Abstract
Blood eosinophilia has been proposed as a surrogate marker for airway eosinophilia and as a predictor of treatment response in chronic obstructive pulmonary disease (COPD). The aim of the study was to assess the relationship between blood and sputum eosinophils and to investigate the association between blood and sputum eosinophil count and clinical features of mild-to-moderate COPD. We performed a retrospective analysis of blood and sputum eosinophil count, as well as demographic and lung function data in a cohort of 90 stable, steroid-naive (Global Initiative for Chronic Obstructive Lung Disease 1 or 2) COPD patients and 20 control subjects. Blood and sputum eosinophil count did not correlate both in patients with COPD (r = –0.04 p = 0.705) and in controls (r = 0.05, p = 0.838). Sputum eosinophilia (≥3%) was present in 40% of COPD patients. The median blood eosinophil count in patients with COPD was 180 (interquartile range 90–270)/μL; patients with low blood eosinophils (<180/μL) did not differ from those with high blood eosinophils (≥180/μL) in terms of forced expiratory volume in 1 second, bronchial reversibility or hyperresponsiveness. This was also the case when COPD patients with and without sputum eosinophilia were compared. At the same time, positive bronchial reversibility and positive bronchial hyperresponsiveness were observed in 2 (11%) COPD patients with high blood eosinophils and in 1 (5%) patient with sputum eosinophilia. There was a weak, albeit significant correlation (r = 0.22 p = 0.041) between blood eosinophil count and age in patients with COPD. Peripheral eosinophil count poorly reflects sputum eosinophils and lung function in stable steroid-naive mild-to-moderate COPD patients.
Introduction
Chronic, neutrophilic airway inflammation has been regarded as one of the most typical features of chronic obstructive pulmonary disease (COPD) [Citation1, Citation2]. However, in the last decade airway eosinophilia has been increasingly recognised as a distinct inflammatory pattern in some COPD patients [Citation3, Citation4]. According to some studies, eosinophilic airway inflammation seems to be a reliable forecaster of a beneficial response to inhaled corticosteroids (ICS), expressed as dyspnoea alleviation [Citation5] and decreased exacerbation rate [Citation6]. It is also associated with a significant improvement when systemic corticosteroids are used to treat disease exacerbations [Citation7].
A widely recognised diagnostic method to evaluate a type of airway inflammation is an assessment of differential cell count in induced sputum. However, this method has a number of limitations. First, it may not allow to obtain a quality sputum sample in all patients. Second, the method is time consuming and requires a special room setting, equipment and technical expertise [Citation8]. Hence, other minimally invasive and easily accessible methods, which could predict or indicate the eosinophilic airway inflammation, are needed. Peripheral blood cell count seems to have the potential to fulfil the criteria of a reliable surrogate biomarker and could be an attractive alternative to the induced sputum collection and examination. The relationship between airway and blood eosinophilia has been confirmed in asthma, where the ability of blood eosinophils to predict sputum eosinophilia has been extensively examined [Citation9–11].
Despite numerous attempts, clinically important blood eosinophil level in COPD has not yet been defined. As there is no commonly accepted threshold for either sputum or blood eosinophilia in COPD, its incidence rate reported by different authors varies significantly. Blood eosinophilia was present persistently in 15% (cut-off value ≥300 cells/µL) [Citation12] to 37% (cut-off value ≥2%) [Citation13] of COPD patients. In a single blood analysis, it was found in even 68% (cut-off value ≥2%) of patients [Citation14]. The normal value for sputum eosinophil differential count is <3% in the standard protocol [Citation15]. Brightling et al. demonstrated high sputum eosinophilia (defined as >3.9%) in 33% of stable COPD patients [Citation6]. Even higher percentage was reported by Leigh et al. who showed sputum eosinophilia, defined as ≥3% of all non-epithelial sputum cells, in 38% of stable COPD patients [Citation5]. Sputum eosinophilia >3% was also found in 28% of patients with acute exacerbations of COPD [Citation7].
