recognise when recovery has not occurred, to identify when additional interventions could be needed that may shorten the time of recovery following AECOPD, and thus reduce the negative consequences of exacerbations.
The aim of this review was to describe the natural recovery following AECOPD for lung function, inflammatory markers, symptoms, physical activity and quality of life.
Respiratory function
A number of studies have quantified the reduction in lung function at exacerbation onset and the time course of recovery. Data from a cohort study with 73 participants who had moderate to severe COPD indicated the median (interquartile range (IQR)) percentage decline in forced expiratory volume in one second (FEV1 in L) from baseline to exacerbation onset was 5.1% (IQR −15.0 to 6.2%) (Citation12). On average, 88% of the total recovery in lung function occurs in the first week after onset of an AECOPD (), with continued improvement over the following month (Citation9,Citation13). A study of hospitalised patients with an AECOPD found that both FEV1 and forced vital capacity (FVC) improved significantly to day 10 compared to at the onset of exacerbation (Citation9). Both FEV1 and FVC continued improving to day 40 (Citation9). However, the study did not have baseline data for when participants were in a stable condition, and as a result it was not possible to be certain that recovery of FEV1 was complete at this time point. The median time for recovery of PEFR to baseline has been reported as five days (IQR 0–14 days) (Citation2) or six days (1–14 days) (Citation3). The rate at which respiratory function recovers will be influenced by the treatment received. Corticosteroid treatment results in more rapid recovery of FEV1 over the first 72 hours (mean 140 ml greater compared to no corticosteroids, 95% confidence interval (CI) 90–200 ml), however no difference was evident at later time points (Citation14).
Figure 2. Recovery of lung function after AECOPD. Day 0 is onset of exacerbation. Data are mean ± SD unless otherwise stated. (a) Recovery of FEV1 %predicted over time. (b) Recovery of FEV1 (L) over time. FEV1 = forced expiratory volume in one second; AECOPD = acute exacerbation of chronic obstructive pulmonary disease; n = number of participants; L = litres; %predicted = percentage of predicted normal.
Note: Data from Jetmalani et al. (Citation74) are a combination of the means and SD from two groups presented in the study (Citation75). Data from Kocks et al. (Citation46) were derived from the graph, there is no SD.
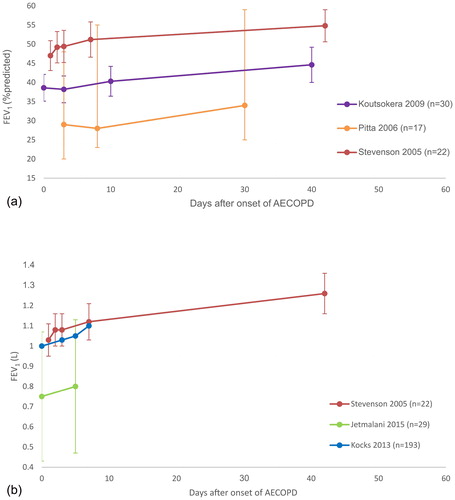
Although improvement in lung function appears to occur most rapidly during the first week after an exacerbation, other authors have reported a longer recovery period, or incomplete recovery. A study of over 100 participants with moderate to severe COPD over 2.5 years found that recovery of PEFR to baseline values was complete in 75% of exacerbations at 35 days, whereas in 7% of exacerbations PEFR recovery had not occurred by 91 days (Citation3). In a cohort of 384 people with moderate to severe COPD followed for 1,039 days, PEFR did not return to baseline by day 99 in 257 out of 3087 exacerbations (8%) (Citation2). Parker et al. (Citation15) assessing patients with a moderate AECOPD found significant changes in FEV1 from baseline did not occur until day 14 after onset of the exacerbation (Citation15). In this study, participants were divided into two groups at 60 days following AECOPD: ‘Symptomatically Recovered’ (dyspnoea returned to baseline levels) and ‘Symptomatically Non-recovered’ (Citation15). Individuals classified as ‘Symptomatically Recovered’ had significant improvement in FEV1 from baseline to the recovery visit at day 60 (0.98 ± 0.08 L to 1.20 ± 0.08 L respectively, p < 0.05). Individuals classified as ‘Symptomatically Non-recovered’ had a small increase in FEV1 after 60 days that was not statistically significant (data not reported) (Citation15). The reasons for incomplete recovery are not clear, but may relate to repeated exacerbations. In one study, repeat exacerbation had occurred in 22% of participants by day 50 (Citation12). This is clinically important, as re-exacerbation is associated with a greater decline in lung function over time (Citation16).
Recently other respiratory function measures have been investigated for their ability to predict onset of an AECOPD and describe the recovery process (Citation17,Citation18). Remote home monitoring of breathing rate showed a decrease in average resting respiratory rate over the first week following an AECOPD, however there was marked inter-individual variation, with some patients showing no changes in respiratory rate, suggesting this measure may not be sufficiently sensitive to physiological change in this period (Citation17). A large randomised controlled trial (n = 312) in people with COPD and GOLD stage II–IV used remote monitoring of airway mechanics with the forced oscillation technique (FOT) to detect the onset of exacerbations, initiate treatment and monitor recovery (Citation19). There was no effect on the primary outcomes of time to first hospitalisation or health-related quality of life, however re-hospitalisation may have been reduced in those undergoing remote monitoring with FOT. Respiratory alerts, triggered by a trend in worsening of at least one FOT parameter, were accompanied by worsening respiratory symptoms on 50% of occasions and prompted a change in treatment on 34% of occasions. These data suggest that FOT may be sensitive for detecting onset of AECOPD, unlike spirometry and PEFR which may not significantly worsen before an exacerbation (Citation3). The clinical utility and cost effectiveness of using FOT in routine care remain to be established.
