Abstract
The value of fractional exhaled nitric oxide (FeNO) in patients with chronic obstructive pulmonary disease (COPD) remains unclear. We aimed to assess whether FeNO is a more valuable biomarker than blood eosinophil count for identifying clinical characteristics of COPD. Stable COPD patients (n = 390) were included and stratified by FeNO and blood eosinophil counts at recruitment. The demographic characteristics, lung functions, St George Respiratory Questionnaire (SGRQ), serum inhaled allergen-specific IgE and the exacerbations in the preceding 12 months were compared. Risk factors for moderate or severe exacerbation in the preceding 12 months were examined by binary regression analysis. The cross-sectional study showed that 167 patients had high level of FeNO (≥25 ppb) and 223 in low level (<25 ppb), while 138 patients had high blood eosinophil count (≥200 cells/μL) and 252 had low (<200 cells/μL). Compared with the high FeNO group, there were higher proportion of patients with GOLD III–IV, higher SGRQ scores, more exacerbations in the preceding 12 months, and with lower positive proportion of sIgE in the low FeNO group (p < 0.05 for all). However, these phenomena above were not associated with blood eosinophil count. Finally, high FeNO level was associated with a lower moderate or severe exacerbation in preceding 12 months (RR: 0.541 [95%CI 0.319–0.917], p = 0.023). In stable COPD patients, FeNO, but not blood eosinophil count was associated with the COPD severity and allergic airway inflammation. However, the role of FeNO in guiding personalized treatment of COPD patients need to be further investigated.
Introduction
Chronic obstructive pulmonary disease (COPD), a chronic airway inflammatory disorder, has become a major health-problem worldwide [Citation1,Citation2]. One of the difficulties in treating and managing COPD is the heterogeneity of this complex disease in terms of severity, progression, exercise tolerance and nature of symptoms [Citation3]. Although most studies have shown that increases in the numbers of neutrophils and macrophages, and in expression of type 1 help T cell (Th1) cytokines are the major feature of airway inflammation in COPD [Citation4,Citation5], some studies have revealed that a subgroup of patients with COPD favored Th2-type inflammation, including elevated sputum and blood eosinophils count, suggesting that Th2 inflammation might also contribute to COPD progression [Citation6,Citation7], at least, in some proportions of subjects with COPD.
Blood eosinophil counts can predict sputum eosinophilia in stable COPD, which have been used as a surrogate maker for airway eosinophils in COPD patients [Citation8]. Some studies have shown that increased eosinophils might be associated with respiratory exacerbation and hyperinflation [Citation9–11], but other report indicates that the increased number of blood eosinophils is not a risk factor for COPD exacerbations [Citation12], suggesting that the blood eosinophil count might not be a reliable biomarker for identifying COPD phenotypes. Although GOLD 2019 has recommended that blood eosinophil counts could be used as a biomarker in conjunction with clinical assessment when making decisions regarding inhaled corticosteroid (ICS) use [Citation13], there are still debated in clinical practice [Citation14].
Fractional exhaled nitric oxide (FeNO) is a well-recognized biomarker of Th2 airway inflammation in asthma [Citation15]. However, the significance of FeNO in COPD is less known. In a previous study, Alcázar-Navarrete and colleagues [Citation16] reported that FeNO could distinguish different phenotypes of COPD. However, the relationship between FeNO and COPD phenotypes is still controversial [Citation17–19]. Furthermore, we investigate whether FeNO, as a biomarker is better than that of blood eosinophils for evaluating clinical characteristics of COPD.
We hypothesized that FeNO is reliable and better biomarker than blood eosinophils in identification of clinical characteristics of COPD. In addition, we verify whether the blood eosinophil count is reliably able to predict FeNO level.