Although the possible use of blood eosinophil count to select patients who would benefit from corticosteroid therapy is an attractive option, conflicting data were published on the relationship between blood eosinophil level and eosinophilic airway inflammation in COPD patients. According to some studies, a cut-off value of 2% for blood eosinophils could serve as a marker of sputum eosinophilia in stable, moderate-to-severe COPD [Citation14] as well as in acute COPD exacerbations [Citation7]. On the other hand, albeit a large SPIROMICS cohort study showed a weak correlation between peripheral and sputum eosinophil counts, it also demonstrated that blood eosinophils is not a reliable marker of sputum eosinophils with a high false discovery rate and a false-negative rate [Citation16]. In the current study, we aimed to assess the relationship between blood and sputum eosinophils and to investigate associations between blood and sputum eosinophil count and other clinical features of mild-to-moderate COPD in steroid-naive patients.
Materials and methods
Study design and participants
In this retrospective study, we analysed data acquired from stable, mild-to-moderate COPD patients and controls who participated in three different projects conducted at the Department of Internal Medicine, Pulmonary Diseases and Allergy, Medical University of Warsaw between 2010 and 2017. The three study projects were approved by the institutional review board (first project: KB/186/2010, second project: KB/82/2014 and third project: KB/236/2015) and one of them was registered at ClinicalTrial.gov (NCT02069054). All participants signed an informed consent. All these patients underwent sputum induction and blood sampling on the same day, with assessment of total and differential leukocyte count in both sputum and peripheral blood. Other data, including demographic and anthropometric characteristics, medical history with special attention to COPD sign and symptoms expressed as modified Medical Research Council (mMRC) score and COPD Assessment Test (CAT) score, smoking history, allergy skin prick test results, lung function, were also collected and analysed.
Definitions and exclusion/inclusion criteria
The specific inclusion criteria for the primary studies were presented elsewhere [Citation17, Citation18]. Briefly, the diagnosis of COPD was made in accordance with the Global Initiative for Chronic Obstructive Lung Disease 2011. The following criteria had to be met to be included in the COPD patients group subjected for current analysis: (1) symptoms typical for COPD, (2) smoking history >10 pack-years, (3) age >40 years, (4) fixed airway obstruction defined as postbronchodilator (salbutamol, 400 µg from pMDI) forced expiratory volume in 1 second (FEV1), to forced vital capacity ratio below the lower limit of normal (i.e. fifth percentile), (5) None of the included COPD subjects had used inhaled and/or systemic corticosteroids in at least three months preceding the inclusion to the study. Of note, COPD patients did not meet diagnostic criteria of asthma-COPD overlap [Citation19].
All control subjects had a negative history of obstructive lung disease and had normal results of lung function tests.
Exclusion criteria for all subjects were disease exacerbation or any symptoms of respiratory tract infection in the previous 6 weeks and current or previous diagnosis of asthma or symptoms suggesting asthma (e.g. wheezing, variability of symptoms, nocturnal symptoms).
The median blood eosinophil count in COPD patients was used to discriminate between those with low (<180 cells/µL) and high (≥180 cells/µL) blood eosinophil counts. In terms of sputum eosinophils, the level of 3% was used as a threshold classifying patients with high (≥3%) and low (<3%) sputum eosinophil percentage. The above level was based on European Respiratory Society (ERS) recommendations [Citation15].
