Abstract
There is a growing body of evidence supporting the use of nasal high flow (NHF) to treat acute respiratory failure, particularly in Chronic Obstructive Pulmonary Disease (COPD) patients. Conversely, there are sparse data evaluating its effects in stable COPD patients.
We identified randomized controlled trial comparing the effects of delivering air or oxygen via NHF, compared with delivering the same gas without NHF, in stable COPD patients through a systematic search using MEDLINE, CENTRAL, Science Direct, and others sources until January 2019. Study selection, data extraction and assessment of the risk of bias (using the Cochrane Risk of Bias tool) was performed by two independent authors.
We included 6 studies (339 participants). Our meta-analysis showed a significant reduction of arterial carbon dioxide pressure (PaCO2) at long (two studies, MD −3 mmHg, [95% Confidence interval (CI) −4 to −2]) and short-term (two studies, MD -3 mmHg [95% CI −4 to −2]). NHF significantly improved quality of life on the St George’s Respiratory Questionnaire (two studies, MD −5 out of 100, [95% CI −8 to −2]). NHF significantly reduced the rate of acute exacerbation at 1 year (one study, rate ratio: 0.6, [95% CI 0.6 to 0.7]). NHF did not significantly improve exercise capacity, hospitalization rate or mortality, but improved breathing pattern.
NHF reduced PaCO2, acute exacerbation and improved quality of life in stable COPD patients. Further long-term studies are needed to confirm the present results and provide more data on patient-centered outcome such as quality of life, exacerbation, hospitalization and mortality.
| List of abbreviations | ||
| COPD | = | chronic obstructive pulmonary disease; |
| HR | = | heart rate |
| FEV1 | = | forced expiratory volume in 1s; |
| FVC | = | forced vital capacity; |
| NHF | = | nasal high-flow; |
| NIV | = | noninvasive ventilation; |
| mMRC | = | modified Medical Research Council dyspnea scale; |
| MV | = | minute ventilation; |
| PaCO2 | = | arterial carbon dioxide partial pressure; |
| Pga | = | gastric pressure; |
| Poes | = | esophageal pressure; |
| PtcCO2 | = | transcutaneous arterial carbon dioxide partial pressure; |
| Ptdia | = | transdiaphragmatic pressure; |
| REM | = | rapid eye movement; |
| RR | = | respiratory rate; |
| RSBI | = | rapid shallow breathing index; |
| SpO2 | = | pulsed oxygen saturation; |
| StO2 | = | transcutaneous oxygen saturation; |
| SaO2 | = | arterial oxygen saturation; |
| TcO2 | = | transcutaneous oxygen; |
| Vt | = | tidal volume. |
Introduction
COPD is a worldwide cause of morbi-mortality with a growing burden [Citation1, Citation2]. This disease progressively leads to chronic respiratory insufficiency which can lead to hypoxia and hypercapnia [Citation3], each of which is associated with poor outcomes [Citation3, Citation4].
Long-term oxygen therapy reduces both hypoxia and mortality significantly in severely hypoxic patient with COPD [Citation5] whereas intermittent noninvasive ventilation (NIV) with high pressure has been used to reduce arterial carbon dioxide and improve mortality in chronic hypercapnic COPD patients (>55 mmHg) [Citation6, Citation7].
Unfortunately, there are important drawbacks associated with the use of NIV, including interface discomfort, excessive high air pressure, sleep disturbance and intolerability due to patient-ventilator asynchrony, each of which can lead to poor compliance or treatment failure [Citation8–11]. Therefore, alternative strategies are warranted.
Nasal high flow (NHF) delivers heated and humidified high flow air (up to 60 L/min) through nasal canula, providing promising physiological benefits such as positive airway pressure [Citation12] or upper airway CO2 washout [Citation13]. It can be used in association with oxygen and offers in this situation the advantage to match the patient’s inspiratory flow, preventing any dilution of the inspired fraction of oxygen [Citation14]. NHF has widely been studied in adult intensive care units and seems better than conventional oxygen therapy and as effective as NIV with regards to mortality to treat hypoxemic acute respiratory failure [Citation15–19]. Moreover, it may be more comfortable than conventional treatments [Citation20] and could help to control hypercapnia in patients with COPD [Citation21].
Despite this promising background evidence about NHF, there are sparse data evaluating its effects on clinically important outcome in stable patients with COPD.