Overall, the body of literature shows a consistent decline in lung function at the onset of AECOPD, with most recovery occurring within the first week. However, in a small number of individuals (less than 10%) full recovery of lung function may not occur by three months. Many studies documenting the recovery of lung function have been of small sample size which could limit the extent to which these results represent the COPD population at large. However, it seems likely that some patients do not experience complete recovery of lung function parameters even many months following an AECOPD, underscoring the importance of these events in the natural history of the disease.
Inflammatory markers
Inflammatory markers increase during an AECOPD. Such markers include interleukin-6 (IL-6), interleukin-8 (IL-8), tumour necrosis factor-alpha (TNF-α) and C-reactive protein (CRP). The systemic inflammatory response during an AECOPD can be measured by blood analysis, while airway inflammation is assessed by the presence of inflammatory markers in sputum or exhaled breath condensate (Citation20). An increase in IL-6 has been associated with a decrease in exercise tolerance in stable patients (Citation21), and high TNF-α has been related to muscle weakness in experimental tests in animal models (Citation22), suggesting that inflammation may be associated with worse functional performance.
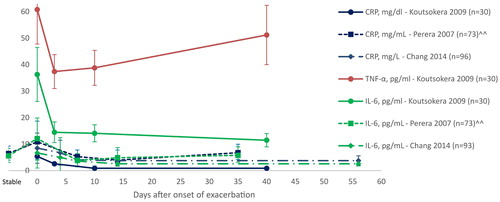
Resolution of airway inflammatory markers after an exacerbation generally occurs before recovery of systemic inflammation (Citation12,Citation23). Chang et al. (Citation23) measured systemic and airway inflammatory markers of hospitalised patients with an AECOPD at days zero (admission), four, seven and fourteen and found that airway inflammatory markers decreased significantly (p < 0.01) on day four and then remain at the same levels over the following ten days (Citation23); while systemic inflammation decreased significantly at day seven and again on day 14 (p < 0.001 for IL-6 and CRP) (Citation23). The decrease in inflammatory markers over this time period is likely to be influenced by treatment for AECOPD, particularly corticosteroids. The more quickly that airway inflammatory markers were reduced was associated with a shorter time to symptom recovery (sputum IL-6: rs = 0.57, p = 0.004; sputum IL-8: rs = 0.48, p = 0.01) and shorter time to lung function improvement (PEFR versus systemic inflammation Mantel R value: 0.770) (Citation12,Citation23). Other authors have reported a longer time for resolution of systemic inflammation in people experiencing an AECOPD () (Citation9, Citation12). Persistently high levels of systemic inflammation may indicate a risk of re-exacerbation. A higher CRP at day 14 is a significant predictor of recurrent exacerbation by day 50 (p = 0.004), independent of disease severity, exacerbation frequency or oral steroid treatment (Citation12). The GOLD strategy reports that in patients with a history of exacerbations, anti-inflammatory treatments with Level A evidence for reduction in future exacerbations are inhaled corticosteroids (in addition to a long acting bronchodilator), phosphodiesterase-4 (PDE-4) inhibitors and long-term antibiotics (azithromycin and erythromycin) (Citation24). The European Respiratory Society/American Thoracic Society guidelines recommend treatment with the PDE-4 inhibitor roflumilast to prevent future exacerbations in patients with severe disease, chronic bronchitis and recurrent exacerbations despite optimal therapy (Citation25). Oral mucolytics and macrolide antibiotics are also recommended for the same purpose in those with moderate to severe disease and recurrent exacerbations despite optimal therapy(Citation25).
Figure 3. Recovery of systemic inflammatory markers over time. Day 0 is onset of exacerbation. Data are mean ± SD, unless otherwise stated. CRP = C-reactive protein; IL-6 = interleukin-6; TNF-α = tumour necrosis factor-alpha; AECOPD = acute exacerbation of chronic obstructive pulmonary disease; pg = picograms; ml = millilitre; mg = milligram; dl = decilitre; n= number of participants. ^^data presented as median.