Sputum induction and processing
Sputum induction was preceded by inhalation of 400 µg of salbutamol and pulmonary function testing. After the postbronchodilator spirometry, the patients inhaled sterile hypertonic saline (NaCl) at increasing concentrations (3, 4 and 5% solutions) at room temperature via an ultrasonic nebuliser (ULTRA-NEB™ 2000, DeVilbiss, USA). The duration of each inhalation was 7 minutes and the induction was stopped after a decrease of FEV1 by at least 20% from baseline (postbronchodilator) FEV1 value. The sputum samples were processed and examined within 2 hours after induction, according to the ERS recommendations [Citation20]. The cells were counted, the percentage of dead cells and epithelial cells was assessed. The differential cell count (the percentage of eosinophils, neutrophils, macrophages, lymphocytes) was determined microscopically in May-Grünwald-Giemsa-stained smears based on the morphology of 300 cells from various fields.
Blood cell count evaluation
The white blood cell count was measured using Sysmex XN-2000 (Sysmex Corporation, Kobe, Japan). The differential cell count was evaluated manually in May-Grünwald-Giemsa stained smears.
Pulmonary function tests
Lung function testing was performed according to the recommendations of the ERS and the American Thoracic Society [Citation21, Citation22]. Changes in FEV1 and/or vital capacity (VC) > 12% and 200 mL compared with baseline identified a positive bronchodilator response. Bronchial hyperresponsiveness (BHR) was tested using methacholine challenge test. Inhalation was interrupted when FEV1 decreased by 20% from its baseline value and the result was considered positive when provocative concentration causing a 20% fall in FEV1 [methacholine concentration causing a 20% drop in FEV1 (PC20)] was 8 mg/mL or less.
Statistical analysis
The statistical analysis was performed using Statistica 13.1 software (StatSoft Inc., Tulsa, USA). The data were presented as median and interquartile range (IQR) or percentage (%). Differences between continuous variables were tested using Mann–Whitney U-test. Fisher’s exact test or χ2 test were used to compare proportions between the groups. Correlations between variables were analysed with Spearman’s rank test. Differences were considered statistically significant at p < 0.05.
Results
Patient characteristics
In total, 90 COPD patients and 20 control subjects were included in the analysis. Comparative characteristics of both study groups are presented in . As the inclusion criteria were as follows FEV1 > 50% of predicted and lack of ICS treatment, final study group had a history with a low rate of exacerbations (<2 per year, no hospitalisation in the previous year), thus, COPD patients were exclusively classified as Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011 stage A (26.7%), and as stage B (73.3%). Long acting beta agonist received 71% of patients and long acting muscarinic antagonist 64.7%. Spirometry revealed that 27% of patients had mild (GOLD 1) and 73% moderate COPD (GOLD 2).
Table 1. Clinical characteristics of patients with COPD and control subjects participating in the study.
Peripheral blood and induced sputum eosinophils in COPD and control
Data on blood eosinophils were available in all patients. As sputum specimens of three COPD patients contained >70% of epithelial cells, these patients were excluded from analyses referring to sputum eosinophils. There was no significant difference between COPD and control subjects in blood and sputum eosinophil count (). Peripheral eosinophil count did not correlate with sputum eosinophil percentage count in either COPD or control group ().
Figure 1. Correlation between percentage sputum eosinophils and absolute blood eosinophil count in COPD patients and controls.
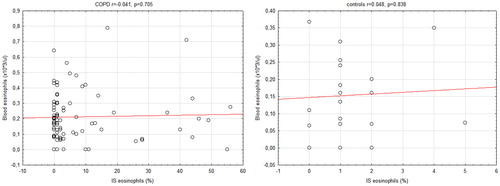
Table 2. Sputum and peripheral cell count in COPD patients and control group.
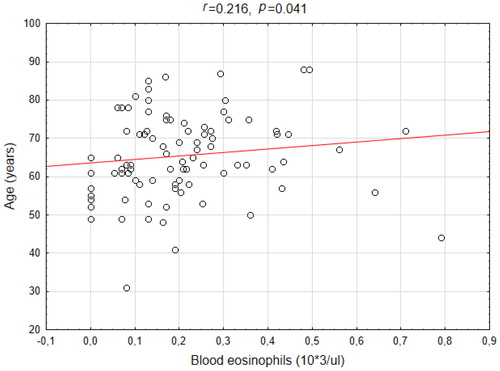
There was a weak but significant correlation (r = 0.216 p = 0.041) between the blood eosinophil level and the age of COPD patients (). No correlation between the sputum eosinophil level and the age (r=–0.198, p = 0.066) was noted. We also did not find any correlation between ΔFEV1 and the eosinophil level in either blood (r = 0.095, p = 0.376) or sputum (r = 0.095, p = 0.380).
