Abstract
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease. The severity grading systems proposed by the Global initiative for Chronic Obstructive Lung Disease (GOLD) have changed over time. The aim of the study was to evaluate if the different GOLD classifications can capture the complexity of the disease by investigating the distribution of lung function and clinical parameters across the GOLD classification systems. This was an observational, retrospective, multicentre study. COPD patients were stratified according to the GOLD severity grading proposed in the 2007, and to the ABCD assessment tool present in the 2011, and 2017 versions of the initiative. Data from body plethysmography, DLCO, comorbidities, exacerbation history, pharmacological therapy and eosinophil counts were collected. A total of 1360 patients (73.4% males) were included in the analysis. Overall, 37% of the patients were severe-very severe according to GOLD 2007. Compared with GOLD 2011, applying the GOLD 2017 criteria, the proportion of the at risk categories (C and D) was reduced by ∼23%. Impairment in inspiratory capacity, DLCO and the prevalence of emphysema paralleled the GOLD 2007 classification only. The proportion of patients with ≥ 200 eosinophils/µL was higher in GOLD 2007 stages 3–4 compared with stages 1–2 (P = 0.008). Eosinophil levels were similar across risk classes in GOLD 2011 and 2017. Overall, 41.8% and 52.4% of the patients in the low risk groups according to GOLD 2011 and 2017 were exposed to inhaled corticosteroids. The GOLD 2011 and 2017 classifications, despite exploring symptoms and exacerbations, might miss other relevant patients’ clinical characteristics such as lung function and phenotypes, which have a significant impact on outcomes and disease severity.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable disease characterized by persistent respiratory symptoms and airflow obstruction. COPD is one of the major causes of chronic morbidity and mortality worldwide and bears a major social and economic burden on healthcare systems [Citation1]. The inhalation of noxious particles (mainly cigarette smoke) [Citation2] causes airway narrowing and loss of elastic recoil, which eventually lead to airflow obstruction, air trapping and hyperinflation [Citation3]. Despite this common pathophysiological substrate, COPD is a complex and heterogeneous disease [Citation4] with different clinical patterns, variable susceptibility to exacerbations, lung function decline, response to treatment and mortality [Citation5]. Currently, the Global Obstructive Lung Disease (GOLD) document is the most used treatment guide worldwide for COPD. The disease classification and severity grading used in this document have changed over time. In GOLD 2007, the level of airflow obstruction, measured as percent predicted forced expiratory volume in 1 s (FEV1), was the only parameter used to classify COPD patients and, consequently, to choose the adequate pharmacological treatment [Citation6]. In GOLD 2011, a new system was proposed to assess the clinical severity of COPD patients. The score grouped patients according to their severity of symptoms (measured using the modified medical research council scale, mMRC scale [Citation7] or CAT score [Citation8]) and future risk of exacerbation (measured as the severity of airflow obstruction and history of exacerbations in the previous year) [Citation9]. Based on these clinical domains, four groups of patients were identified: group A (low symptoms and low exacerbation risk), group B (high symptoms and low risk), group C (low symptoms and high risk) and group D (high symptoms and high risk). The so-called “ABCD score” was proposed not only to evaluate the severity of the disease but also to drive the choice of pharmacological regimens in these patients. From GOLD 2017 onward [Citation10–12], the “ABCD score” has been modified. In the latter versions of the GOLD documents, the assessment of the severity of COPD patients is based first on the evaluation of lung function impairment and second on the “ABCD score” that considers the magnitude of symptoms and the history of exacerbations irrespective of the severity of airflow limitations. In these new versions of the GOLD document, the ABCD guides the initial pharmacological approach [Citation10]. The “ABCD score” systems have been highly debated. Indeed, the new GOLD ABCD classifications demonstrated poor prognostic power, particularly for patient-reported outcomes [Citation13–17]. Moreover, the score does not include several functional and clinical aspects of the disease with a recognized significant impact on the short- and long-term prognosis and outcomes, such as: lung hyperinflation [Citation3], impairment of peripheral airways [Citation18], specific clinical phenotypes (chronic bronchitis and/or emphysema, [Citation19,Citation20]) or biomarkers of systemic inflammation possibly linked to disease progression and response to treatment [Citation21–24]. The aim of the study is to investigate if “ABCD” categorization in GOLD 2011 and 2017, in comparison with GOLD 2007, improved clinicians’ ability to capture the complexity of the disease. Specifically, we aimed to investigate the distribution of lung function parameters, clinical phenotypes, and serum eosinophils in a cohort of COPD patients assessed according to the GOLD 2007, GOLD 2011, and GOLD 2017 classification criteria. Furthermore, we evaluated how the relative composition of the disease severity groups changed with the evolving classification of COPD.
Methods
Study design
This was a multicentre, observational, retrospective study conducted in several academic secondary- and tertiary-care hospitals in northern, central and southern Italy. A complete list of the participating centres is detailed in the Supplementary file.