Therefore, the overall aim of this review was to summarize the available evidence assessing the effects of delivering air or oxygen via NHF, compared with delivering the same gas without NHF, in people with stable COPD.
Method
Study registration and methodology
The protocol of this systematic review and meta-analysis was prospectively registered at PROSPERO (www.crd.york.ac.uk/prospero; CRD: 42018103358). It has been designed according to the Cochrane Handbook for Systematic Reviews of Interventions [Citation22] and reported according to the PRISMA statement.
Criteria for considering studies for this review
Types of studies
Parallel and cross-over randomized trials, included those in the format of an abstract, assessing one or more of the considered outcomes.
Type of participants
Adult patients with stable COPD (no acute exacerbation in the previous 3 weeks), of any age, diagnosed based on the individual study’s criteria. Studies with a mixed population of COPD and other respiratory disease(s) could be included if the data for the participants with COPD could be extracted separately.
Type of intervention
NHF with air, or supplemental oxygen if indicated by the patient’s clinical status, compared with the same gas delivered without NHF.
Type of outcome measures
Primary outcomes
Arterial carbon dioxide partial pressure measured transcutaneously (PtcCO2) or by arterial blood gases (PaCO2);
Arterial oxygen partial pressure measured transcutaneously (TcO2) or by arterial blood gases (PaO2);
pH measured by arterial blood gases;
Quality of life (assessed either with a general or a disease-specific questionnaire);
Deaths;
Number of acute exacerbations per year;
Number of hospitalizations per year;
Secondary outcomes
Oxygen saturation measured by pulse or transcutaneous oximetry (SpO2 and StO2 respectively) or by arterial blood gases (SaO2);
Cardiorespiratory function (heart rate (HR); forced expired volume in 1s (FEV1); forced vital capacity (FVC);
Breathing pattern (volume tidal (Vt); respiratory rate (RR); minute ventilation (MV); rapid shallow breathing index (RSBI));
Respiratory mechanics (gastric pressure (Pga); esophageal pressure (Poes); transdiaphragmatic pressure (Ptdia); end-expiratory lung impedance);
Dyspnea assessed either during or after an exercise or at rest;
Exercise capacity measured either by a maximal cardiopulmonary exercise testing or field tests;
Objective or self-reported physical activity;
Comfort and patient preference;
Adverse events.
Search methods for identification of studies
Electronic searches
MEDLINE, CENTRAL, Science Direct, Scopus, PEDro, OpenGrey and Greylit were searched from inception up to January 2019 for relevant studies in English or French. Reference list of the included studies were also checked for eligible studies.
Detailed searching strategy is shown in eAppendix 1.
Data collection and analysis: see eAppendix 1.
Study selection, data extraction and assessment of the risk of bias (using the Cochrane Risk of Bias tool[Citation22]) was performed by two independent authors. Any disagreement was resolved by discussion or the intervention of a third author.
Data synthesis
Heterogeneity was assessed using the I2 and Chi2 statistic, considering values I2 ≥ 50% as a sign of moderate to high heterogeneity. Meta-analysis was performed with a fixed or a random-effect model according to heterogeneity. RevMan 5.3.5 was used for analysis. The quality of evidence was rated using the GRADE system.
Results
Descriptions of studies and participants
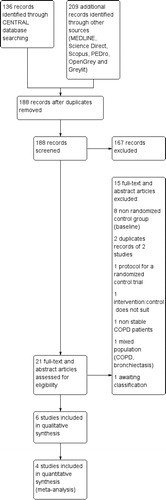
188 records were retrieved from database searching after removal of duplicates and 6 studies [Citation23–28] were included giving a total of 339 participants. See .
There was a good agreement between authors for the study selection (kappa score: 0.71).
Characteristics (methods, participants, intervention and outcomes) of the included studies are shown in .
Table 1. Characteristics of included studies.
Five studies were reported as full-text publications [Citation23–25, Citation27, Citation28] while one was available as an abstract only [Citation26]. Three assessed the short-term effects of NHF [Citation24–26], two assessed the long-term effects [Citation27, Citation28] and the remaining study assessed NHF during exercise [Citation23].