Symptoms
Exacerbations of COPD are characterised by an increase in symptom severity compared to baseline. Increased symptoms are associated with worse health status, poorer health-related quality of life and reduced exercise capacity (Citation26,Citation27). Symptom assessment may comprise questionnaires, scales, or total symptom count – which is a sum of the number of increased symptoms (e.g. dyspnoea, cough, sputum production, wheezing and night-time awakenings) usually presented as a total score per day (Citation3,Citation12). presents the time course of symptom resolution after onset of AECOPD across eight studies. Although a number of studies show the resolution of symptoms before two weeks (Citation2,Citation3,Citation9,Citation12,Citation15,Citation23,Citation28,Citation29), there are also patients for whom it takes much longer. Perera et al. (Citation12) found that 23% of their 73 participants did not have full symptom recovery by day 35 after the onset of an AECOPD (Citation12). Parker et al. (Citation15) also found that participants who reported full symptom recovery (n = 12) took a mean of 41 days for dyspnoea intensity to return to pre-exacerbation level (Citation15). However, the small number of participants in these studies makes it uncertain if this is representative of the wider COPD population. Data from a cohort study with a larger population (334 participants) found that symptoms were fully recovered in a mean of 14.5 days (SD 8.5 days) after the onset of AECOPD (Citation2). Another study in 101 patients with moderate to severe COPD reported symptomatic recovery in 86% of patients by day 35 (Citation3). Symptom recovery may lag behind the recovery of lung function, with PEFR recovery preceding symptom recovery by an average of 3.6 days (Citation2). A longer symptom duration has been associated with poorer health status (measured by St. Georges Respiratory Questionnaire) and a significantly shorter time to the onset of the next exacerbation (Citation2). Patients with more symptoms during an AECOPD also spend more time confined to the home (Citation26). A combination of sore throat with either dyspnoea or cough increase the number of days per week patients stay at home (Citation26). The prolonged effect of AECOPD on symptom severity, as well as the impact of symptoms on quality of life and function, highlights the need for actions to improve symptoms as quickly as possible following an AECOPD.
Table 1. Symptoms recovery in days and tools used for assessment.
Health status and quality of life
Poor health-related quality of life (HRQOL) is associated with increased mortality in COPD, particularly in the first year following a severe acute exacerbation (Citation30,Citation31). Thus, measurement of HRQOL and health status are important to understand the impact of the disease on daily life (Citation32,Citation33). There are many reliable and valid questionnaires to assess long-term changes in health status and HRQOL in COPD, however whether these are sensitive to change in the small window of an AECOPD is less clear (Citation34). One of the tools used to measure health status is the COPD Assessment Tool (CAT) (Citation35). Studies show that the CAT is responsive to change during recovery from an AECOPD (Citation36–38). Mackay et al. (Citation39) found that health status measured on the CAT recovered to baseline in a median of 11 days (IQR 5 to 17 days) (Citation39). illustrates the changes in health status measured by the CAT after an exacerbation and demonstrates that health status improves during the first month after an AECOPD with the most rapid improvement detected in the first two weeks (Citation23,Citation38–43). In patients hospitalised with a severe exacerbation, the CAT items that recovered most rapidly related to breathlessness, sputum and energy levels, whilst the item related to confidence was slowest to recover (Citation44). The degree of recovery in CAT score may also important prognostic information. In 106 patients admitted with a severe exacerbation of COPD, an improvement in CAT score of less than four points between admission and discharge was an independent predictor of treatment failure at three months (new exacerbation, hospital readmission or death) (Citation41).
Figure 4. Recovery of health-related quality of life during following AECOPD. Baseline indicates pre-exacerbation data. Day 0 is onset of exacerbation. Data expressed as mean ± SD unless otherwise stated. (a) Demonstrates the recovery curve measured by CAT score. (b) Demonstrates the recovery curve measured by SRGQ score.
SGRQ score = Saint George’s Respiratory Questionnaire; CAT score = chronic obstructive pulmonary disease assessment test score; AECOPD = acute exacerbation of chronic obstructive pulmonary disease; n = number of participants; QoL: Quality of life.
Note: Ansari et al. (Citation47) presented only results from the hospitalised participants group. Data from Kocks et al. (Citation46), Mackay et al. (Citation39) and Chang et al. (Citation23) were extracted from the graph.
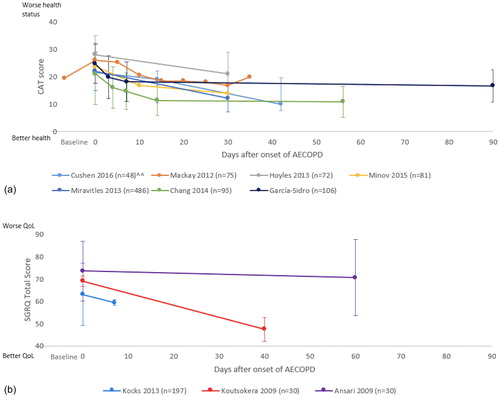
Health-related quality of life can be assessed using disease specific health related questionnaires such as St George’s Respiratory Questionnaire (Citation36) and the Chronic Respiratory Questionnaire (CRQ) (Citation45). Studies using disease specific questionnaires to assess quality of life generally show improvement during the first month after exacerbation () (Citation9,Citation46). One study showed only a slight improvement in quality of life (St George Respiratory Questionnaire) at two months after onset of AECOPD, and the improvement did not exceed the minimum important difference (MID) of four points (Citation47). The difference in findings of this study compared to other studies (Citation9,Citation46) may be the long time to reassessment (two to three months) in a population with a progressive disease and susceptible to recurrent exacerbations. Also, the study did not assess quality of life during the early stages of recovery to give a clear picture of the recovery process (Citation47). Parker et al. (Citation15) assessed changes from baseline in HRQOL using the CRQ in 20 patients hospitalised for AECOPD (Citation15). The study reported statistically significant improvement in the CRQ-dyspnoea domain on days 14, 30 and 60 when compared to the onset of exacerbation (change from day 0: 1.5 ± 0.3; 1.4 ± 0.4 and 1.8 ± 0.5 respectively p < 0.01) (Citation15); an improvement that exceeded the MID (difference of 0.5) (Citation48). Unfortunately, most studies do not present data from the early stages of the recovery process (), possibly because assessments of HRQOL are difficult to administer during hospitalisation when the relevance of the questions to daily activities is less clear. While the overall trajectory of recovery in HRQOL is not clear, studies consistently showing an improvement over the first two weeks after onset of an AECOPD.
