Abstract
Combining measurements of impaired lung mechanics (inspiratory constraints) with an index of increased respiratory stimuli to metabolic demand (poor ventilatory efficiency) might enhance the ability of cardiopulmonary exercise testing (CPET) in exposing a mechanistic role for ventilation on exertional dyspnea in COPD. In addition to the standard approach to suggest ventilatory limitation to exercise – a low breathing reserve (1−(peak ventilation (V̇E)/maximal voluntary ventilation × 100 < 20%) – we assessed the presence of critical inspiratory constraints (end-inspiratory lung volume (EILV)/total lung capacity (TLC) ≥ 0.9) and ventilatory inefficiency (V̇E/CO2 output (V̇CO2) nadir > 34) in 288 patients with mild to very severe COPD (FEV1 ranging from 18 to 121% predicted). We found that ∼50% of the patients with preserved breathing reserve developed critical inspiratory constraints. A low breathing reserve was weakly related to a lower peak O2 uptake (V̇O2) and/or a higher dyspnea burden; for instance, patients with low breathing reserve but without critical inspiratory constraints had similar dyspnea and peak V̇O2 than those with preserved breathing reserve (p > 0.05). In contrast, critical inspiratory constraints and ventilatory inefficiency were strongly associated with a negative outcome (likelihood ratio = 42.3 and 47.7, respectively; p < 0.001). A multiple logistic regression analysis revealed that only EILV/TLC ≥ 0.9 and V̇E/V̇CO2 nadir >34 predicted a severely reduced peak V̇O2 due to a high dyspnea burden (p < 0.001). Measurements of dynamic mechanical constraints and ventilatory inefficiency during incremental CPET are key to determine the impact of COPD on dyspnea and exercise tolerance across the spectrum of disease severity.
Introduction
Cardiopulmonary exercise testing (CPET) has long been used to assess the consequences of chronic obstructive pulmonary disease (COPD) on a key patient-related outcome: exertional dyspnea (Citation1). The traditional approach has been largely focused on assessing whether a patient has or not crossed the threshold for a low breathing reserve, i.e., peak ventilation (V̇E)/estimated maximal voluntary ventilation (MVV) ratio × 100 < 15% (Citation2), <20% (Citation3,Citation4) or <30% (Citation5). Despite widespread criticism (Citation6–8), the breathing reserve remains seen by most practitioners as indicative of a “dyspnea index” (Citation9). In fact, influential textbooks (Citation3), recent investigations on the role of CPET in the assessment of exertional dyspnea of unclear etiology (Citation10), and the recommendations endorsed by American Thoracic Society/American College of Chest Physicians (Citation2) and American Heart Association (Citation11) still consider the breathing reserve as a key variable to determine whether patient’s dyspnea is or not mechanistically related to ventilation.
In this context, there is mounting evidence that dyspnea markedly increases when the patient’s limits for tidal expansion are reached (as shown, for instance, by an end-inspiratory lung volume (EILV)/total lung capacity (TLC) ratio ≥ 0.9) (Citation1,Citation12,Citation13); of note, such a crucial exercise intensity may not coincide with a low breathing reserve (Citation14). Similarly, excessive ventilatory stimuli relative to metabolic demand (high submaximal V̇E/CO2 output (V̇CO2) ratio or poor ventilatory efficiency) (Citation15) – another important modulator of exertional dyspnea – (Citation1) does not necessarily lead to a low breathing reserve (Citation16). It follows that a COPD patient may show a preserved breathing reserve despite limiting dyspnea due to critically high inspiratory constraints and/or poor ventilatory efficiency (Citation17). On the other hand, a motivated and relatively fit patient may cross the threshold for an abnormally low breathing reserve as a mere consequence of reaching high work rates (Citation18). In the latter scenario, a low breathing reserve might be rather inconsequential to patient’s activity-related dyspnea.
It is therefore conceivable that CPET-based variables which better expose the neurobiological underpinnings of dyspnea in COPD (mechanical constraints plus increased ventilatory stimuli) (Citation19) might prove superior to breathing reserve in assessing the impact of the symptom on patient’s exercise tolerance. We, therefore, reviewed a large dataset of incremental CPETs with the intent of answering the following research question: do dynamic measurements of inspiratory constraints (Citation14) and ventilatory inefficiency (Citation16) outperform the breathing reserve in assessing the severity of dyspnea and exercise intolerance across the spectrum of COPD severity? We hypothesized that the breathing reserve either underestimates or overestimates the presence and severity of dyspnea and exercise intolerance which are better exposed by measurements of neuromechanical (Citation14) and ventilatory-metabolic (Citation16) uncoupling.