Patients with chronic hypoxemia [Citation24, Citation28], or both chronic hypoxemia and hypercapnia [Citation26, Citation27] were specifically included in four studies. When patients had oxygen during the control intervention, the oxygen flow during NHF was adjusted as necessary to maintain the same baseline transcutaneous oxygen saturation (SpO2) [Citation23, Citation24, Citation27, Citation28].
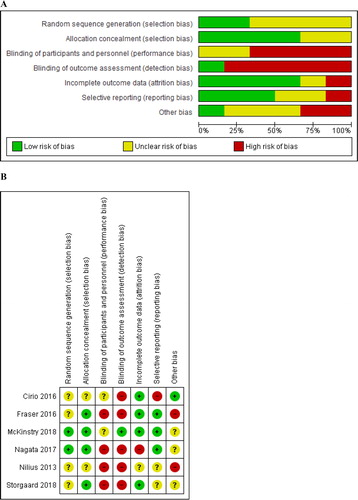
Risk of bias in included studies
The risk of bias among the included studies is depicted in and . The detailed evaluation is available in eAppendix 2.
Effects of interventions
The results for the three categories of trials (long-term, short-term, and during exercise) are detailed below. The overall findings are also summarized in .
Table 2. Summary of findings.
Long-term effects
Two studies including 229 participants contributed data on the long-term effects of NHF [Citation27, Citation28]. The follow-up period for these studies ranged from 6 weeks to 12 months.
Primary outcomes
Arterial carbon dioxide pressure
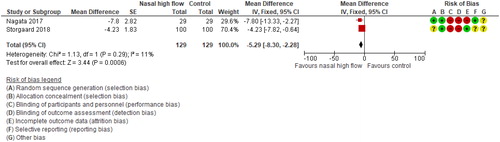
Meta-analysis of two studies showed a statistically significant benefit for NHF on PaCO2 (pooled MD −3 mmHg, 95% CI −4 to −2; I2=0%; .
Figure 3. Long-term (A) and short-term (B) effects of nasal high-flow on arterial carbon dioxide pressure (mmHg).
Nagata et al. also assessed single night PtcCO2 (without NHF) [Citation27]. They found a significant improvement in the mean median PtcCO2 (MD −5mmHg, 95% CI −8 to −2) in favor of NHF.
Arterial oxygen pressure
One study was available for this outcome (total 29 participants) [Citation27]. There was no difference between NHF and control in PaO2 (MD −3 mmHg, 95% CI −9 to 3).
pH
One cross-over study (total 29 participants) found a significant increase in pH in favor of NHF (MD 0.02, 95% CI 0 to 0.04) [Citation27]. Conversely, Storgaard et al. (total 200 participants) found no significant difference in baseline-adjusted changes in pH (detailed data not available on the original report and we had no answer from the author). The quality of evidence was “very low”.
Quality of life
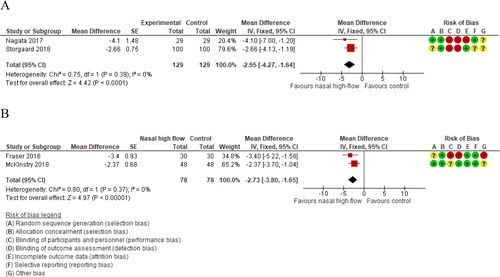
The two studies that measured quality of life both used the Saint George’s Respiratory Questionnaire, which is measured from 0 (no impairment) to 100 (maximum impairment). The result showed a statistically significant effect in favor of NHF (pooled MD −5, 95% CI −8 to −2; I2=11%; ).
Mortality
One study was available for this outcome (total 200 participants) [Citation28]. There was no difference between NHF and control in death events (risk ratio = 1.25, 95% CI 0.62 to 2.53).
Acute exacerbation
Because of the relatively short-term evaluation for this particular outcome in one study (6 weeks) and its cross-over design [Citation27], meta-analysis was deemed inappropriate and results are reported narratively instead.
One cross-over study (total 29 participants) [Citation27] reported three exacerbation’s of COPD with long-term oxygen (10.3%) and none with NHF over a 6-week period of treatment. One parallel study (total 200 participants) reported a significant reduction in acute exacerbations of COPD (rate ratio: 0.63, 95% CI 0.55 to 0.72) at 1 year.
Hospitalization
One study was available for this outcome (total 200 participants) [Citation28]. There was no difference between NHF and control in patient-year hospitalization rate (rate ratio: 0.89, 95% CI 0.69 to 1.15).