Physical activity and exercise capacity
Regular physical activity participation is important for reducing morbidity and mortality in people with COPD (Citation49,Citation50). However, during and immediately following an AECOPD physical activity participation declines (Citation50,Citation51). A longitudinal study of 18 patients with COPD (mean FEV1 52 ± 20% predicted) found a 17% reduction (p < 0.0001) in the time spent in ‘higher level physical activity’ (vector magnitude units – VMU of ≥3,000) during an exacerbation compared to baseline (Citation52). During the recovery period following an exacerbation, the number of daily steps also decreased, with a median of 3.5 days (IQR 1–8 days) from exacerbation onset to return to baseline step count (Citation28). Physical inactivity during an AECOPD is greater when patients are hospitalised (Citation51). Borges et al. (Citation51) monitored 20 patients during hospitalisation and found they spent 83% of their time inactive, either lying down or sitting (Citation51). The same study compared time spent walking during a hospital stay compared to one month after discharge, and found a significant increase in the minutes of walking per day one month after discharge (mean 7 minutes/day to 42 minutes/day respectively, p < 0.001) (Citation51). It was not possible to determine whether the time spent walking per day one-month post AECOPD was comparable to the stable baseline condition as participants were only recruited at the hospital after onset of AECOPD. A similar study with a larger population (76 participants) also found that patients hospitalised for an AECOPD spent little time in weight-bearing activities (walking and standing) (Citation50). At one month following discharge participants in this study increased their time spent in standing or walking (137 minutes/day) compared to walking time measured on the second (50 minutes/day) and seventh (65 minutes/day) day after admission to hospital (Citation50). Despite the improved walking time one month after discharge, the amount of walking completed remained low when compared to people with COPD in a stable condition (Citation50,Citation53).
During an AECOPD, reduced time spent in weight-bearing activities has been associated with lower quadriceps force (r = 0.47; p = 0.048) and participants with weaker quadriceps during hospitalisation had less improvement in walking time in the first month after discharge (r = 0.58; p = 0.03) (Citation50). Pitta et al. (Citation50) noted that lower physical activity levels in the first month after hospital discharge increased the risk of hospital readmission in the following year (p = 0.03) (Citation50). Physical activity is also a predictor of mortality in people with COPD, with people with very low or worsening physical activity two months after AECOPD having a higher mortality risk (Citation54). Given these relationships between physical inactivity and longer term critical outcomes, restoration of physical activity levels following AECOPD may be an important target for interventions.
People with COPD experience progressive deterioration in exercise capacity during the disease course (Citation55). However only a few studies have investigated the impact of AECOPD on functional exercise capacity and the time course of recovery, possibly because it is difficult to perform exercise tests during this time period. In patients experiencing an AECOPD, the distance walked in six minutes decreased by an average of 49 metres (m) (13.1%) on day three after onset of exacerbation when compared to when they were stable (p = 0.001), but increased significantly at day seven compared to day three (p < 0.001) (Citation56). Six-minute walk distance (6MWD) was lower at day eight in patients who were admitted to hospital in the previous year compared to those without previous hospitalisation (median 200 m (IQR 155–298 m) vs 351 m (IQR 164–520 m); p = 0.12) (Citation50). One month after discharge 6MWD increased by a median of 73m (IQR, 27 to 149 m; p = 0.01) compared with the hospitalisation period (day eight after exacerbation) however it is not known if functional capacity had returned to baseline level at this point (Citation50).
The influence of functional impairment on readmissions and mortality following AECOPD demonstrates the importance of measuring recovery in function in these patients. Recently a number of new measures of exercise capacity have emerged that may be useful in the setting of AECOPD. Sit to stand tests, step tests and 4-metre gait speed tests are easier to perform than a 6-minute walk test in highly symptomatic patients and more suitable for the inpatient setting (Citation57–59). However these measures are not yet widely used and as yet there are no data pertaining to the sensitivity of these tests for monitoring recovery from an AECOPD.