Materials and methods
Subjects
Two hundred and eighty-eight patients (166 males) who were followed in the institutional outpatient service for COPD (FEV1/FVC < lower limit of normal with other clinical and imaging findings consistent with the disease) (Citation20) and performed an incremental CPET with serial measurements of dynamic (exercise) inspiratory capacity (ICdyn) and dyspnea. Subjects were unnamed and identified by unique identification numbers. Analysis of anonymous data collected as part of the standard of care was approved by the Queen’s University Research Ethics Board (DMED-1588-13).
Procedures
Spirometry, static lung volumes and lung transfer capacity for carbon monoxide were performed using automated equipment (Vmax229d; SensorMedics, Yorba Linda, CA): values were compared to those predicted by specific regression equations (Citation21–23). After pretest spirometry, the incremental CPET (5–15 W/every 2 minutes according to patient’s reported dyspnea burden) was conducted on an electronically braked cycle ergometer (Ergoline 800s™; SensorMedics, Yorba Linda, CA) using the SensorMedics Vmax229d™ system. Dyspnea and leg discomfort scores (0–10 category-ratio Borg scale) (Citation24) and ICdyn (L) (Citation12) were obtained in the last minute of each stage. Standard breath-by-breath cardiorespiratory (including O2 saturation by pulse oximetry (SpO2, %)) were also obtained (Citation25). Peak oxygen uptake (V̇O2) was classified as “preserved” (≥lower limit of normal (LLN)) or mildly- (70% predicted-LLN), moderately- (50–70% predicted) or severely impaired (<50% predicted) (Citation26). The breathing reserve was classified as abnormally low using the cut-offs proposed in the literature: <15% (Citation2), <20% (Citation3) or <30% (Citation5)) with MVV (L/minutes) being estimated as FEV1 × 35 (MVV35) or FEV1 × 40 (MVV40) (Citation2). The lowest (nadir) V̇E/CO2 output (V̇CO2) ratio > 34 established the presence of ventilatory inefficiency (Citation2); moreover, we calculated ΔV̇E − ΔV̇CO2 slope and intercept by linear regression (Citation27). EILV/TLC ≥ 0.9 defined the presence of critically high inspiratory constraints, i.e., inspiratory constraints which, as shown in our previous studies, have been consistently associated with unique quantitative (upward inflection in dyspnea ratings as a function of V̇E) and qualitative (from “work and effort” to “features”) (Citation1,Citation12,Citation13).
Statistical analysis
The statistical software package used was IBM™ SPSS™ Statistics version 24. The χ2 test was used to compare frequencies. Inter-method agreement in determining an abnormal test result (low breathing reserve versus critical inspiratory constraints and excess ventilation) were assessed by Cohen’s kappa (κ) (Citation28). The level of agreement was rated according to the cut-offs proposed by Altman: κ < 0.20 = very poor, 0.21–0.40 = poor, 0.41–0.60 = moderate, 0.61–0.80 = good, 0.81–1.00 = very good (Citation29). One-way ANOVA (followed by Bonferroni’s post-hoc if required) was used to contrast resting and exercise variables among groups. Two-way ANOVA with repeated measures was used to compare key physiological variables and dyspnea intensity amongst patient groups separated by EILV/TLC (<0.9 or ≥0.9) and V̇E/V̇CO2 nadir (≤34 or >34) at progressively high work rates. Multiple logistic regression analysis determined the predictors of “lower” exercise tolerance (peak V̇O2<sample’s median (1.04 L/minutes) and <50% predicted) (Citation26) and a high dyspnea burden (dyspnea/work rate > sample’s median (0.09 Borg unit/W) plus peak dyspnea ≥ leg discomfort ratings) (Citation8). The probability of a type I error was set at 5% (p < 0.05).