Patients
Patients with an established diagnosis of COPD were retrospectively recruited from October 2014 to November 2016. The inclusion criteria were: (a) age of ≥40 years; (b) smoking history > 20 pack years [Citation25,Citation26], and (c) a post-bronchodilator forced expiratory volume in one second to slow vital capacity ratio (FEV1/VC) < the lower limit of normal (LLN) criteria [Citation27,Citation28]. In line with previous literature [Citation29], nonsmokers with a significant history of exposure to noxious particles or pollutants that satisfied the criteria for COPD diagnosis were also included. The exclusion criteria were: (a) a diagnosis of interstitial lung disease or another clinically significant respiratory disease other than COPD, (b) a restrictive ventilatory pattern with a total lung capacity (TLC) < LLN [Citation28], and (c) a current diagnosis of asthma at the time of enrolment representing the main respiratory disorder and for which the patient was taking asthma treatments. Patients with a previous history of asthma or asthma-like symptoms that met the criteria for a diagnosis of COPD were not excluded from the study.
Clinical and functional variables
All eligible patients were recruited from the outpatient clinics of the participating centres, and the clinical and functional variables were referred to as the stable state conditions.
The following demographic and clinical characteristics were collected for all of the recruited patients: age, sex, height, weight, body mass index, smoking history (pack years), dyspnoea (assessed by the mMRC scale) [Citation7]), number of mild/moderate and severe COPD exacerbations in the 12 months prior to study enrolment, comorbidities (assessed by the Charlson Comorbidity Index [Citation30]), and inhaled and oral chronic respiratory medications.
The following function parameters were considered in the analysis: FEV1, VC, forced vital capacity (FVC), functional residual capacity (FRC), inspiratory capacity (IC), TLC, residual volume (RV) and specific total airway resistance (sRawtot). The presence of air trapping was defined as an RV ≥ upper limit of normal [Citation31]. The lung function tests were standardized and performed according to international recommendations [Citation28,Citation32]. Lung diffusion capacity for carbon monoxide (DLCO) measured during the single breath manoeuvre [Citation33], the alveolar volume (VA) and the transfer factor (KCO) were also evaluated.
If available within 3 months before the index visit, the following data were also analysed: percentage over total blood white cells and total number/mcl of blood eosinophils, blood gas analysis, peripheral oxygen saturation (SpO2) at rest and distance covered during the 6-minute walk test (6MWT) [Citation34]. The reference values were taken from the 1993 European Community for Steel and Coal statement [Citation35].
Definition of COPD exacerbation
A mild COPD exacerbation (AECOPD) was defined as a worsening of respiratory symptoms (cough, dyspnea, wheezing, or sputum) requiring a change of inhaled therapy. A moderate AECOPD was defined as a symptomatic deterioration requiring antibiotic therapy or medium to high dose systemic corticosteroids. A severe AECOPD was an exacerbation requiring hospitalization [Citation36].
GOLD classifications and clinical phenotypes
The patients were stratified according to the GOLD 2007, 2011 and 2017 recommendations [Citation2,Citation6,Citation9] (see the Supplementary file for further details) using the clinical and functional data obtained at the time of the index visit. We evaluated and compared the functional parameters and clinical data in each grading system in order to assess the changes in each group population.
A clinical characterization of the enrolled patients was performed according to the predominant clinical phenotype following the multifactorial model proposed by Pistolesi et al. [Citation37]. Briefly, for each patient, the presence of chronic cough, sputum, and sputum purulence, adventitious sounds and hyper-resonance at physical examination, chest X-ray parameters such as increased vascular markings, bronchial wall thickening, increased lung volume and reduced lung density, together with the FEV1/VC value were recorded. The aforementioned parameters were used as independent variables to calculate the clinical and radiological score. A score > 0.56 was considered as suggestive of dominant emphysema and ≤ 0.56 of predominant chronic bronchitis [Citation37].
A pre-specified analysis according to the peripheral eosinophil counts was performed. As no validated thresholds have been recommended to date [Citation38], two different cutoffs were used for both the absolute and relative eosinophil counts: (a) ≥ 200 cells/µL [Citation39] and ≥ 300 cells/µL [Citation40] and (b) ≥ 2% [Citation41] and ≥ 3% [Citation42], respectively.
Statistics
An electronic ad hoc form was prepared to collect the aforementioned qualitative and quantitative variables. The descriptive analysis included the absolute and relative (percentage) frequencies for the categorical variables and the means (standard deviations, SD) and medians (interquartile ranges, IQR) for the quantitative parametric and non-parametric variables, respectively. Differences between the groups were computed using the chi-squared and Fisher’s exact tests for the qualitative variables and the Mann–Whitney or Kruskal–Wallis tests for the non-normal variables. Spearman’s correlation was calculated to assess the correlation between the variables.
Stata statistical software version 15 (StataCorp, College Station, TX) was used to perform the statistical computations. A two-tailed, p-value < 0.05 was considered statistically significant.
Results
A total of 1360 patients were included in the study. The demographic, clinical and functional characteristics are summarized in . Overall, the study population was characterized by moderate airflow obstruction (median [IQR] FEV1: 62% predicted [44–77]), elevated functional parameters reflecting air trapping (median [IQR] RV: 134% predicted [105–170]) and mild DLCO impairment (median [IQR]: 62% predicted [43–77]), and the proportion of patients with air trapping was 65.3%. When considering the entire population, inspiratory capacity (IC) was positively correlated with FEV1 (rho: 0.51; P < 0.0001). Both FEV1 (rho: 0.40; P < 0.0001) and IC (rho: 0.33; P < 0.0001) showed positive correlation with DLCO.