Outcomes following AECOPD
Risks of hospital readmission and death are increased following an AECOPD. In the year following an AECOPD the risk of readmission has been reported as 63% (Citation60) and the risk of death 23% (Citation61). Long-term use of oral corticosteroids, hypercapnia, older age (Citation61), and exacerbation frequency (Citation8) have been identified as risk factors associated with higher mortality after AECOPD (Citation8,Citation61). A recent study provides insight into how this risk is modified over the time course of recovery (Citation62). Amongst more than 2 million Medicare beneficiaries in the United States who were discharged from hospital following AECOPD, the one-year readmission rate was 64% and mortality was 26% (Citation62). The risk of death was highest at 3–4 days following hospital discharge. The average time for the risk of death to decline by 50% was 17 days in those who did not receive ventilatory support, compared to 4 and 3 days in those who required non-invasive and invasive ventilation respectively (Citation62). The time required for the risk of hospital readmission to fall by 50% was much longer, at 43 days, 39 days and 28 days, and remained elevated at one year when compared to Medicare beneficiaries without COPD (Citation62). This illustrates that the risk of adverse outcomes remains high for some time after AECOPD, and although ‘recovery’ of a more favourable risk profile occurs, it is prolonged and may be incomplete. This is consistent with data showing incomplete recovery of respiratory function and symptoms in a significant minority of patients (Citation3,Citation12). Treatment of the initial exacerbation with systemic corticosteroids reduces the risks of both treatment failure and re-exacerbation at 30 days, with relative risks (RR) 0.48 (95% CI 0.35–0.67) and 0.78 (0.63–0.97) respectively (Citation14). Similarly, treatment with antibiotics reduces the risk of treatment failure in ambulatory patients (RR 0.67, 0.51–0.87) (Citation63), with a similar trend in admitted patients (RR 0.65, 0.38–1.12) (Citation64). However not all patients require treatment with antibiotics and efforts to accurately identify those who will benefit are ongoing (Citation63).
Which patients with AECOPD are at risk of prolonged recovery?
Recovery after an AECOPD may be prolonged in some subgroups. This is important as delayed or incomplete recovery is associated with extended hospitalisation and poor long-term outcomes (Citation2). Hospitalisation costs form the largest component of the total costs of COPD exacerbations and thus a prolonged inpatient stay has critical health economic implications (Citation65). It may be useful to identify the characteristics of those at risk of prolonged recovery in whom additional interventions could be required, to minimise hospital days and improve longer term outcomes. The factors related to prolonged recovery are presented in . Demographic features of those with prolonged recovery following AECOPD include older age, more severe lung disease, presence of chronic bronchitis, lower body mass index and more chronic dyspnoea. Exacerbation features associated with prolonged recovery are symptoms of the common cold at exacerbation onset, evidence of viral infection, more severe dyspnoea during the exacerbation and persistent systemic inflammation.
Table 2. Factors associated with prolonged recovery from an AECOPD.
Implications for research and clinical practice
Data presented in this review indicate that most people with COPD have a substantial improvement in physiological parameters (lung function, inflammatory markers) within the first week after onset of an exacerbation. Improvement in symptoms, quality of life and physical activity may take longer, with average recovery periods of at least two weeks. It is unclear whether improvement in physiological variables following AECOPD documented in the literature represents a complete recovery after AECOPD, due to the lack of baseline measurements in most studies, however it seems likely that in a small number of patients (<10%) recovery of respiratory function has not occurred by three months (Citation2,Citation3). Recovery following an AECOPD is not commonly tracked during clinical research (Citation2,Citation3,Citation26,Citation66), making it challenging to understand the process of recovery and the best timing of interventions by clinicians.
The data presented here indicate that clinicians should assume that most patients take at least two weeks for a ‘complete’ symptomatic recovery. However, it must be acknowledged that in some people with COPD, symptoms will persist for longer periods. Prolonged recovery is observed in frequent exacerbators who take longer to recover symptoms and health status (Citation2). Prolonged recovery is also more likely in those who are older, have more severe disease and more severe symptoms (). Patients who take longer to recover have more frequent hospitalisations (Citation67) which may further exacerbate the decline in functional status and physical inactivity that is evident during an AECOPD. Inactivity increases the future risk for hospitalisation, which in turn may impact upon functional capacity and quality of life, further increasing the risk of readmission to hospital. Therefore, patients may end up in a cycle of prolonged recovery, inactivity and re-admissions. The cycle needs to be discontinued and interventions in the early stages after AECOPD may be an option to break the inactivity cycle.
To break the cycle of prolonged recovery-inactivity-hospitalisation, interventions that commence during or immediately after hospital admission may be warranted. It is not clear whether treatments aimed at identified risk factors (e.g. persistent systemic inflammation) are of value. It is possible that treatments aimed at the consequences of an AECOPD, such as physical inactivity, may be more practical. Although some studies have shown that exercise training during hospitalisation is of benefit after AECOPD the evidence is inconsistent (Citation6). Some randomised controlled trials show that patients who undergo early exercise-based rehabilitation have reduced hospital admissions in the following year (Citation68,Citation69), whilst others do not (Citation70,Citation71). Uptake of exercise programmes early following an AECOPD is very low, suggesting it may not be acceptable to patients who are still highly symptomatic (Citation72). Day four of an AECOPD is when a reduction in airway inflammation is seen to occur and is related to a gradual decrease in symptoms, suggesting exercise may be more feasible after this time (Citation23). Comprehensive pulmonary rehabilitation programmes may be more feasible when started at least 14 days from onset of AECOPD, when systemic inflammation is reduced, symptoms have largely recovered, and patients may have greater tolerance of exercise interventions. This is well aligned with data showing greater benefits of pulmonary rehabilitation delivered at this time than when commenced in the inpatient period (Citation73).