Results
Sample characteristics
The sample presented with a predominance of males (57.6%) with an ample distribution of age (from 44 to 82 years). FEV1 ranged from 18 to 121% predicted: there was a sizeable number of patients in each GOLD stage of functional severity (25.1%, 36.5%, 26.8%, 17.6% prevalence for spirometry stages 1, 2, 2 and 4, respectively) (Citation20). As expected, exercise tolerance also varied widely: prevalence of preserved, mild, moderate and severe impairment in peak V̇O2 were 13.9%, 9.4%, 25.3% and 51.4%, respectively.
Breathing reserve versus inspiratory constraints and ventilatory inefficiency
Regardless of the threshold for a low breathing reserve, there was a poor agreement between this variable and EILV/TLC. For instance, ∼50% of the patients with preserved breathing reserve (>20% based on MVV40, the most-commonly used cut-off) (Citation3,Citation26,Citation30,Citation31)) showed critical inspiratory constraints and ventilatory inefficiency. Conversely, critical inspiratory constraints and ventilatory inefficiency was not observed in ∼30% and ∼50% of those with a low breathing reserve, respectively ().
Table 1. Contingency table showing the level of agreement between low or preserved breathing reserve (maximal voluntary ventilation estimated as FEV1 × 40) versus the presence of not of critical inspiratory constraints (end-inspiratory lung volume (EILV)/total lung capacity (TLC) ratio) or excess ventilation (ventilation (V̇E)/CO2 output (V̇CO2) ratio) in patients with COPD.
Lung function according to method (dis)agreement
The group of patients in whom breathing reserve and inspiratory constraints agreed in indicating abnormality presented with significantly lower spirometry values compared to their counterparts (p < 0.05; ). Of note, patients with preserved breathing reserve who reached critical inspiratory constraints had higher resting operating lung volumes and lower transfer factor than patients who did not reach these constraints ().
Table 2. Resting and exercise characteristics of COPD patients separated according to preserved or low breathing reserve (BR > or ≤20%, respectively) versus absence or presence of critical inspiratory constraints (end-inspiratory lung volume (EILV)/total lung capacity (TLC) ratio < or ≥0.9, respectively) at the peak of incremental cardiopulmonary exercise testing (CPET).
CPET responses according to method (dis)agreement
As shown in and , the patients with preserved breathing reserve who developed critical inspiratory constraints had lower exercise tolerance, poorer ventilatory efficiency, higher O2 desaturation and higher dyspnea burden than patients who did not reach these constraints. Conversely, patients with a low breathing reserve who did not present with critical inspiratory constraints showed the best exercise tolerance among the groups (p < 0.05). and Citation3 show that most patients with higher dyspnea burden or lower exercise tolerance attained critical inspiratory constraints despite preserved breathing reserve, respectively. On the other hand, those with a low breathing reserve but with no evidences of reaching critical inspiratory constraints were unlikely to present with these negative outcomes. Ventilatory inefficiency was particularly useful to identify patients who presented with higher dyspnea burden or lower exercise tolerance but did not have a low breathing reserve and/or did not reach critical inspiratory constraints (lower right quadrant in the upper panels of and Citation3).
Figure 1. Metabolic (panel A), cardiovascular (panel B), ventilatory (panels C–E) and sensory (panel F) responses to symptom-limited incremental CPET in COPD patients presenting or not with a low breathing reserve (BR ≤ or >20%, respectively) and/or high inspiratory constraints (end-inspiratory lung volume (EILV)/total lung capacity (TLC) ≥ or <0.9, respectively). Commonly used ranges for severe physiological and sensory impairment are highlighted (shadow areas in panels C–F). The arrows in panels C, D and F emphasize the exercise intensity associated with a disproportionate increase in dyspnea relative to metabolic and ventilatory demand.
Note: p < 0.05. *versus the other groups; †versus the remaining groups. Data are mean ± SEM. O2= oxygen uptake, HR: heart rate, EELV: end-expiratory lung volume; VT: tidal volume, TLC: total lung capacity, V˙E: minute ventilation,V˙CO2: carbon dioxide output.