Differences between COPD patients with higher and lower blood and sputum eosinophil levels
and show comparative characteristics of COPD patients with high and low blood and sputum eosinophil levels, respectively. Forty percent of COPD patients had ≥3% eosinophils in induced sputum. COPD patients with higher and lower blood and sputum eosinophil level did not differ with regard to age, body mass index (BMI), smoking status (pack-years), CAT points, FEV (% pred.), FEV1/VC ratio (%), residual volume (RV) and total lung capacity (TLC, % pred.) values. Also, no difference was found in PC20 and ΔFEV1 (% pred.) after bronchodilation. The only exception was a dyspnoea level expressed as mMRC points which was higher in patients with the elevated blood eosinophil count (p = 0.049). However, we did not find a significant correlation between mMRC and blood eosinophils (r = 0.142, p = 0.236).
Table 3. Comparison of clinical data of COPD patients according to peripheral eosinophilia.
Table 4. Comparison of clinical data of COPD patients according to sputum eosinophilia.
Relationships between COPD patients with blood and sputum eosinophilia, BHR and bronchial reversibility
The results of bronchial reversibility test were available in all COPD patients but only 40 of them underwent methacholine challenge, therefore the relationship between blood and sputum eosinophilia, bronchial reversibility and BHR could be assessed in 40 COPD patients ().
Figure 3. Venn diagrams showing the distribution of COPD patients with increased blood, sputum eosinophil level, bronchial hyperresponsiveness (BHR+) and positive bronchial reversibility test.
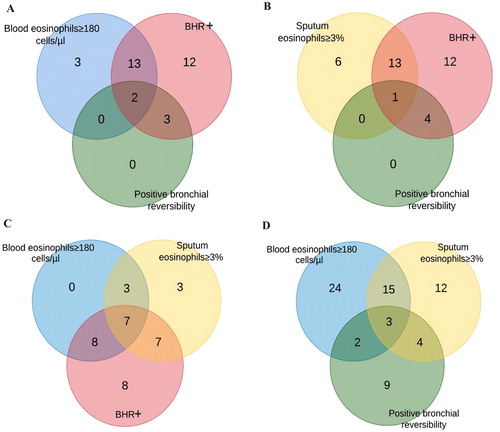
Among the patients with high blood or high sputum eosinophil count positive BHR was prevalent in 15 (83%) and in 14 (70%) patients, respectively ( and . Positive bronchial reversibility and positive BHR were observed in 2 (11%) COPD patients with higher peripheral eosinophil level and in 1 (5%) patient with higher sputum eosinophil level ( and . In a group of patients with increased blood and sputum eosinophil level, 7 patients (70%) had positive BHR () and 3 (17%) had positive bronchial reversibility test (.
Discussion
This study showed that blood eosinophil count is not a reliable marker of eosinophilic airway inflammation in mild-to-moderate COPD patients who had not used inhaled or systemic corticosteroids. We believe, that in the face of a heated debate on blood eosinophils as a potential predictor of effective ICS therapy in COPD, our results might be an interesting, but indirect, counterargument. We were not able to demonstrate a correlation between blood and sputum eosinophil count. Further, patients with and without elevated sputum eosinophil level did not differ in regard to clinical characteristics and lung function. We also did not find any characteristic clinical features related to blood eosinophilia. Importantly, the study was performed on a selected group of steroid-naive COPD patients who did not have asthma at present or in the past. Of note, the prevalence of positive skin prick tests was comparable in COPD patients and control subjects (31% versus 30%) and even lower than in general Polish population (48%) [Citation23]. Therefore, we believe that our study group represents a population of “pure” COPD patients without co-existing asthma and is not overrepresented by patients with allergies. This is critically important in the context of bias reduction associated with a possible impact of asthma or allergy coexistence on the sputum and peripheral blood eosinophil count.