Understanding the natural course of recovery following an AECOPD is important to be able to recognise when recovery is prolonged and when patients are at risk of poor outcomes. Current data provide good evidence for the time course of recovery for lung function and inflammation. However, there is limited literature describing the natural recovery process for functional capacity, symptoms, quality of life and strength. Another limitation of the literature is that most studies have not presented baseline data from prior to the onset of AECOPD, which prevents the understanding of the complete recovery process. Research that systematically documents the recovery process over the first month following an AECOPD is needed. A better understanding of the recovery process after an AECOPD may, in the future, enable delivery of therapeutic interventions at the most appropriate point in the recovery process. Most of the studies presented in this review considered exacerbation severity, which allowed us to assess whether ‘recovery’ following AECOPD had occurred across domains of respiratory function, inflammation, symptoms, health status and function. However, it is also important to consider the frequency of exacerbations, as those with frequent AECOPD may be slower to recoverCitation9 and have worse long-term outcomes (Citation16). The conclusions of this review regarding recovery from AECOPD are therefore limited to exacerbation severity rather than frequency.
Conclusions
Acute exacerbations are common events in the natural history of COPD. Physiological measures such as lung function and inflammation improve during the first week after onset of an AECOPD, however other aspects of recovery such as symptoms, health status, quality of life and functional capacity take longer to improve. Limited information regarding the recovery of functional capacity, strength and health status make it challenging to provide interventions at the most effective time point following an AECOPD. Characteristics of patients who experience delayed recovery after AECOPD have been identified. In clinical practice efforts should be made to recognise when recovery is prolonged, putting patients at risk of poor outcomes, and to address the consequences of AECOPD including physical inactivity and skeletal muscle weakness. Further research is required to understand whether additional interventions could enhance recovery and improve long term outcomes in these individuals.
Disclosure statement
The authors certify that they have no affiliations with or involvement in any organisation or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Funding
References
- Vestbo J, Hurd S, Agustí A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2013;187(4).
- Donaldson GC, Law M, Kowlessar B, et al. Impact of Prolonged Exacerbation Recovery in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2015;192(8):943–950. doi:10.1164/rccm.201412-2269OC.
- Seemungal TA, Donaldson GC, Bhowmik A, et al. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–1613. doi:10.1164/ajrccm.161.5.9908022.
- Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi:10.1164/ajrccm.157.5.9709032.
- Piquet J, Chavaillon JM, David P, et al. High-risk patients following hospitalisation for an acute exacerbation of COPD. Eur Respir J. 2013;42(4):946–955. doi:10.1183/09031936.00180312.
- Puhan MA, Gimeno-Santos E, Cates CJ, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;12:CD005305.
- Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41:46s–53s.
- Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527.
- Koutsokera A, Kiropoulos TS, Nikoulis DJ, et al. Clinical, functional and biochemical changes during recovery from COPD exacerbations. Respir Med. 2009;103(6):919–926. doi:10.1016/j.rmed.2008.12.006.
- Liang Y, Chen Y, Wu R, et al. Chronic bronchitis is associated with severe exacerbation and prolonged recovery period in Chinese patients with COPD: a multicenter cross-sectional study. J Thorac Dis. 2017;9(12):5120–5130. doi:10.21037/jtd.2017.11.54.
- Dalal AA, Patel J, D'Souza A, et al. Impact of COPD Exacerbation Frequency on Costs for a Managed Care Population. J Manag Care Spec Pharm. 2015;21(7):575–583. doi:10.18553/jmcp.2015.21.7.575.
- Perera WR, Hurst JR, Wilkinson TMA, et al. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J. 2007;29(3):527–534. doi:10.1183/09031936.00092506.
- Stevenson NJ, Walker PP, Costello RW, et al. Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(12):1510–1516. doi:10.1164/rccm.200504-595OC.
- Walters JA, Tan DJ, White CJ, et al. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014(9):CD001288.
- Parker CM, Voduc N, Aaron SD, et al. Physiological changes during symptom recovery from moderate exacerbations of COPD. Eur Respir J. 2005;26(3):420–428. doi:10.1183/09031936.05.00136304.
- Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi:10.1136/thorax.57.10.847.
- Rubio N, Parker RA, Drost EM, et al. Home monitoring of breathing rate in people with chronic obstructive pulmonary disease: observational study of feasibility, acceptability, and change after exacerbation. Int J Chronic Obstr Pulm Dis. 2017;12:1221–1231. doi:10.2147/COPD.S120706.
- Shah SA, Velardo C, Farmer A, et al. Exacerbations in chronic obstructive pulmonary disease: identification and prediction using a digital health system. J Med Internet Res. 2017;19(3):e69. doi:10.2196/jmir.7207.
- Walker PP, Pompilio PP, Zanaboni P, et al. Telemonitoring in COPD: The CHROMED Study, a Randomized Clinical Trial. Am J Respir Crit Care Med. 2018;198(5):620–628.
- Larsson K. Inflammatory markers in COPD. Clin Respir J. 2008;2(Suppl 1):84–87. doi:10.1111/j.1752-699X.2008.00089.x.
- Yende S, Waterer GW, Tolley EA, et al. Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax. 2006;61(1):10–16. doi:10.1136/thx.2004.034181.
- Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002;166(4):479–484. doi:10.1164/rccm.2202005.
- Chang C, Guo ZG, Shen N, et al. Dynamics of inflammation resolution and symptom recovery during AECOPD treatment. Sci Rep. 2014;4.