Table 1. Clinical and functional characteristics of the study population.
A total of 307 patients (22.6%) were on long-term oxygen therapy because of chronic respiratory failure. The most prevalent comorbid conditions were peripheral vascular disease (reported by 20.5% of the patients), followed by ischemic heart disease (20.3%) and chronic heart failure (19.9%). Overall, 434 patients (31.9%) reported at least 2 moderate to severe exacerbations in the year prior to the index visit. The median (IQR) eosinophil count was 130 (100–210) cells/µL, corresponding to a median (IQR) of 1.8% of the total leucocytes (1.1–2.9%). Overall, 55.7% of the patients were treated with inhaled corticosteroid (ICS)-containing regimens, whereas only 4.3% were on long-acting anti-muscarinic agents (LAMAs) alone ().
Stratification of patients according to GOLD classifications
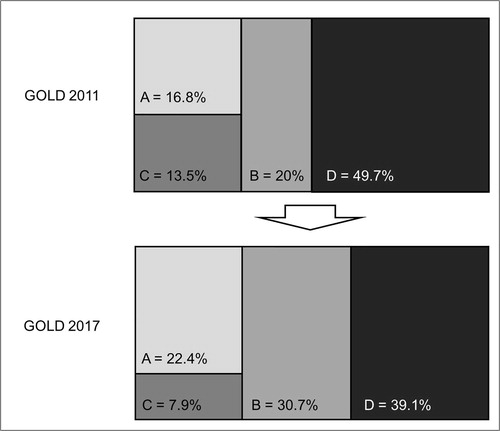
According to the GOLD 2007 classification [Citation6], the patients were stratified as follows: 189 (13.9%) were GOLD 1, 661 (48.7%) were GOLD 2, 367 (27.0%) were GOLD 3 and 141 (10.4%) were GOLD 4. When the GOLD 2011 classification [Citation9] was considered, the patients were grouped as follows: 214 (16.8%) in group A, 255 (20.0%) in group B, 172 (13.5%) in group C and 634 (49.7%) in group D. When the GOLD 2017 classification [Citation2] was considered, the patient distribution was as follows: 285 (22.4%) patients in group A, 391 (30.7%) patients in group B, 101 (7.9%) patients in group C and 498 (39.1%) patients in group D. The percentage of patients in high risk groups (C and D) was significantly reduced by 23% in the GOLD 2017 compared with the GOLD 2011 classification (). In particular, group C decreased by ∼41% while group D decreased by ∼27%.
Lung function parameters in COPD patients stratified according to GOLD 2007, GOLD 2011 and GOLD 2017 classifications
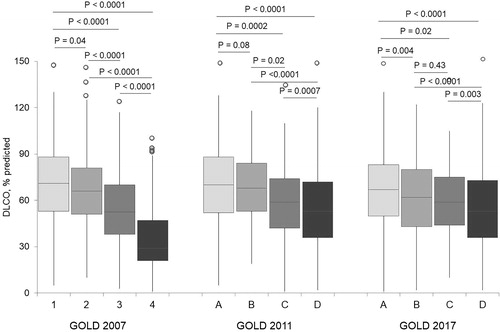
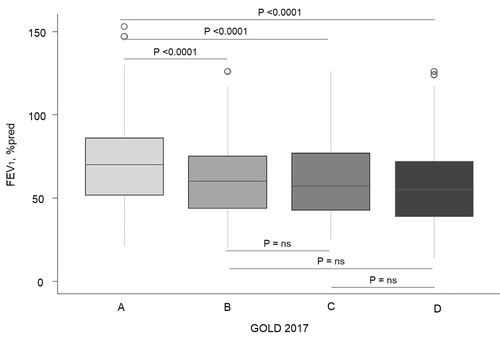
The 2017 GOLD classification does not include the FEV1 assessment for the ABDC score. Therefore, we first evaluated the lung function as the FEV1% predicted in the A, B, C and D groups stratified according to the GOLD 2017 classification. We found that the patients in group A had significantly higher FEV1 (70% predicted, IQR: 52–86) compared to the B (60% predicted, IQR: 44–75; P < 0.0001), C (57% predicted. IQR: 43–77; P = 0.002) and D (55% predicted, IQR: 39–72; P < 0.0001) patients. No significant differences were found between groups B, C and D, except that the patients in group B had higher FEV1 compared with those in group D (P = 0.04) ().
Figure 2. Box plot showing the distribution of the forced expiratory volume in 1 second according to the GOLD 2017 subgroups.