The recent GOLD 2019 report suggests that blood eosinophilia could be a marker of the exacerbation risk and the effectiveness of ICS therapy [Citation24]. This is important because the differential blood cell count is a less expensive and more accessible alternative compared to the sputum induction and analysis. A close correlation between sputum and peripheral blood eosinophils has been found in several studies in asthma patients [Citation3, Citation10, Citation11]. Likewise, such an association has also been shown in some COPD studies [Citation13, Citation14, Citation16, Citation25]. However, our study that included mild-to-moderate COPD patients did not confirm the relationship between the sputum and blood eosinophil levels. It is probable that the discrepancy between the results of the study could be accounted for by the differences in the inclusion criteria in those studies: including patients with history of asthma [Citation16, Citation25], different GOLD stages or during an exacerbation [Citation7]. Noteworthy, despite the significant correlation between sputum and blood eosinophil levels, these studies also showed a high false-positive rate of detecting increased sputum eosinophilia. Negewo et al. reported false-positive rate of 40% when the cut-off value of blood eosinophils was set at 300 cells/µL [Citation25]. In the studies by Kim et al. and Hastie et al., the false-positive rate was almost 70% for the cut-off value of 2% [Citation14, Citation16]. Such results may have a great implication for clinical practice and lead to unnecessary prescribing of ICS to patients who would not benefit from such a treatment, and on the other hand, may be prone to pneumonia [Citation26].
The age-related changes in the blood differential cell count in healthy subjects should be considered when discussing the relationship between sputum and blood eosinophils [Citation27, Citation28]. In our study, we found a weak positive correlation between blood eosinophils and age of COPD patients. Valiathan et al. analysed the effect of ageing on the immune system in five different healthy groups: infants, children, adolescent, adults and the elderly [Citation28]. Although the percentage of blood eosinophils decreased continuously to adulthood, the authors noted an increase in the elderly group. This result might be explained by “inflamm-aging” – a state of low-grade chronic inflammation commonly observed in the elderly. Interestingly, Mathur et al. showed the impairment in eosinophil function in older asthma patients (55–80 years) compared to younger individuals (20–40 years) [Citation29]. Moreover, there is evidence that blood eosinophil levels change over time and some studies observed that a significant number of patients had intermittent eosinophilia, from 40.5% (≥300 cells/µL) [Citation12] to 49% of patients (≥2%) [Citation13]. The increase in peripheral blood eosinophils may thus be related to the disease as well as to age-associated inflammatory changes.
In contrast to the SPIROMICS cohort, our study did not demonstrate an association between high sputum eosinophils level and worse lung function [Citation16]. Although the study by Hastie et al. suggested that eosinophilic patients had a significantly greater postbronchodilator improvement in FEV1 (% pred.) than non-eosinophilic patients, we did not find any difference in FEV1 change in the high and low sputum eosinophil groups. However, the SPIROMICS study included a significant number of patients with GOLD stage 0 and did not exclude patients with the history of asthma and the patients using ICS.