Secondary outcomes
Results for the secondary outcomes are shown in eAppendix 3. The quality of evidence ranged from “moderate” (significant improvement in the mMRC dyspnea scale with NHF) to “low” (no significant effects FEV1(%)) and “very low” (no significant effects on SpO2, exercise capacity, and physical activity).
Short-term effects
Three cross-over studies including 98 participants contributed data on the short-term effects of NHF [Citation24–26].
Primary outcomes
Arterial carbon dioxide pressure
Two studies including 78 participants contributed data on arterial carbon dioxide pressure [Citation24, Citation25]. Meta-analysis showed statistically significant effects of nasal high flow on arterial carbon dioxide pressure (pooled MD −3 mmHg, 95% CI −4 to −2; I2=0%; .
One study including 20 participants assessed single-night nocturnal PtcCO2 [Citation26]. Nilius et al. found a significant improvement both in the non-REM PtcCO2 (MD −1 mmHg, 95% CI −3 to −0) and in the REM PtcCO2 (MD −2 mmHg, 95% CI −3 to −1) with NHF.
Arterial oxygen pressure
One study was available for this outcome (total 30 patients) [Citation24]. There was a significant difference between NHF and control in TcO2 in defavor of the COPD patients randomized to the NHF group (MD −4 mmHg, 95% CI −7 to −1). The quality of evidence was “very low”.
Secondary outcomes
Results for the secondary outcomes are shown in eAppendix 3. The quality of evidence ranged from “very low” (no significant effects on SpO2, HR and MV, significant improvement in Vt and end-expiratory lung impedance and significant worsening in dyspnea and comfort with NHF) to “low” (significant improvement in RR with NHF).
During exercise
Only one cross-over study (12 participants) assessed the effects of during exercise NHF (constant workload exercise testing) compared with oxygen [Citation23]. The overall quality of evidence was “very low” (significant improvement in endurance time, isotime dyspnea and isotime SaO2).
Detailed results are shown in eAppendix 4.
Discussion
The main findings of this meta-analysis were that NHF significantly reduced PaCO2 (about -3mmHg) compared with usual care. Moreover, it significantly increased health-related quality of life and breathing pattern, and reduced acute exacerbations of COPD. NHF had no significant effects on mortality, hospitalization rate, pulmonary function and exercise capacity. One short-term study revealed that NHF could reduce TcO2 and worsen dyspnea and comfort. Finally, the use of NHF during exercise might improve endurance capacity and dyspnea.
The improvement in PaCO2 was found both at short and long term. The main explanation for the short-term improvement lies in the improvement of the breathing pattern which is supported by our results suggesting an increase in Vt and a decrease RR. Since minute ventilation was not significantly different between conditions, the improvement in RR and Vt contributed to improve alveolar ventilation. This deeper breathing pattern likely arises from a combination of several factors such as a reduction of inspiratory resistance, improved respiratory mechanics [Citation29], humidification [Citation30] and a positive expiratory pressure effect [Citation14, Citation29, Citation31]. Moreover, the flow and leakage dependent washout of the upper airway has been highlighted as an important factor to reduce PaCO2 [Citation13]. The mechanisms of long-term improvement are unclear and remain to be elucidated. It could be hypothesized that a better NHF-mediated humidification improved airway clearance and inflammatory status [Citation14, Citation30] which in turn decreased dynamic hyperinflation and inspiratory load [Citation32], while improving breathing pattern (which was also observed in this review) [Citation32], and finally improved ventilation-perfusion matching [Citation33]. The magnitude of improvement of PaCO2 with NHF was relatively modest (-3 mmHg, [95% CI -4to −2]) compared to that found with high pressure and rather high backup rate NIV (−7 mmHg [CI 95% −9 to −4], recalculated data from Tables 3 and 4) [Citation34]. This difference could in part be explained by the difference in baseline PaCO2 in the study by Kohnlein et al. (about 58 mmHg) compared to that of the studies included in the present meta-analysis (< 50 mmHg). Despite this, the indirect comparison of these confidence intervals shows some degree of overlapping so that an equivalent effect cannot totally be ruled out. This is strengthened by a recent study which compared the effects of 6 weeks of NHF with 6 weeks of NIV on PaCO2 and showed substantial improvement with both treatments without any significant difference between them [Citation35]. Therefore, NHF could be relevant for hypercapnic COPD patients who do not tolerate high-pressure NIV and long-term studies comparing the effects of NHF and NIV are now warranted.