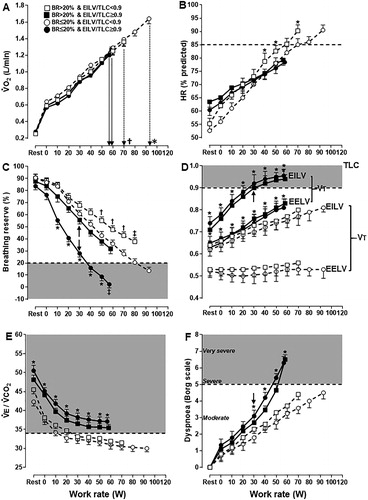
Figure 2. Distribution of COPD patients presenting or not with a higher dyspnea burden (peak dyspnea/work rate ratio > sample’s median (0.09 Borg unit/W) and peak dyspnea ≥ peak leg effort) relative to the breathing reserve (maximal voluntary ventilation = FEV1 × 40) and inspiratory constraints. Patients were separated by the presence or not of excess ventilation (high ventilation (V̇E)/CO2 output (V̇CO2) nadir). The dotted lines are the cut-offs for ventilatory limitation according to a low breathing reserve or critically high inspiratory constraints. The upper right shaded quadrant represents the patients with preserved breathing but critically high inspiratory constraints: the lower left shaded quadrant depicts the opposite.
Note: EILV: end-inspiratory lung volume; TLC: total lung capacity.
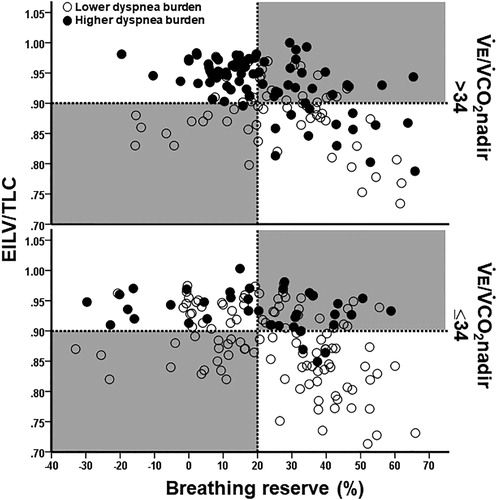
Figure 3. Distribution of COPD patients presenting or not with a lower exercise tolerance (peak O2 uptake < sample’s median (1.04 L/minutes) and <50% predicted) relative to the breathing reserve (maximal voluntary ventilation = FEV1 × 40) and inspiratory constraints. Patients were separated by the presence or not of excess ventilation (high ventilation (V̇E)/CO2 output (V̇CO2) nadir). The dotted lines are the cut-offs for ventilatory limitation according to a low breathing reserve or critically high inspiratory constraints. The upper right shaded quadrant represents the patients with preserved breathing but critically high inspiratory constraints: the lower left shaded quadrant depicts the opposite.
Note: EILV: end-inspiratory lung volume, TLC: total lung capacity.
In line with these premises, the likelihood ratio of an abnormal test result to be associated with higher dyspnea burden and/or lower exercise tolerance was ∼5 times greater for the inspiratory constraints and ventilatory inefficiency than the breathing reserve (). In fact, a multiple logistic regression analysis controlling for FEV1 (GOLD functional criteria) (Citation20) found that EILV/TLC and V̇E/V̇CO2 nadir – but not age, gender, body mass index, a low breathing reserve (regardless of its metrics) or exercise-induced hypoxemia – predicted a severely reduced peak V̇O2 due to a high dyspnea burden (p < 0.001; ).
Table 3. Association between CPET-based negative outcomes (“higher” dyspnea burden = peak dyspnea/work rate ratio > sample’s median and peak dyspnea ≥ peak leg effort and/or “lower” exercise tolerance (peakV˙O2 < sample’s median and <50% predicted) with abnormal or preserved breathing reserve (maximal voluntary ventilation estimated as FEV1 × 40), end-inspiratory lung volume (EILV)/total lung capacity (TLC) and the nadir of ventilation (V̇E)/CO2 output (V̇CO2) ratio in patients with COPD.
Table 4. Final outcome of a multiple logistic regression analysis to predict the association of a high dyspnea burden (dyspnea/work rate ratio > study population’s median value and peak dyspnea ≥ leg effort scores) with a low peak O2 uptake (peakV˙O2 < sample’s median and <50% predicted) (Citation26).