Although BHR is a typical hallmark of asthma, it is also quite common in COPD [Citation30]. In our study, BHR was found in 75% out of 40 COPD patients who underwent the methacholine challenge, hence its prevalence lies within the wide range reported by other studies 41–94% [Citation31, Citation32]. The exact mechanisms responsible for BHR in COPD remains unknown, however, they seem to be different from that in asthma, where BHR is closely related to eosinophilic airway inflammation [Citation33]. Although we noted that among the COPD patients with BHR, 27% had also high blood eosinophil level, 23% had high sputum eosinophil level, and 23% had coexisting these two characteristics, we did not find any correlations between eosinophils and BHR. Large-scale studies showed that smoking was an important risk factor for BHR [Citation34, Citation35]. In a multivariate analysis published by Juusela et al., smoking was demonstrated to be an independent determinant for BHR, after adjustment for other covariates [Citation36]. Van Berge et al. who studied clinical and inflammatory determinants of BHR in COPD showed a strong and independent association between BHR and neutrophilic airway inflammation reflected by the number of sputum neutrophils [Citation32]. These results are consistent with those published by Dima et al. who observed a positive correlation between a sputum neutrophil percentage and airway hyperresponsiveness in COPD patients [Citation37]. However, we cannot compare results as a relationship between BHR and neutrophils was outside the scope of our study.
Eosinophilic airway inflammation is also linked to a bronchodilator response [Citation38]. In our study, 20% of all COPD patients had a positive result of the bronchial reversibility test, and of those 39% had a high sputum eosinophil level. In turn, in the study by Zanini et al. 37.8% of COPD patients had a positive bronchodilator response and almost one third of them had sputum eosinophilia (≥3%) [Citation31]. However, they excluded patients with positive skin prick tests so we could expect more individuals with bronchial reversibility in our study, especially that both study groups had similar inclusion criteria. The reason for different prevalence of eosinophilia in bronchodilator responders might be a sample size. Contrary to Zanini et al., we did not find a correlation between the change in FEV1 and eosinophil level in either blood or sputum. Our findings are consistent with those presented by Dima et al. and Papi et al. where the bronchial reversibility (or partial reversibility) in COPD did not correlate with inflammatory cells counts in sputum [Citation37, Citation38].
We realise that our study has some limitations. First, it was a retrospective analysis of patient cohorts participating in three earlier studies and some data were missing. In particular, the results of the methacholine challenge were available only for less than 50% of all patients. Second, it is recommended that systemic eosinophilia should not be evaluated by a single blood measurement because it may be not stable and influenced by many variable factors (e.g. parasites, treatment, infection). However, the aim of this study was to compare the systemic and local eosinophil inflammation in COPD which was collected at the same time point. Third, our study group included a relatively small number of patients. However, it should be emphasised, that the number of patients in this group was limited by the retrospective nature of the study and the exclusion of patients treated with ICS. Moreover, all patients had a mild or moderate disease with no frequent exacerbations. Thus, we could not reliably evaluate the association between blood eosinophils and COPD exacerbations. On the other hand, as the majority of previous studies were focussed on patients with more severe disease, our current analysis of mild-to-moderate COPD patients can be also construed as one of the study advantages. Nevertheless, we realise that including more severe stages of COPD could deliver slightly different results.
In conclusion, our study demonstrated no relationship between the peripheral eosinophil count and the sputum eosinophil percentage in steroid-naive, mild-to-moderate COPD patients. We also did not find a relationship between blood or sputum eosinophils, BHR, and bronchial reversibility. Our study showed that sputum eosinophilia is relatively common in this patient group and is not always reflected by blood eosinophilia. Our findings highlight the significance of assessment of the local airway eosinophil level.
Declaration of Interest
KG reports fees for lectures and travel expenses from Boehringer Ingelheim, Chiesi, AstraZeneca, Polpharma and Roche, outside the submitted work. RK reports fees for lectures and travel expenses from Boehringer Ingelheim, Chiesi, AstraZeneca and Polpharma, outside the submitted work. MP, KM, MP-G, PN-G, PJ, and NZ declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author’s contributions
MP study design, data analysis and interpretation, manuscript writing, critical revision; KM data acquisition, data analysis, literature review, manuscript writing; MPG study design, data analysis and interpretation, critical revision; KG study design, patients’ recruitment, creation of a database, supervision; PNG, PJ, NZ data acquisition; RK manuscript writing and critical revision.