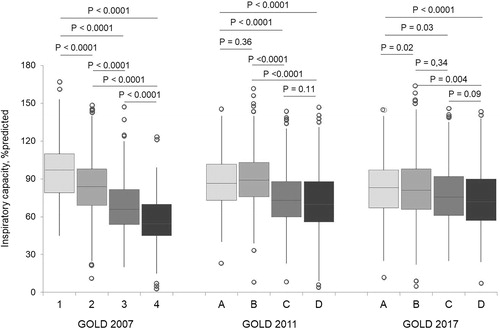
The disease severity according to the GOLD 2007 criteria was reflected by the progressive reduction in IC from GOLD 1 to GOLD 4 patients (GOLD 1 median [IQR]: 95% predicted [78–110], GOLD 2: 82% predicted [67–96], GOLD 3: 66% predicted [54–81] and GOLD 4: 54.5% predicted [44–72]; overall P < 0.0001) (). In fact, a statistically significant difference was found between every group of patients according to the GOLD 2007 classification (). When the GOLD 2011 stratification criteria were adopted, IC was similar in groups A and B (86.5 versus 86% predicted; P = 0.36) and in groups C and D (73 versus 69% predicted; P = 0.11) (). No difference was found in the IC levels comparing the patients in groups B and C (median IC, 78 versus 75.5% predicted; P = 0.34) and in groups C and D (median IC, 75.5 versus 72% predicted; P = 0.09) according to the GOLD 2017 classification ().
Figure 3. Box plot showing the distribution of the inspiratory capacity according to the GOLD 2007, GOLD 2011 and GOLD 2017 criteria.
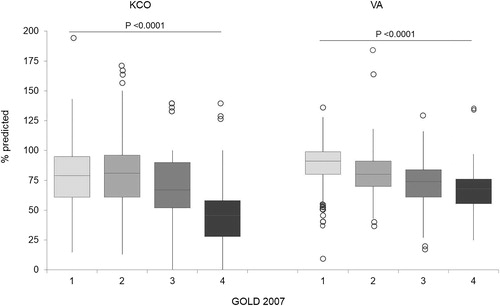
Similar results were found when the % predicted DLCO distribution was considered in the GOLD 2007 groups (). When adopting the GOLD 2011 criteria, no difference in DLCO values was found between groups A and B (median [IQR] IC % predicted, 69 [52–85] versus 65 [53–78]; P = 0.08) (), while DLCO among the GOLD 2017 classification groups did not differ when comparing groups B and C (60% predicted [44–76] versus 59% predicted [44–75]; P = 0.43). According to the GOLD 2007 criteria, VA gradually and significantly decreased from milder to more severe disease stages (P < 0.0001), while the KCO showed a marked reduction starting from the patients in the GOLD 3 group ().
Clinical phenotype assessment
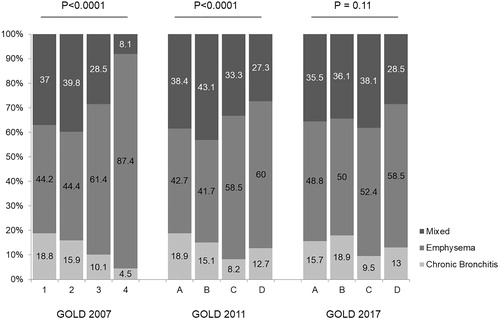
Overall, the most frequent phenotype was the emphysema-prevalent phenotype (n = 614, 53.2%), followed by the mixed phenotype (n = 383, 33.1%) and chronic bronchitis (n = 157, 13.6%). When the patients were stratified according to GOLD 2007, the prevalence of the emphysema phenotype progressively increased as the severity of airflow obstruction increased (). In the GOLD 2011 classification, the emphysema-prevalent phenotype was less prevalent in the A and B subgroups than in the patients at higher risk. Finally, the phenotype distribution in the GOLD 2017 classification was similar across the ABCD subgroups ().
Eosinophil count distribution
The blood eosinophil count was available for 639 patients (47%). No difference was found in the blood eosinophil levels (absolute value and %) between the patients with mild-moderate (GOLD 1 and 2 according to the 2007 classification) compared to those with severe-very severe airflow obstruction (GOLD 3 and 4 according to the 2007 classification). However, the proportion of patients with eosinophils ≥200 or ≥300 or ≥3% was significantly higher in the patients with severe–very severe compared to those with mild-moderate airflow obstruction (GOLD 2007). No difference was found in the blood eosinophil levels (absolute value and %) between the patients with low (A and B) compared with high risk (C and D) of exacerbation according to GOLD 2011. Significantly higher levels of blood eosinophils were found in the patients with low (A and B) compared to high (C and D) risk of exacerbation according to GOLD 2017 ().
Table 2. Distribution of the eosinophil counts and inhaled treatments according to the different GOLD classifications.
The patients with the emphysema-prevalent phenotype showed higher levels of blood eosinophil levels compared to those with chronic bronchitis and mixed phenotypes ().
Table 3. Distribution of the eosinophil counts according to the patients’ clinical phenotype.
Inhaled therapy according to phenotype and GOLD classification
Overall, 43.6% of the entire study population was treated with LABA/ICS + LAMA, 21.4% with LABA/LAMA, 12.1% with LABA/ICS, 18.6, and 4.3% with LABA or LAMA alone, respectively. The patients with reported frequent exacerbations were more frequently on triple therapy (P = 0.003); 51.5% of the patients with ≤1 exacerbation in the previous year were on ICS-containing regimens ().
Table 4. Inhaled treatment distribution in frequent and non-frequent exacerbations.
According to the GOLD 2007 classification, LAMA monotherapy was the most frequently administered regimen in the patients with mild disease, whereas for those ranked GOLD stage ≥ 2, the most frequently prescribed regimen was triple combination therapy (34.5, 63.3 and 70.0% for GOLD stages 2, 3 and 4, respectively). Therapy with LAMA/LABA was evenly distributed among the functional classes ().