Discussion
This seems to constitute the first study to specifically contrast a widely used index of ventilatory limitation to exercise (a low breathing reserve) (Citation3–7) with markers of mechanical inspiratory constraints (Citation8) and poor intra-pulmonary gas exchange efficiency (ventilatory inefficiency) (Citation16) in a large group of patients with mild to end-stage COPD who underwent incremental CPET. Our main original findings are: (a) a large fraction of patients with preserved breathing reserve did present with critical inspiratory constraints and/or ventilatory inefficiency, (b) regardless of its metrics, a low breathing reserve was weakly related to a high dyspnea burden and exercise intolerance; in fact, a low breathing reserve was associated with these negative outcomes only if it coexisted with critical inspiratory constraints and (c) the combination of critical inspiratory constraints with ventilatory inefficiency was highly predictive of dyspnea and exercise tolerance across the range of COPD severity. These data, therefore, indicate that the clinical value of CPET in assessing the subjective and objective burden of COPD can be significantly improved by a systematic assessment of readily available indexes of constrained inspiration (critically high EILV/TLC) and excess ventilatory stimuli (high V̇E/V̇CO2 nadir).
Several studies convincingly demonstrated that exertional dyspnea rises as the inspiratory neural drive increases in the face of an ever-reducing capacity of the ventilatory pump to respond because of excessive mechanical loading (Citation12,Citation14,Citation32). This is worsened in the presence of increased ventilation to overcome an enlarged dead space (Citation33) and/or the patient controls his PaCO2 at a lower set-point (Citation34). Those considerations provide the mechanistic bases for our findings that dyspnea intensified () as mechanical constraints on tidal volume critically worsened and inspiratory reserve volume decreased () in patients with higher V̇E/V̇CO2 (). Of note, these abnormalities were poorly related to the submaximal () or end-exercise () breathing reserve thereby providing a rationale for the superior ability of critically high inspiratory constraints and/or ventilatory inefficiency in identifying the most dyspneic and disabled patients (). It should be noted that we defined a high dyspnea burden considering its importance relative to leg effort in a given subject but also its severity comparative to other patients facing a similar challenge (work rate). Thus, we obtained an index of intra- and inter-subject dyspnea severity (Citation19).
Our results indicate that ventilatory inefficiency does carry an independent (from impaired lung mechanics) role in contributing to exertional dyspnea in COPD. Although poor ventilatory efficiency hastens the attainment of critically high inspiratory constraints (Citation35), V̇E/V̇CO2 nadir remained an independent predictor of exertional dyspnea and poor exercise tolerance when controlled for EILV/TLC (). In fact, ventilatory inefficiency alone did identify some patients with higher dyspnea burden or lower exercise tolerance who neither present with a low breathing reserve nor reach critical inspiratory constraints ( and Citation3). As COPD progresses, severe mechanical constraints may preclude increased afferent stimuli to be fully translated into high ventilatory output: though this may blunt the rate of increase in V̇E (i.e., V̇E-V̇CO2 slope) the response “gain” is increased (high V̇E-V̇CO2 intercept) () (Citation16). It follows that the V̇E/V̇CO2 nadir (which depends on both slope and intercept) (Citation36) remains an important index of excessive ventilation across the range of disease severity (Citation16). Nevertheless, this might not be the case in the patients at the extremes of disease severity in whom a blunted slope may drive the nadir low despite a high intercept. Thus, a high V̇E/V̇CO2 nadir may lose its role in predicting a high dyspnea burden in end-stage COPD (Citation37).
What are the scenarios in which the frequent disagreement between ventilatory limitation suggested by the breathing reserve and the measured dyspnea burden and exercise tolerance ( and Citation3) are more likely to impact on clinical decision making? CPET is widely regarded as a useful tool to ascertain an etiologic role for COPD to explain exertional dyspnea in patients with multiple co-morbidities (Citation25). Over- or under-calling the role of ventilatory abnormalities to explain patient’s dyspnea may negatively influence on patient’s management vis-à-vis the correct identification of dyspnea cause(s) or sub-optimal COPD treatment, respectively (Citation1). Claiming that an apparently “low” breathing reserve fully explain patient’s symptoms may hinder the identification of life-threatening co-morbidities (such as heart failure) (Citation38) or pulmonary hypertension (Citation39). In patients with milder disease, in particular, there was a noticeable trend of breathing reserve to indicate lack of ventilatory limitation in dyspneic patients with extensive mechanical constraints and high ventilation to metabolic demand (Citation40). Thus, if ICdyn is not performed and/or V̇E/V̇CO2 is ignored, the patient’s exertional dyspnea may be erroneously ascribed to mere “lack of fitness”.