- Rebordosa C, Plana E, Aguado J, et al. GOLD assessment of COPD severity in the Clinical Practice Research Datalink (CPRD). Pharmacoepidemiol Drug Saf. 2019;28(2):126–133. doi:10.1002/pds.4448.
- Wedzicha JA, Calverley PMA, Albert RK, et al. Prevention of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;50(3).
- Donaldson GC, Wilkinson TMA, Hurst JR, et al. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(5):446–452. doi:10.1164/rccm.200408-1054OC.
- Wedzicha JA, Bestall JC, Garrod R, et al. Randomized controlled trial of pulmonary rehabilitation in severe chronic obstructive pulmonary disease patients, stratified with the MRC dyspnoea scale. Eur Respir J. 1998;12(2):363–369. doi:10.1183/09031936.98.12020363.
- Alahmari AD, Patel AR, Kowlessar BS, et al. Daily activity during stability and exacerbation of chronic obstructive pulmonary disease. BMC Pulmonary Medicine. 2014;14:98.
- Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi:10.1164/ajrccm.164.9.2105011.
- Almagro P, Calbo E, Ochoa de Echaguen A, et al. Mortality after hospitalization for COPD. Chest. 2002;121(5):1441–1448.
- Mekov E, Slavova Y, Tsakova A, et al. One-year mortality after severe COPD exacerbation in Bulgaria. PeerJ. 2016;4:e2788. doi:10.7717/peerj.2788.
- Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622–669.
- Zamzam MAA, N.Y; El Wahsh, R.A; Ragab, A.Z; Allam, E.M. Quality of life in COPD patients. Egypt J Chest Dis Tuberc. 2012;61(4):281–289. doi:10.1016/j.ejcdt.2012.08.012.
- Reda AA, Kotz D, Kocks JWH, et al. Reliability and validity of the clinical COPD questionniare and chronic respiratory questionnaire. Respir Med. 2010;104(11):1675–1682. doi:10.1016/j.rmed.2010.04.023.
- Agusti A, Soler-Cataluna JJ, Molina J, et al. Does the COPD assessment test (CAT(TM)) questionnaire produce similar results when self- or interviewer administered? Qual Life Res. 2015;24(10):2345–2354. doi:10.1007/s11136-015-0983-x.
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509.
- Jones PW, Harding G, Wiklund I, et al. Tests of the responsiveness of the COPD assessment test following acute exacerbation and pulmonary rehabilitation. Chest. 2012;142(1):134–140. doi:10.1378/chest.11-0309.
- Minov J, Karadzinska-Bislimovska J, Vasilevska K, et al. Course of COPD Assessment Test (CAT) scores during bacterial exacerbations of chronic obstructive pulmonary disease treated in outpatient setting. Open Respir Med J. 2015;9:39–45. doi:10.2174/1874306401509010039.
- Mackay AJ, Donaldson GC, Patel AR, et al. Usefulness of the Chronic Obstructive Pulmonary Disease Assessment Test to evaluate severity of COPD exacerbations. Am J Respir Crit Care Med. 2012;185(11):1218–1224. doi:10.1164/rccm.201110-1843OC.
- Cushen B, McCormack N, Hennigan K, et al. A pilot study to monitor changes in spirometry and lung volume, following an exacerbation of Chronic Obstructive Pulmonary Disease (COPD), as part of a supported discharge program. Respir Med. 2016;119:55–62. doi:10.1016/j.rmed.2016.08.019.
- Garcia-Sidro P, Naval E, Martinez Rivera C, et al. The CAT (COPD Assessment Test) questionnaire as a predictor of the evolution of severe COPD exacerbations. Respir Med. 2015;109(12):1546–1552. doi:10.1016/j.rmed.2015.10.011.
- Hoyles KH, Sheehan AS, Forrester DLF, et al. The Chronic Obstructive Pulmonary Disease Assessment Tool (Cat) in Patients Admitted to Hospital for Exacerbation. Thorax. 2013;68:A204–A.
- Miravitlles M, Garcia-Sidro P, Fernandez-Nistal A, et al. Course of COPD assessment test (CAT) and clinical COPD questionnaire (CCQ) scores during recovery from exacerbations of chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2013;11:147. doi:10.1186/1477-7525-11-147.
- Feliz-Rodriguez D, Zudaire S, Carpio C, et al. Evolution of the COPD Assessment Test score during chronic obstructive pulmonary disease exacerbations: determinants and prognostic value. Can Respir J. 2013;20(5):e92–e97.
- Wijkstra PJ, Van Altena R, Kraan J, et al. Quality of life in patients with chronic obstructive pulmonary disease improves after rehabilitation at home. Eur Respir J. 1994;7(2):269–273. doi:10.1183/09031936.94.07020269.
- Kocks JW, van den Berg JW, Kerstjens HA, et al. Day-to-day measurement of patient-reported outcomes in exacerbations of chronic obstructive pulmonary disease. Int J Chronic Obstr Pulm Dis. 2013;8:273–286.
- Ansari K, Shamssain M, Farrow M, et al. Hospital-at-home care for exacerbations of chronic obstructive pulmonary disease: an observational cohort study of patients managed in hospital or by nurse practitioners in the community. Chron Respir Dis. 2009;6(2):69–74. doi:10.1177/1479972309102728.