According to GOLD 2011, LAMA monotherapy was the most frequently prescribed therapy in group A, while the patients in groups B, C, and D were most frequently on LABA/ICS and LAMA (30.8, 51.8 and 55.1% respectively). ICS-containing regimens were prescribed in 34% of the patients in group A, 41.% in group B, 62.4% in group C and 67.3% in group D ().
According to GOLD 2017, in all of the groups, LABA/ICS + LAMA combinations were the most prescribed therapeutic regimens (28.6, 41.1, 47.5 and 53.6% for groups A, B, C and D respectively). Considering all ICS-containing regimens, the proportion of patients on inhaled steroids was 41.8% in GOLD 2017 group A, 52.4% in group B, 60.6% in group C and 65.5% in group D ().
No significant differences were found in the levels of blood eosinophil counts in the patients treated with different inhaled regimens (P = 0.45, data not shown).
Discussion
COPD is a heterogeneous disease. The GOLD scoring of disease severity has changed over time from a pure FEV1-based functional assessment to a more sophisticated (“ABCD”) score that includes symptom assessment and exacerbation history. In this retrospective, observational, multicentre study, we found that: (i) the functional GOLD 2007 classification better reflected the pathophysiological abnormalities of the patients with COPD compared with the GOLD ABCD criteria; (ii) the transition from GOLD 2011 to GOLD 2017 led to a significant shift from high risk (C and D) to low risk categories (A and B); (iii) the ABCD criteria poorly reflected the clinical heterogeneity (the presence of chronic bronchitis and/or emphysema) of COPD; (iv) the absolute eosinophil counts were higher in the patients with severe disease, but the peripheral eosinophil count was not associated with the ABCD grading system; and (v) ICS-containing pharmacological regimens were the most frequently adopted therapeutic strategies, irrespective of the disease severity.
Pathological pulmonary deterioration in COPD patients leads to a decreased availability of operating volumes and eventually causes dyspnoea and reduced exercise tolerance [Citation3]. IC is one of the most reliable indexes of hyperinflation. IC is a powerful functional predictor of all-cause and respiratory mortality and exacerbation-related hospital admissions in COPD patients [Citation43], also correlating with a patient’s dyspnoea perception [Citation31,Citation44]. On the other hand, DLCO is associated with exercise capacity [Citation45] and its decline over time [Citation46]. Our data show that the 2007 functional COPD classification also reflects IC and DLCO impairment while similar levels of IC and DLCO were found in the ABCD patient groups according to the GOLD 2011 and 2017 classifications. Moreover, VA appeared to be correlated with the GOLD 2007 functional groups, confirming previous reports showing that the degree of ventilation heterogeneity may reflect the overall disease severity in patients with COPD [Citation45,Citation47].
Chronic bronchitis and emphysema are frequent and important clinical manifestations of the disease. Interestingly, patients with COPD and chronic bronchitis have an increased risk of exacerbations and respiratory mortality compared to patients without chronic phlegm production [Citation48]. Similarly, emphysema predominance has been associated with a rapid annual decline in FEV1 and poor response to treatment in COPD [Citation49]. Assessment of chronic bronchitis and emphysema are therefore mandatory when treating a patient with COPD. In the present study, in line with previous reports [Citation18], we found that the emphysema-prevalent phenotype progressively increased as the severity of airflow obstruction increased. The same pattern was not found in the ABCD categories, which showed a homogeneous phenotype distribution across the risk classes.
In line with recent studies [Citation15,Citation50], we found that the change from the GOLD 2011 to 2017 criteria was associated with a consistent shift of the patients (23% in our cohort) from higher (C and D) to lower (A and B) risk categories. This modification was mainly related to the exclusion of the airflow obstruction criteria in the assessment of the exacerbation risk. This redistribution to apparently milder categories flattens the between-patient functional and clinical differences and might jeopardize the predictability of important patient-centred outcomes, such as mortality [Citation13,Citation15]. Overall, the data suggest that the GOLD 2011 and particularly the 2017 version might miss important functional and clinical information with a relevant impact on the severity of disease manifestation [Citation14]
ICS-containing regimens have been suggested for patients with frequent exacerbations and elevated eosinophil counts. However, different cutoffs for blood eosinophils have been proposed as well as absolute rather than relative values. Our data show that the functional classification can better describe the eosinophilic pattern than the GOLD 2011 and 2017 criteria. GOLD stages 3 and 4 had a higher proportion of patients with > 200 and > 300 eosinophils/µL. This is in line with data from the SPIROMICS cohort [Citation23], which demonstrated that sputum eosinophil levels had a better correlation with exacerbations and disease severity [Citation51]. Unexpectedly, a higher eosinophil level was related to the emphysema phenotype. This is in contrast with previous studies [Citation5], even if comparability can be argued based on different study designs and emphysema diagnosis. Interestingly, a recent in vitro and animal model study demonstrated that eosinophil derived interleukin-13 promotes alveolar macrophage matrix metalloprotease-12 production, the latter being a predictor of emphysema [Citation52].