Our study has the inherent limitations of a retrospective investigation performed in a reference research laboratory for CPET. For instance, some of our patients were likely familiar with exercise studies involving ICdyn maneuvers; thus, they might have performed better on these maneuvers than the typical COPD outpatient. Nevertheless, our sample is highly representative of the COPD patients usually referred for clinical CPET, showing an ample range of resting functional impairment (). Most commercially available CPET systems nowadays allow ICdyn measurement and detailed technical and interpretative considerations have been published (Citation1,Citation12). It follows that the present results have a clear potential to directly impact on the clinical interpretation of CPET in this patient population.
In conclusion, measurements of dynamic mechanical constraints (critically high EILV/TLC) and ventilatory inefficiency (a high V̇E/V̇CO2 nadir) provide unique pathophysiological information which is germane to exertional dyspnea and exercise intolerance across the spectrum of COPD severity. By considering these measurements on clinical CPET interpretation, the pulmonologist can avoid the pitfalls of under or overestimation of the role of ventilatory abnormalities in eliciting dyspnea in patients with mild to advanced COPD. This might prove relevant to improve the clinical applicability of CPET, a potentially useful, but largely underused, investigative tool in respiratory medicine.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- O’Donnell DE, Elbehairy AF, Faisal A, Webb KA, Neder JA, Mahler DA. Exertional dyspnoea in COPD: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev. 2016;25(141):333–47. doi: 10.1183/16000617.0054-2016.
- American Thoracic Society, American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–77.
- Wasserman K, Hansen J, Sue D, Stringer W, Sietsema K, Sun X-G, Whipp B. Principles of exercise testing and interpretation: including pathophysiology and clinical applications. 5th ed., Philadelphia (PA): Lippincott Williams & Wilkins; 2011. 592 p.
- Wasserman K, Whipp BJ. Exercise physiology in health and disease. Am Rev Respir Dis. 1975;112(2):219–49. doi: 10.1164/arrd.1975.112.2.219.
- Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 Focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2016;133(24):e694–711. doi: 10.1161/CIR.0000000000000406.
- Johnson BD, Weisman IM, Zeballos RJ, Beck KC. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest. 1999;116(2):488–503. doi: 10.1378/chest.116.2.488.
- Casaburi R, Rennard SI. Exercise limitation in chronic obstructive pulmonary disease. The O’Donnell threshold. Am J Respir Crit Care Med. 2015;191(8):873–5. doi: 10.1164/rccm.201501-0084ED.
- O’Donnell DE, Elbehairy AF, Berton DC, Domnik NJ, Neder JA. Advances in the evaluation of respiratory pathophysiology during exercise in chronic lung diseases. Front Physiol. 2017;8:82.
- Fishman AP, Ledlie JF. Dyspnea. Bull Eur Physiopathol Respir. 1979;15(5):789–804.
- Oldham WM, Lewis GD, Opotowsky AR, Waxman AB, Systrom DM. Unexplained exertional dyspnea caused by low ventricular filling pressures: results from clinical invasive cardiopulmonary exercise testing. Pulm Circ. 2016;6(1):55–62. doi: 10.1086/685054.
- Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126(18):2261–74. doi: 10.1161/CIR.0b013e31826fb946.
- Guenette JA, Chin RC, Cory JM, Webb KA, O’Donnell DE. Inspiratory capacity during exercise: measurement, analysis, and interpretation. Pulm Med. 2013;2013:956081. doi: 10.1155/2013/956081.
- Laveneziana P, Webb KA, Ora J, Wadell K, O’Donnell DE. Evolution of dyspnea during exercise in chronic obstructive pulmonary disease: impact of critical volume constraints. Am J Respir Crit Care Med. 2011;184(12):1367–73. doi: 10.1164/rccm.201106-1128OC.
- O’Donnell DE, Guenette JA, Maltais F, Webb KA. Decline of resting inspiratory capacity in COPD: the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest. 2012; 141(3):753–62. doi: 10.1378/chest.11-0787.
- Whipp BJ, Ward SA. Cardiopulmonary coupling during exercise. J Exp Biol. 1982;100:175–93.
- Neder JA, Arbex FF, Alencar MCN, O’Donnell CDJ, Cory J, Webb KA, et al. Exercise ventilatory inefficiency in mild to end-stage COPD. Eur Respir J. 2015;45(2):377–87. doi: 10.1183/09031936.00135514.