The present results also suggest that NHF did not improve SpO2 both at short and long-term. This was also the case for long-term PaO2. This is strengthened by a recent study which did not support the use of NHF with room air as a stand-alone therapy to oxygenate hypoxemic COPD patients who already benefit from long-term oxygen therapy [Citation36]. Surprisingly, one short-term study reported a significant fall in TcO2 when using NHF supplemented with an equivalent fraction of inspired oxygen compared with 2 to 4 L/min of oxygen through nasal cannula (about -4mmHg) [Citation24]. Reassuringly, the mean TcO2 in the NHF group remained largely within a normal range (97 mmHg (SD 24)). Nonetheless, this result raises concern about the necessity to titrate oxygen during NHF to avoid any flushing effect of the usual oxygen prescription as previously described [Citation29].
Interestingly, long-term NHF was associated with a decrease in the rate of acute exacerbation of COPD of about 40% in the only long-term study, which assessed this outcome at 1 year. Considering the burden of exacerbations on disease progression and functional outcome [Citation37, Citation38], even a 25% reduction in this relative risk, using a conservative approach based on the lower bound of the 95% CI, would still be particularly relevant. The underlying mechanisms remain to be elucidated but the implication of humidification in the management of airway secretions retention is a plausible explanation [Citation30]. Since exacerbation and quality of life are closely related, the improvement of the former can explain the improvement of health-related quality of life with NHF (-5% [95% CI: -8 to -2]) which exceeded the minimum clinically important difference (MCID) of -4% for the SGRQ. These results are in line with those of Rea et al., who found a significant reduction in the rate of acute exacerbation and improvement in quality of life with NHF in a mixed population of COPD and bronchiectasis patients [Citation39]. However, the 95% CI of the present estimate is relatively wide and its lower bound falls below this MCID, so a clinically trivial effect cannot be excluded.
In the context of intensive care unit, NHF is frequently reported as better tolerated (comfort and dyspnea) than the treatment it is compared with (oxygen or NIV) [Citation15, Citation16, Citation40]. Conversely, the results presented here, from a single study, highlight that it may be less comfortable and elicit more dyspnea than conventional oxygen in stable COPD patients [Citation24]. This may be attributable to the initiation of a new treatment and seems to be reversible with time because long-term dyspnea was improved. Also, the choice of the NHF flow and temperature is of real concern with regards to comfort. 30 L/min and temperature below 37 °C seems to be a good compromise between efficiency in reducing PaCO2 and comfort [Citation25, Citation41]. Despite this, NHF was not associated with an increase in adverse event.
Finally, while the improvement in exercise capacity associated with the use of long-term NHF was not significant, its 95% CI encompassed the MCID (25 to 33 m [Citation42]). Because of a high level of heterogeneity, the quality of evidence was downgraded to “very low” and further studies are warranted to refine this estimate.
Limitations: The main limitation of this study was the number of included studies, reducing the meta-analysis to the combination of a maximum of two studies. Major outcomes, including hospitalization rate and mortality were only assessed in one study. Moreover, the confidence in the estimates was restricted due to methodological issues, particularly because of inadequate blinding of the participants and outcome assessors. Although some outcomes such are exacerbation are most likely not influenced by the lack of blinding, the overall quality of evidence ranged from moderate to very low.
Conclusion
NHF has the potential to reduce PaCO2, acute exacerbation and improve long-term health related quality of life in stable COPD patients. Although these estimates are promising, further evidence could refine them. Caution should be taken when setting up NHF in those patients with oxygen to avoid any flushing effect of oxygen. Further long-term studies are needed to confirm the present results and should now compare the effects of NHF with those from NIV.
Declaration of interest
TB reports grant from Fisher & Paykel. Dr MP reports grants from B&D Electromedical, personal fees from ResMed and Philips Respironics, grants and nonfinancial support from Fisher & Paykel, nonfinancial support from MSD, nonfinancial support from Asten, and grants from ADIR Association, outside the submitted work. The other authors report no conflicts of interest in this work.
Ethical approval and consent to participate
Written informed consent was not required for this study.