Material and methods
Subjects
Patients involved in the present study were selected from the cohort study for COPD in China—Identification of prognostic biomarkers for the disease (COMFORT study), which is an ongoing prospective observational study at multiple centers in China. The details of study design refer to http://chinacopd.com/#/hot (ClinicalTrials.gov ID: NCT03044847). Data involved in the present study were obtained from April 2017 to November 2018. For inclusion, all individuals with diagnosed COPD conformed to the following criteria: aged 40–75 years with current or former history of tobacco use (≥10 pack-years); and post-bronchodilator forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) ratio of less than 0.7 according to the diagnostic criteria of GOLD [Citation13]. Furthermore, we have now performed a sensitivity analysis by using lower limits of normal (LLN) with Chinese reference values to define airflow limitation [Citation20], and all subjects recruited in this study had FEV1/FVC less than LLN. Individuals with any of the following were excluded: acute exacerbation COPD during the 3 months prior; ever physician-diagnosed asthma; other respiratory diseases with lung tissue destruction (such as severe tuberculosis or bronchiectasis) and uncontrolled systemic diseases. 483 patients recruited in COMFORT study, 14 patients were excluded by unavailable blood counts or FeNO testing, 7 patients for FEV1/FVC ≥0.7, 5 patients with history of tobacco use <10 pack-years, and 67 patients excluded with physician-diagnosed asthma, so 390 patients date available for final analysis ().
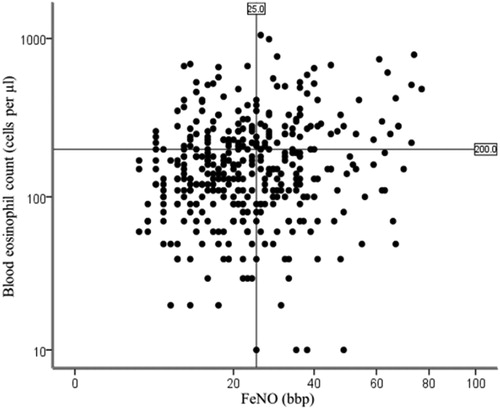
Figure 1. Distribution of FeNO with respect to blood eosinophils.
Notes: The coordinate axes are expressed as logarithms of the corresponding data. Although a significant association between FeNO and blood eosinophils was seen (Pearson correlation coefficient r = 0.192, P < 0.001), use of the cutoff of 200 cells/μL or more blood eosinophils (horizontal line) to predict FeNO of at least 25 ppb (vertical line) will mistakenly identify many patients with lower FeNO (upper left quadrant) and miss many patients with actual FeNO of 25 ppb or more (lower right quadrant).
The present study was approved by the Ethics Committee of Beijing Chao-Yang Hospital (2016-KE-183) and conducted in accordance with the Declaration of Helsinki. All subjects provided written informed consent.
Clinical and physiologic measurements
The relevant data were collected by trained staff, including demographic information of all participants at recruitment, and recorded St George Respiratory Questionnaire (SGRQ), COPD assessment test (CAT), medications usage (at least use regularly for nearly 3 months) and moderate or severe exacerbation defined as exacerbation required treated with antibiotics and/or oral corticosteroids, or required hospitalization or visited emergency room in the preceding 12 months.
Pulmonary function test (PFT) was performed using a MasterScreen spirometer (VIASYS Healthcare GmbN, Germany) before and after inhaled salbutamol 400 µg via MDI following the guideline [Citation21].
The FeNO was measured at a constant flow (50 mL·s−1) in accordance with ATS/ERS recommendation [Citation22] using a nitric oxide analyzer (NIOXMINO, Aerocrine, Sweden) before spirometry, and required stopping smoking at least 24 h prior to measure for current smokers.
Meanwhile, peripheral blood eosinophils were counted, and the serum total IgE (tIgE), and inhaled allergen specific IgE (sIgE, Phadiatop) were assessed using UniCAP 250 (ThermoFisher Scientific).
Statistical analysis
Analyses were performed using IBM SPSS Statistics 19.0. Results are expressed as mean ± standard deviation or median (interquartile range) or n (%). Continuous variables were analyzed by parametric (Student’s t-test) or non-parametric tests (Mann-Whitney U test), while categorical variables with the chi-squared or Fisher’s exact tests. Kruskal-Wallis was applied between multiple groups of data. All tests of statistical significance were two-sided, and P values of less than 0.05 were considered statistically significant. Pearson correlation analysis was used to examine the correlation between FeNO and blood eosinophil counts. Receiver operating characteristic (ROC) analysis was used for blood eosinophil prediction of FeNO. A regression models with adjustment for potential confounders was applied to examine the influence of groups stratified by FeNO on moderate or severe exacerbation for COPD in the preceding 12 months.