- Schunemann HJ, Puhan M, Goldstein R, et al. Measurement properties and interpretability of the Chronic respiratory disease questionnaire (CRQ). COPD – Journal of Chronic Obstructive Pulmonary Disease. 2005;2(1):81–9. doi:10.1081/COPD-200050651.
- Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61(9):772–778. doi:10.1136/thx.2006.060145.
- Pitta F, Troosters T, Probst VS, et al. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129(3):536–544. doi:10.1378/chest.129.3.536.
- Borges RC, Carvalho CRF. Physical activity in daily life in Brazilian COPD patients during and after exacerbation. COPD – Journal of Chronic Obstructive Pulmonary Disease. 2012;9(6):596–602. doi:10.3109/15412555.2012.705364.
- Ehsan M, Khan R, Wakefield D, et al. A longitudinal study evaluating the effect of exacerbations on physical activity in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(6):559–564. doi:10.1513/AnnalsATS.201304-100OC.
- Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–977. doi:10.1164/rccm.200407-855OC.
- Esteban C, Garcia-Gutierrez S, Legarreta MJ, et al. One-year Mortality in COPD After an Exacerbation: The Effect of Physical Activity Changes During the Event. Int J Chronic Obstr Pulm Dis. 2016;13(6):718–725. doi:10.1080/15412555.2016.1188903.
- Oga T, Nishimura K, Tsukino M, et al. Exercise capacity deterioration in patients with COPD: longitudinal evaluation over 5 years. Chest. 2005;128(1):62–69. doi:10.1378/chest.128.1.62.
- Alahmari AD, Kowlessar BS, Patel AR, et al. Physical activity and exercise capacity in patients with moderate COPD exacerbations. Eur Respir J. 2016;48(2):340–349. doi:10.1183/13993003.01105-2015.
- Kofod LM, Dossing M, Steentoft J, et al. Resistance training with ankle weight cuffs is feasible in patients with acute exacerbation of COPD. J Cardiopulm Rehabil Prev. 2017;37(1):49–56. doi:10.1097/HCR.0000000000000230.
- Kon SS, Jones SE, Schofield SJ, et al. Gait speed and readmission following hospitalisation for acute exacerbations of COPD: a prospective study. Thorax. 2015;70(12):1131–7. doi:10.1136/thoraxjnl-2015-207046.
- Torres-Sanchez I, Cabrera-Martos I, Diaz-Pelegrina A, et al. Physical and functional impairment during and after hospitalization in subjects with severe COPD exacerbation. Respir Care. 2017;62(2):209–214. doi:10.4187/respcare.04597.
- Garcia-Aymerich J, Farrero E, Felez MA, et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58(2):100–105.
- Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124(2):459–467.
- Lindenauer PK, Dharmarajan K, Qin L, et al. Risk Trajectories of Readmission and Death in the First Year after Hospitalization for Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2018;197(8):1009–1017. doi:10.1164/rccm.201709-1852OC.
- Wedzicha JAEC-C, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49(3).
- Vollenweider DJ, Frei A, Steurer-Stey CA, et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;10:CD010257.
- Toy EL, Gallagher KF, Stanley EL, et al. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD – Journal of Chronic Obstructive Pulmonary Disease. 2010;7(3):214–228. doi:10.3109/15412555.2010.481697.
- Murray LT, Leidy NK. The short-term impact of symptom-defined COPD exacerbation recovery on health status and lung function. Chronic Obstr Pulm Dis. 2018;5(1):27–37.
- Anzueto A, Miravitlles M, Ewig S, et al. Identifying patients at risk of late recovery (≥ 8 days) from acute exacerbation of chronic bronchitis and COPD. Respir Med. 2012;106(9):1258–1267. doi:10.1016/j.rmed.2012.06.002.
- Man WD, Polkey MI, Donaldson N, et al. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ. 2004;329(7476):1209. doi:10.1136/bmj.38258.662720.3A.
- Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax. 2010;65(5):423–428. doi:10.1136/thx.2009.124164.
- Eaton T, Young P, Fergusson W, et al. Does early pulmonary rehabilitation reduce acute health-care utilization in COPD patients admitted with an exacerbation? A randomized controlled study. Respirology. 2009;14(2):230–238. doi:10.1111/j.1440-1843.2008.01418.x.
- Ko FW, Dai DL, Ngai J, et al. Effect of early pulmonary rehabilitation on health care utilization and health status in patients hospitalized with acute exacerbations of COPD. Respirology. 2011;16(4):617–624. doi:10.1111/j.1440-1843.2010.01921.x.
- Jones SE, Green SA, Clark AL, et al. Pulmonary rehabilitation following hospitalisation for acute exacerbation of COPD: referrals, uptake and adherence. Thorax. 2014;69(2):181–182. doi:10.1136/thoraxjnl-2013-204227.
- Holland AE. Physiotherapy management of acute exacerbations of chronic obstructive pulmonary disease. J Physiother. 2014;60(4):181–188. doi:10.1016/j.jphys.2014.08.018.
- Jetmalani K, Timmins S, Brown NJ, et al. Expiratory flow limitation relates to symptoms during COPD exacerbations requiring hospital admission. Int J Chronic Obstr Pulm Dis. 2015;10:939–945.
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011] ed. http://handbook.cochrane.org/chapter_7/7_7_3_8_combining_groups.htm: Cochrane; 2011.