| Abbreviations | ||
| BHR | = | bronchial hyperresponsiveness |
| BMI | = | body mass index |
| CAT | = | COPD Assessment Test |
| COPD | = | chronic obstructive pulmonary disease |
| ERS | = | European Respiratory Society |
| FEV1 | = | forced expiratory volume in 1 second |
| GOLD | = | Global Initiative for Chronic Obstructive Lung Disease |
| ICS | = | inhaled corticosteroids |
| mMRC | = | modified Medical Research Council |
| PC20 | = | methacholine concentration causing a 20% drop in FEV1 |
| RV | = | residual volume |
| TLC | = | total lung capacity |
| VC | = | vital capacity |
Additional information
Funding
References
- Hoenderdos K, Condliffe A. The neutrophil in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2013;48(5):531–539. doi:10.1165/rcmb.2012-0492TR.
- Górska K, Maskey-Warzęchowska M, Krenke R. Airway inflammation in chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2010;16(2):89–96. doi:10.1097/MCP.0b013e3283341ba0.
- Górska K, Paplińska-Goryca M, Nejman-Gryz P, et al. Eosinophilic and neutrophilic airway inflammation in the phenotyping of mild-to-moderate asthma and chronic obstructive pulmonary disease. COPD. 2017;14(2):181–189. doi:10.1080/15412555.2016.1260539.
- Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(1):39–47.
- Leigh R, Pizzichini MMM, Morris MM, et al. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. 2006;27(5):964–971.
- Brightling CE, McKenna S, Hargadon B, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60(3):193–198. doi:10.1136/thx.2004.032516.
- Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi:10.1164/rccm.201104-0597OC.
- Baines KJ, Pavord ID, Gibson PG. The role of biomarkers in the management of airways disease. Int J Tuberc Lung Dis. 2014;18(11):1264–1268. doi:10.5588/ijtld.14.0226.
- Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70(2):115–120. doi:10.1136/thoraxjnl-2014-205634.
- Fowler SJ, Tavernier G, Niven R. High blood eosinophil counts predict sputum eosinophilia in patients with severe asthma. J Allergy Clin Immunol. 2015;135(3):822–824.e2. doi:10.1016/j.jaci.2014.09.034.
- Westerhof GA, Korevaar DA, Amelink M, et al. Biomarkers to identify sputum eosinophilia in different adult asthma phenotypes. Eur Respir J. 2015;46(3):688–696. doi:10.1183/09031936.00012415.
- Casanova C, Celli BR, de-Torres JP, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J. 2017;50(5):1701162. doi:10.1183/13993003.01162-2017.
- Singh D, Kolsum U, Brightling CE, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–1700. doi:10.1183/09031936.00162414.
- Kim VL, Coombs NA, Staples KJ, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J. 2017;50.
- Weiszhar Z, Horvath I. Induced sputum analysis: step by step. Breathe. 2013;9(4):300–306. doi:10.1183/20734735.042912.
- Hastie AT, Martinez FJ, Curtis JL, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967.
- Gorska K, Nejman-Gryz P, Paplinska-Goryca M, et al. Comparative study of IL-33 and IL-6 levels in different respiratory samples in mild-to-moderate asthma and COPD. COPD. 2018;15(1):36–45. doi:10.1080/15412555.2017.1416074.
- Gorska K, Krenke R, Domagala-Kulawik J, et al. Comparison of cellular and biochemical markers of airway inflammation in patients with mild-to-moderate asthma and chronic obstructive pulmonary disease: an induced sputum and bronchoalveolar lavage fluid study. J Physiol Pharmacol Off J Pol Physiol Soc. 2008;59 Suppl 6:271–283.