Whether eosinophilic inflammation can identify specific phenotypes of COPD in terms of clinical presentation and response to treatment has been highly debated [Citation23,Citation53–55]. Similar to previous European cohorts [Citation56,Citation57], we showed poor adherence to international guidelines and recommendations for maintenance therapy: in fact, 76% of patients with mild and moderate disease were on ICS-containing regimens. Although ICS is currently recommended only in patients with ≥ 2 exacerbations per year and ≥ 300 peripheral eosinophils [Citation12], almost 38 and 48% of the patients classified in group A and B according to GOLD 2011 and GOLD 2017, respectively, were exposed to combinations of LABA/ICS. This finding was unexpected due to the decrease in the high risk patients in the GOLD 2017 classification. Furthermore, it cannot be explained by ICS administration based on a “phenotype approach” [Citation58], as the chronic bronchitis phenotype had a lower prevalence in our cohort.
This study has some limitations that should be discussed. First, the findings on treatment regimens and adherence to guidelines should be cautiously interpreted; due to the study’s retrospective design, the GOLD 2017 guidelines could not be applied. Second, the number of patients with eosinophil counts higher than 300 cells/µL was low, limiting the reliability of the estimates based on the adopted cutoffs. Third, although the patients’ characteristics appear comparable with large prospective interventional [Citation23] and observational studies [Citation41,Citation50,Citation59], the COPD population recruited in our study was referred to secondary and tertiary academic centres; thus, the results may lack generalizability for populations managed by general practitioners. Fourth, the clinical phenotypes described in the present study were not based on chest high definition computed tomography (HRCT) findings. In fact, in the original work by Pistolesi et al. [Citation37], the HRCT was used only in the validation group, as a tool to prospectively validate the model and the methods used in the study. The validation allowed to establish the cutoff for the classification score. The HRCT was thus not included among the variables used in the multivariate model, which comprised, among clinical criteria and the FEV1/VC ratio, only chest X-ray derived parameters as independent variables (please see the methods section for a detailed list). The adequacy of the model developed by Pistolesi et al. [Citation37] was further supported by data from Pellegrino and colleagues [Citation60], that found similar differences in DLCO and TLC in patients with dominant chronic bronchitis and emphysema, the former being strong correlates of anatomical emphysema [Citation61]. Finally, an updated version of the GOLD document was made available in early 2019 [Citation12]. However, we believe that our results are still actual and important because the ABCD distribution remained unchanged in the final version of the GOLD document.
Conclusions
In conclusion, GOLD 2007, although classifying COPD patients based on FEV1 only, correlated better, in comparison with GOLD 2011 and 2017, with important pathophysiological parameters associated with exercise capacity, functional decline and dyspnoea. The GOLD 2011 and 2017 classifications, despite exploring important COPD determinants corresponding to symptoms and exacerbations, might thus miss relevant clinical characteristics with a significant impact on disease severity and short- to long-term prognosis such as lung function and COPD phenotypes.
Acknowledgments
This study was conducted according to the amended Declaration of Helsinki and approved by the ethical committee of the Coordinator Center (no. 220-2016). All of the participants gave their written informed consent.
Disclosure statement
D.R. reports personal fees from AstraZeneca, Boehringer Ingelheim and Neopharmed Gentili. M.C. reports grants from Chiesi and personal fees from Chiesi, AstraZeneca, Boehringer Ingelheim, Novartis, Menarini, Mundipharma, Almirall, and Zambon. F.D.M. reports fees for research from Boehringer Ingelheim Italia S.p.A. and Novartis and grants for board participation or lectures from Boehringer Ingelheim, GlaxoSmithKline, Chiesi, Zambon, Novartis, Guidotti/Malesci, AstraZeneca, Menarini, Mundipharma, and TEVA. F.B. has received honoraria for lectures and advisory board membership from AstraZeneca, Boehringer Ingelheim, and Chiesi Farmaceutici, Dompè, Guidotti/Malesci, Glaxo Smith Kline, Menarini, Zambon, Novartis. C.M. has participated as a lecturer, speaker, and/or advisor in scientific meetings and courses under the sponsorship of AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, Glaxo Smith Kline, Guidotti, Menarini, and Novartis. PR participated as a lecturer and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis. Her department was funded by Almirall, Boehringer Ingelheim, Novartis, Zambon, and Chiesi Farmaceutici. N.S. has participated as a lecturer, speaker, and/or advisor in scientific meetings and courses under the sponsorship of AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Guidotti/Malesci, Menarini Group, and Zambon and has received financial support for research and for congress attendance from AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Guidotti/Malesci, and Novartis. P. Santus reports grants and personal fees from Chiesi Farmaceutici, grants from AirLiquide, Pfizer, Almirall, and AstraZeneca, and personal fees from AstraZeneca, Boehringer Ingelheim, Novartis, Menarini, Malesci/Guidotti, Mundipharma, Valeas, Berlin-Chemie, and Zambon. P. Solidoro has received honoraria for lectures and advisory board membership from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi Farmaceutici, Dompè, Guidotti/Malesci, GlaxoSmithKline, Menarini, MSD, Mundipharma, Novartis, Biofutura Pharma (Alpha Sigma), and Biotest. GS, GP, AGC, and LS declare that they have no competing interests.
Data availability statement
The data are available upon request.