- Neder, JA, Berton, DC, Rocha, A, Arbex, F, Alencar, MC, Degani-Costa, LH, et al. Abnormal patterns of response to Incremental CPET. In: 2018 Clinical Exercise Testing. European Respiratory Society Journals; 2018. p. 34–58. (European Respiratory Monograph; vol. 80).
- Dempsey JA, Smith CA. Pathophysiology of human ventilatory control. Eur Respir J. 2014;44(2):495–512. doi: 10.1183/09031936.00048514.
- Mahler DA, O’Donnell DE. Recent advances in dyspnea. Chest. 2015;147(1):232–41. doi: 10.1378/chest.14-0800.
- Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65. doi: 10.1164/rccm.201204-0596PP.
- Coates AL, Wong SL, Tremblay C, Hankinson JL. Reference equations for spirometry in the Canadian population. Ann Am Thorac Soc. 2016;13(6):833–41. doi: 10.1513/AnnalsATS.201508-569OC.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J. 1993;16:5–40.
- Stanojevic S, Graham BL, Cooper BG, Thompson BR, Carter KW, Francis RW, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J. 2017;50(3). pii: 1700010. doi: 10.1183/13993003.00010-2017. Print 2017 Sep.
- Borg G. A category scale with ratio properties for intermodal and interindividual comparisons. In: Geissler HG, Petzold P, editors. Psychophysical judgment and the process of perception. Amsterdam: VEB Deutscher Verlag der Wissenschaften; 1982. p. 25–34.
- ERS Task Force, Palange P, Ward SA, Carlsen K-H, Casaburi R, Gallagher CG, et al. Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007;29(1):185–209. doi: 10.1183/09031936.00046906.
- Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis. 1985;131(5):700–8. doi: 10.1164/arrd.1985.131.5.700.
- Whipp BJ. The bioenergetic and gas exchange basis of exercise testing. Clin Chest Med. 1994;15(2):173–92.
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. doi: 10.1177/001316446002000104.
- Altman DG. Practical statistics for medical research. London: Chapman and Hall; 1991.
- Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129(2 Pt 2):S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49.
- Jones N. Clinical exercise testing. 3rd ed. Philadelphia, PA: W.B Saunders Company; 1988. 323 p.
- O’Donnell DE. Ventilatory limitations in chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2001;33(7 Suppl):S647–S655.
- Neder JA, Berton DC, Müller P de T, Elbehairy AF, Rocha A, Palange P, et al. Ventilatory inefficiency and exertional dyspnea in early chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(Suppl_1):S22–S29 doi: 10.1513/AnnalsATS.201612-1033FR.
- Rocha A, Arbex FF, Sperandio PA, Souza A, Biazzim L, Mancuso F, et al. Excess ventilation in COPD-heart failure overlap: implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. 2017;196(10):1264–1274. doi: 10.1164/rccm.201704-0675OC.
- O’Donnell DE, Laveneziana P, Webb K, Neder JA. Chronic obstructive pulmonary disease: clinical integrative physiology. Clin Chest Med. 2014;35(1):51–69. doi: 10.1016/j.ccm.2013.09.008.
- Whipp BJ. The hyperpnea of dynamic muscular exercise. Exerc Sport Sci Rev. 1977;5:295–311.
- Neder JA, Berton DC, Arbex FF, et al. Physiological and clinical relevance of exerciseventilatory efficiency in COPD. Eur Respir J 2017; 49: 1602036 [https://doi.org/10.1183/13993003.02036-2016].
- Arbex FF, Alencar MC, Souza A, Mazzuco A, Sperandio PA, Rocha A, et al. Exercise ventilation in COPD: influence of systolic heart failure. COPD. 2016;12;1–8.
- Boerrigter BG, Bogaard HJ, Trip P, Groepenhoff H, Rietema H, Holverda S, et al. Ventilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertension. Chest. 2012;142(5):1166–74. doi: 10.1378/chest.11-2798.
- Chin RC, Guenette JA, Cheng S, Raghavan N, Amornputtisathaporn N, Cortés-Télles A, et al. Does the respiratory system limit exercise in mild chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2013;187(12):1315–23. doi: 10.1164/rccm.201211-1970OC.