Supplemental Material
Download Zip (1 MB)Acknowledgments
We thank Gregory Reychler for his support during data analysis.
Additional information
Funding
Notes on contributors
Tristan Bonnevie
TB, ME, CP, FEG, and GP have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; have drafted the submitted article or revised it critically for important intellectual content; have provided final approval of the version to be published; and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CM, YC, MP, JFM, and AC have drafted the submitted article or revised it critically for important intellectual content; have provided final approval of the version to be published; and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Khakban A, Sin DD, FitzGerald JM, et al. The projected epidemic of chronic obstructive pulmonary disease hospitalizations over the next 15 years. A population-based perspective. Am J Respir Crit Care Med. 2017;195(3):287–291. doi:10.1164/rccm.201606-1162PP.
- Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi:10.1183/09031936.06.00124605.
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [Internet]. 2018. Available from: August 1st, 2019 https://goldcopd.org/gold-reports/
- Steer J, Gibson GJ, Bourke SC. Predicting outcomes following hospitalization for acute exacerbations of COPD. QJM. 2010;103(11):817–829. doi:10.1093/qjmed/hcq126.
- COPD Working Group. Long-term oxygen therapy for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser. 2012;12(7):1–64.
- Chen H, Liang BM, Xu ZB, et al. Long-term non-invasive positive pressure ventilation in severe stable chronic obstructive pulmonary disease: a meta-analysis. Chin Med J. 2011;124(23):4063–4070.
- Altintas N. Update: Non-invasive positive pressure ventilation in chronic respiratory failure due to COPD. COPD. 2016;13(1):110–121. doi:10.3109/15412555.2015.1043520.
- Cheng SL, Chan VL, Chu CM. Compliance with home non-invasive ventilation. Respirology. 2012;17(4):735–736. doi:10.1111/j.1440-1843.2012.02169.x.
- Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care. 2009;54(1):71–84.
- Adler D, Perrig S, Takahashi H, et al. Polysomnography in stable COPD under non-invasive ventilation to reduce patient-ventilator asynchrony and morning breathlessness. Sleep Breath. 2012;16(4):1081–1090. doi:10.1007/s11325-011-0605-y.
- Fanfulla F, Taurino AE, Lupo ND, et al. Effect of sleep on patient/ventilator asynchrony in patients undergoing chronic non-invasive mechanical ventilation. Respir Med. 2007;101(8):1702–1707. doi:10.1016/j.rmed.2007.02.026.
- Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care. 2007;20(4):126–131. doi:10.1016/j.aucc.2007.08.001.
- Braunlich J, Mauersberger F, Wirtz H. Effectiveness of nasal highflow in hypercapnic COPD patients is flow and leakage dependent. BMC Pulm Med. 2018;18(1):14doi:10.1186/s12890-018-0576-x.
- Pisani L, Vega ML. Use of nasal high flow in stable COPD: rationale and physiology. COPD. 2017;14(3):346–350. doi:10.1080/15412555.2017.1315715.
- Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: a systematic review and meta-analysis. Chest. 2017;151(4):764–775. doi:10.1016/j.chest.2017.01.004.
- Monro-Somerville T, Sim M, Ruddy J, et al. The effect of high-flow nasal cannula oxygen therapy on mortality and intubation rate in acute respiratory failure: a systematic review and meta-analysis. Crit Care Med. 2017;45(4):e449–e456. doi:10.1097/CCM.0000000000002091.
- Lin SM, Liu KX, Lin ZH, et al. Does high-flow nasal cannula oxygen improve outcome in acute hypoxemic respiratory failure? A systematic review and meta-analysis. Respir Med. 2017;131:58–64. doi:10.1016/j.rmed.2017.08.005.
- Corley A, Rickard CM, Aitken LM, et al. High-flow nasal cannulae for respiratory support in adult intensive care patients. Cochrane Database Syst Rev. 2017;5:CD010172.
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi:10.1056/NEJMoa1503326.
- Maggiore SM, Idone FA, Vaschetto R, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med. 2014;190(3):282–288. doi:10.1164/rccm.201402-0364OC.
- Pilcher J, Eastlake L, Richards M, et al. Physiological effects of titrated oxygen via nasal high-flow cannulae in COPD exacerbations: a randomized controlled cross-over trial. Respirology. 2017;22(6):1149–1155. doi:10.1111/resp.13050.