References
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602.
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease updated 2017. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018. [cited 2019 Jan 6] Available from: http://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf.
- Milic-Emili J, Pecchiari M, D’ Angelo E. Pathophysiology of chronic obstructive pulmonary disease. Curr Respir Med Rev. 2008;4:249–256.
- Tantucci C, Pini L. COPD: it is time to change! Int J Chron Obstruct Pulmon Dis. 2015;10:2451–2457. doi:10.2147/COPD.S87696.
- Papaioannou AI, Loukides S, Gourgoulianis KI, et al. Global assessment of the COPD patient: time to look beyond FEV1? Respir Med. 2009;103:650–660. doi:10.1016/j.rmed.2009.01.001.
- Rabe KF, Hurd S, Anzueto A, Global Initiative for Chronic Obstructive Lung Disease, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi:10.1164/rccm.200703-456SO.
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi:10.1378/chest.93.3.580.
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–654. doi:10.1183/09031936.00102509.
- Global strategy for the diagnosis, management and prevention of chronic pulmonary disease, 2011. Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2011. [cited 2019 Jan 6]. Available from: http://www.goldcopd.org/Guidelines/guidelines-resources.html.
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi:10.1164/rccm.201701-0218PP.
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease updated 2018. Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2018. [cited 2019 Jan 6] Available from: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease updated 2019. Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2019. [cited 2019 Jan 6] Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf
- Braido F, Di Marco F, Santus P, et al. COPD classification methods and informativeness on mortality: contrasting evidences. Minerva Med. 2013;104: 1–5.
- Leivseth L, Brumpton BM, Nilsen TI, et al. GOLD classifications and mortality in chronic obstructive pulmonary disease: the HUNT Study, Norway. Thorax. 2013;68:914–921. doi:10.1136/thoraxjnl-2013-203270.
- Cabrera López C, Casanova Macario C, Marín Trigo JM, et al. Comparison of the 2017 and 2015 global initiative for chronic obstructive lung disease reports. Impact on grouping and outcomes. Am J Respir Crit Care Med. 2018;197:463–469. doi:10.1164/rccm.201707-1363OC.
- Dusser D, Wise RA, Dahl R, et al. Differences in outcomes between GOLD groups in patients with COPD in the TIOSPIR(®) trial. Int J Chron Obstruct Pulmon Dis. 2016;11:133–145. doi:10.2147/COPD.S97924.
- Kobayashi S, Hanagama M, Ishida M, et al. Clinical characteristics and outcomes in Japanese patients with COPD according to the 2017 GOLD classification: the Ishinomaki COPD Network Registry. COPD. 2018;13:3947–3955. doi:10.2147/COPD.S182905.
- Bhatt SP, Soler X, Wang X, COPDGene Investigators, et al. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194:178–184. doi:10.1164/rccm.201511-2219OC.
- Golpe R, Suárez-Valor M, Martín-Robles I, et al. Mortality in COPD patients according to clinical phenotypes. COPD. 2018;13:1433–1439. doi:10.2147/COPD.S159834.
- Cosio BG, Soriano JB, López-Campos JL, CHAIN study, et al. Distribution and outcomes of a phenotype-based approach to guide COPD management: results from the CHAIN cohort. PLoS One. 2016;11:e0160770. ;. doi:10.1371/journal.pone.0160770.
- Barnes NC, Sharma R, Lettis S, et al. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J. 2016;47:1374–1382. doi:10.1183/13993003.01370-2015.
- Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3:435–442. doi:10.1016/S2213-2600(15)00106-X.
- Hastie AT, Martinez FJ, Curtis JL, SPIROMICS investigators, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5:956–967. doi:10.1016/S2213-2600(17)30432-0.
- Pinto-Plata V, Casanova C, Müllerova H, et al. Inflammatory and repair serum biomarker pattern: association to clinical outcomes in COPD. Respir Res. 2012;13:71. doi:10.1186/1465-9921-13-71.
- Burrows B, Knudson RJ, Cline MG, et al. Quantitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis. 1977;115:195–205. doi:10.1164/arrd.1977.115.2.195.
- Ohar JA, Sadeghnejad A, Meyers DA, et al. Do symptoms predict COPD in smokers? Chest. 2010;137:1345–1353. doi:10.1378/chest.09-2681.
- Hansen JE, Sun XG, Wasserman K. Spirometric criteria for airway obstruction: use percentage of FEV1/FVC ratio below the fifth percentile, not <70%. Chest. 2007;131:349–355.
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi:10.1183/09031936.05.00035205.
- Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi:10.1016/S0140-6736(09)61303-9.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Santus P, Radovanovic D, Henchi S, et al. Assessment of acute bronchodilator effects from specific airway resistance changes in stable COPD patients. Respir Physiol Neurobiol. 2014;197:36–45. doi:10.1016/j.resp.2014.03.012.
- Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi:10.1183/09031936.05.00035005.
- Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi:10.1183/09031936.05.00034905.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117.
- Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40.
- Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41:46s–53s.
- Pistolesi M, Camiciottoli G, Paoletti M, et al. Identification of a predominant COPD phenotype in clinical practice. Respir Med. 2008;102:367–376. doi:10.1016/j.rmed.2007.10.019.