- Diagnosis of Diseases of Chronic Airflow limitation: Asthma, COPD and Asthma-COPD Overlap Syndrome (ACOS) [Internet]. Glob. Initiat. Asthma – GINA; 2015 [cited 2019 Apr 26]. Available from: https://ginasthma.org/asthma-copd-and-asthma-copd-overlap-syndrome-acos/.
- Vignola AM, Rennar SI, Hargreave FE, et al. Standardised methodology of sputum induction and processing. Future directions. Eur Respir J Suppl. 2002;37:51s–55s.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805.
- Guidelines for Methacholine and Exercise Challenge Testing—1999. Am J Respir Crit Care Med. 2000;161:309–329.
- Samoliński B, Raciborski F, Lipiec A, et al. Epidemiologia Chorób Alergicznych w Polsce (ECAP). Alergol Pol – Pol J Allergol. 2014;1(1):10–18. doi:10.1016/j.alergo.2014.03.008.
- Gold Reports 2018 [Internet]. Glob. Initiat. Chronic Obstr. Lung Dis. – GOLD. [cited 2019 Jan 25]. Available from: https://goldcopd.org/gold-reports/.
- Negewo NA, McDonald VM, Baines KJ, et al. Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. COPD. 2016;11:1495–1504. doi:10.2147/COPD.S100338.
- Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–223. doi:10.1016/S2213-2600(13)70040-7.
- Fagiolo U, Cossarizza A, Scala E, et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23(9):2375–2378. doi:10.1002/eji.1830230950.
- Valiathan R, Ashman M, Asthana D. Effects of ageing on the immune system: infants to elderly. Scand J Immunol. 2016;83(4):255–266. doi:10.1111/sji.12413.
- Mathur SK, Schwantes EA, Jarjour NN, et al. Age-related changes in eosinophil function in human subjects. Chest. 2008;133(2):412–419. doi:10.1378/chest.07-2114.
- DiSantostefano RL, Hinds D, Le HV, et al. Relationship between blood eosinophils and clinical characteristics in a cross-sectional study of a US population-based COPD cohort. Respir Med. 2016;112:88–96. doi:10.1016/j.rmed.2016.01.013.
- Zanini A, Cherubino F, Zampogna E, et al. Bronchial hyperresponsiveness, airway inflammation, and reversibility in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1155–1161.
- van den Berge M, Vonk JM, Gosman M, et al. Clinical and inflammatory determinants of bronchial hyperresponsiveness in COPD. Eur Respir J. 2012;40(5):1098–1105. doi:10.1183/09031936.00169711.
- Wardlaw AJ, Dunnette S, Gleich GJ, et al. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis. 1988;137(1):62–69. doi:10.1164/ajrccm/137.1.62.
- Janson C, Chinn S, Jarvis D, et al. Effect of passive smoking on respiratory symptoms, bronchial responsiveness, lung function, and total serum IgE in the European Community Respiratory Health Survey: a cross-sectional study. Lancet Lond Engl. 2001;358(9299):2103–2109. doi:10.1016/S0140-6736(01)07214-2.
- Schwartz J, Schindler C, Zemp E, et al. Predictors of methacholine responsiveness in a general population. Chest. 2002;122(3):812–820. doi:10.1378/chest.122.3.812.
- Juusela M, Pallasaho P, Rönmark E, et al. Dose-dependent association of smoking and bronchial hyperresponsiveness. Eur Respir J. 2013;42(6):1503–1512. doi:10.1183/09031936.00073712.
- Dima E, Rovina N, Gerassimou C, et al. Pulmonary function tests, sputum induction, and bronchial provocation tests: diagnostic tools in the challenge of distinguishing asthma and COPD phenotypes in clinical practice. Int J Chron Obstruct Pulmon Dis. 2010;5:287–296.
- Papi A, Romagnoli M, Baraldo S, et al. Partial reversibility of airflow limitation and increased exhaled NO and sputum eosinophilia in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(5):1773–1777. doi:10.1164/ajrccm.162.5.9910112.