- Higgins JPT, Green A. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. www.cochrane-handbook.org. The Cochrane Collaboration; 2011.
- Cirio S, Piran M, Vitacca M, et al. Effects of heated and humidified high flow gases during high-intensity constant-load exercise on severe COPD patients with ventilatory limitation. Respir Med. 2016;118:128–132. doi:10.1016/j.rmed.2016.08.004.
- Fraser JF, Spooner AJ, Dunster KR, et al. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax. 2016;71(8):759–761. doi:10.1136/thoraxjnl-2015-207962.
- McKinstry S, Pilcher J, Bardsley G, et al. Nasal high flow therapy and PtCO2 in stable COPD: a randomized controlled cross-over trial. Respirology. 2018;23(4):378–384. doi:10.1111/resp.13185.
- Nilius G, Domanski U, Franke KJ, et al. Nasal high flow oxygen therapy attenuates nocturnal hypoventilation in COPD patients with hypercapnic respiratory failure [Abstract]. Am J Respir Crit Care Med. 2013;187:A3102.
- Nagata K, Kikuchi T, Horie T, et al. Domiciliary high-flow nasal cannula oxygen therapy for patients with stable hypercapnic chronic obstructive pulmonary disease. A multicenter randomized crossover trial. Annals ATS. 2018;15(4):432–439. doi:10.1513/AnnalsATS.201706-425OC.
- Storgaard LH, Hockey HU, Laursen BS, et al. Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. COPD. 2018;13:1195–1205. doi:10.2147/COPD.S159666.
- Pisani L, Fasano L, Corcione N, et al. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax 2017;72(4):373–375. doi:10.1136/thoraxjnl-2016-209673.
- Hasani A, Chapman TH, McCool D, et al. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis. 2008;5(2):81–86. doi:10.1177/1479972307087190.
- Dysart K, Miller TL, Wolfson MR, et al. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400–1405. doi:10.1016/j.rmed.2009.04.007.
- Diaz O, Begin P, Torrealba B, et al. Effects of noninvasive ventilation on lung hyperinflation in stable hypercapnic COPD. Eur Respir J. 2002;20(6):1490–1498. doi:10.1183/09031936.02.00034402.
- De Backer L, Vos W, Dieriks B, et al. The effects of long-term noninvasive ventilation in hypercapnic COPD patients: a randomized controlled pilot study. Int J Chron Obstruct Pulmon Dis. 2011;6:615–624.
- Kohnlein T, Windisch W, Kohler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698–705. doi:10.1016/S2213-2600(14)70153-5.
- McKinstry S, Singer J, Baarsma JP, et al. Nasal high-flow therapy compared with non-invasive ventilation in COPD patients with chronic respiratory failure: a randomized controlled cross-over trial. Respirology. 2019; doi:10.1111/resp.13575.
- Atwood CW, Jr., Camhi S, Little KC, et al. Impact of heated humidified high flow air via nasal cannula on respiratory effort in patients with chronic obstructive pulmonary disease. J COPD F. 2017;4(4):279–286. doi:10.15326/jcopdf.4.4.2016.0169.
- Ramon MA, Ter Riet G, Carsin AE, et al. The dyspnoea-inactivity vicious circle in COPD: Development and external validation of a conceptual model. Eur Respir J. 2018;52(3):1800079.
- Wedzicha JA, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med. 2014;35(1):157–163. doi:10.1016/j.ccm.2013.11.001.
- Rea H, McAuley S, Jayaram L, et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med. 2010;104(4):525–533. doi:10.1016/j.rmed.2009.12.016.
- Lee CC, Mankodi D, Shaharyar S, et al. High flow nasal cannula versus conventional oxygen therapy and non-invasive ventilation in adults with acute hypoxemic respiratory failure: a systematic review. Respir Med. 2016;121:100–108. doi:10.1016/j.rmed.2016.11.004.
- Mauri T, Galazzi A, Binda F, et al. Impact of flow and temperature on patient comfort during respiratory support by high-flow nasal cannula. Crit Care. 2018;22(1):120. doi:10.1186/s13054-018-2039-4.
- Puente-Maestu L, Palange P, Casaburi R, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J. 2016;47(2):429–460. doi:10.1183/13993003.00745-2015.