- Pascoe S, Pavord I, Hinds D, et al. The association between blood eosinophils and risk and treatment outcome in COPD is not dichotomised. Lancet Respir Med. 2018;6:e18doi:10.1016/S2213-2600(18)30137-1.
- Couillard S, Larivée P, Courteau J, et al. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. 2017;151:366–373. doi:10.1016/j.chest.2016.10.003.
- Casanova C, Celli BR, de-Torres JP, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J. 2017;50:1701162. doi:10.1183/13993003.01162-2017.
- Vedel-Krogh S, Nielsen SF, Lange P, et al. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193:965–974. doi:10.1164/rccm.201509-1869OC.
- Cosío BG, Pérez de Llano L, Lopez Viña A, et al. on behalf of the CHACOS study group. Th-2 signature in chronic airway diseases: towards the extinction of asthma-COPD overlap syndrome?. Eur Respir J. 2017;49:1602397. doi:10.1183/13993003.02397-2016.
- Tantucci C, Donati P, Nicosia F, et al. Inspiratory capacity predicts mortality in patients with chronic obstructive pulmonary disease. Respir Med. 2008;102:613–619. doi:10.1016/j.rmed.2007.11.004.
- Celli BR, Decramer M, Lystig T, et al. Longitudinal inspiratory capacity changes in chronic obstructive pulmonary disease. Respir Res. 2012;13:66. doi:10.1186/1465-9921-13-66.
- Santus P, Radovanovic D, Balzano G, et al. Improvements in lung diffusion capacity following pulmonary rehabilitation in COPD with and without ventilation inhomogeneity. Respiration. 2016;92:295–307. doi:10.1159/000448847.
- Díaz AA, Pinto-Plata V, Hernández C, et al. Emphysema and DLCO predict a clinically important difference for 6MWD decline in COPD. Respir Med. 2015;109:882–889. doi:10.1016/j.rmed.2015.04.009.
- Pecchiari M, Radovanovic D, Santus P, et al. Airway occlusion assessed by single breath N2 test and lung P-V curve in healthy subjects and COPD patients. Respir Physiol Neurobiol. 2016;234:60–68. doi:10.1016/j.resp.2016.09.006.
- Lahousse L, Seys LJM, Joos GF, et al. Epidemiology and impact of chronic bronchitis in chronic obstructive pulmonary disease. Eur Respir J. 2017;50:1602470. doi:10.1183/13993003.02470-2016.
- Nishimura M, Makita H, Nagai K, et al. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:44–52. doi:10.1164/rccm.201106-0992OC.
- Safka KA, Wald J, Wang H, et al. GOLD stage and treatment in COPD: A 500 patient point prevalence study. Chronic Obstr Pulm Dis. 2016;4:45–55. doi:10.15326/jcopdf.4.1.2016.0126.
- Tashkin DP, Celli B, Senn S, et al. UPLIFT study investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi:10.1056/NEJMoa0805800.
- Doyle AD, Mukherjee M, LeSuer WE, et al. Eosinophil-derived IL-13 promotes emphysema. Eur Respir J. 2019;53:1801291. doi:10.1183/13993003.01291-2018.
- Calverley PMA, Magnussen H, Miravitlles M, et al. Triple therapy in COPD: what we know and what we don't. COPD. 2017;14:648–662. doi:10.1080/15412555.2017.1389875.
- Bafadhel M, Greening NJ, Harvey-Dunstan TC, et al. Blood eosinophils and outcomes in severe hospitalized exacerbations of COPD. Chest. 2016;150:320–328. doi:10.1016/j.chest.2016.01.026.
- Zysman M, Deslee G, Caillaud D, et al. Relationship between blood eosinophils, clinical characteristics, and mortality in patients with COPD. COPD. 2017;12:1819–1824. doi:10.2147/COPD.S129787.
- Glaab T, Vogelmeier C, Hellmann A, et al. Guideline-based survey of outpatient COPD management by pulmonary specialists in Germany. Int J Chron Obstruct Pulmon Dis. 2012;7:101–108.
- Corrado A, Rossi A. How far is real life from COPD therapy guidelines? An Italian observational study. Respir Med. 2012;106:989–997. doi:10.1016/j.rmed.2012.03.008.
- Contoli M, Corsico AG, Santus P, et al. Use of ICS in COPD: from blockbuster medicine to precision medicine. COPD. 2017;14:641–647. doi:10.1080/15412555.2017.1385056.
- Tudoric N, Koblizek V, Miravitlles M, et al. GOLD 2017 on the way to a phenotypic approach? Analysis from the Phenotypes of COPD in Central and Eastern Europe (POPE) Cohort. Eur Respir J. 2017;49:1602518. doi:10.1183/13993003.02518-2016.
- Pellegrino R, Crimi E, Gobbi A, et al. Severity grading of chronic obstructive pulmonary disease: the confounding effect of phenotype and thoracic gas compression. J Appl Physiol (1985). 2015;118:796–802. doi:10.1152/japplphysiol.00801.2014.
- Burrows B, Fletcher CM, Heard BE, et al. The emphysematous and bronchial types of chronic airways obstruction. A clinicopathological study of patients in London and Chicago. Lancet. 1966;1:830–835. doi:10.1016/S0140-6736(66)90181-4.