Results
All patients initially recruited to this study had the available FeNO and blood eosinophil counts (n = 390). The mean FeNO value was 25.3 ppb (SD: 14.4; median: 22 ppb [IQR 15–33]; range: 5–77 ppb), the mean blood eosinophil count was 189 cells/μL (SD: 153; median: 150 cells/μL [IQR 90–240]; range: 0–1050 cells/μL). In this case, 25 ppb for FeNO and 200 cells/μL for blood eosinophil counts were used as the cutoff for further analysis. In addition, a higher FeNO cutoff of 30 ppb or a higher cutoff of 300 cells/μL of blood eosinophils was also examined.
The demographic characteristics of patients in different group were summarized in . The proportion of current smokers was higher in the low FeNO group (100 [44.8%] vs 43 [25.7%], p < 0.001), while the concentrations of tIgE (p = 0.019) and sIgE (p = 0.004) were significantly higher in the high FeNO group. In addition, the high FeNO group had higher positive proportion of sIgE (p = 0.006). In contrast, unlike the FeNO groups, there were no significant differences in positive proportion of the serum sIgE between the low and high blood eosinophil groups (p = 0.333). In addition, there was no difference in the use of ICS, LABA and LAMA (at least use regularly for nearly 3 months) between FeNO groups and eosinophil groups.
Table 1. Demographics for patients stratified by the mean FeNO or blood eosinophil counts at baseline.
Similar results derived from the FeNO and blood eosinophil stratification were also observed in the patients with the higher cutoffs of FeNO (≥30 ppb) or of blood eosinophils (≥300 cells/μL) ().
Compared with the high FeNO group, there was higher proportion of patients with GOLD III–IV in the low FeNO group (p = 0.063 for 25 ppb as the cutoff, p = 0.035 for 30 ppb as the cutoff, respectively) ( and ECitation2 (Supplementary material)). For example, 42.8% of patients were GOLD III–IV in the FeNO <30 ppb group and 32.0% in the FeNO ≥30 ppb group (). However, there was no significant difference in the GOLD stages between the high and low eosinophil groups, regardless whether the eosinophils were divided by 200 cells/μL (p = 0.560) or 300 cells/μL (p = 0.739).
Table 2. Clinical characteristics for patients stratified by the mean FeNO or blood eosinophil counts.
The SGRQ symptoms, impact, activity and total score were significantly higher in the FeNO < 25 ppb group than those in the FeNO ≥ 25 ppb group (38.3 vs 31.8, p = 0.025 for SGRQ symptoms, 24.2 vs 19.3, p = 0.050 for SGRQ impact, 41.4 vs 35.5, p = 0.042 for SGRQ activity, 31.0 vs 27.0, p = 0.014 for SGRQ total), but which were not observed between the high and low blood eosinophil groups (p = 0.991, p = 0.424, p = 0.637, p = 0.379, respectively).
Moderate and severe exacerbations in the preceding 12 months were higher in the FeNO < 25 ppb group than that in the FeNO ≥ 25 ppb group (65 [29.1%] of 223 vs 31 [18.6%] of 167, p = 0.016) (), which were, however, not associated with blood eosinophil counts (p = 0.138). Similar results were also observed in the groups with the higher cutoff of 30 ppb FeNO or 300 cells/μL of blood eosinophil counts () (p = 0.018, p = 0.416, respectively).
Although a significant association between FeNO and blood eosinophils was seen (r = 0.192, p < 0.001, ), ROC analysis for blood eosinophil prediction of FeNO showed a slightly significant prediction of FeNO of 25 ppb or more (AUC 0.565, [95% CI 0.506–0.623], p = 0.028; ); while there were no significances in ROC to predict FeNO of 30 ppb or more (AUC 0.560, [95% CI 0.495–0.625], p = 0.060; ). Comparison of the four concordant and discordant groups for different cutoffs of lung function ( and ECitation3 (Supplementary material)) revealed that there was no significant difference in lung function and airway reversibility between groups, even between the groups of patients with high FeNO and high blood eosinophil counts and patients with low FeNO and low blood eosinophil counts. The proportion of patients with moderate or severe exacerbation in the preceding 12 months among the four groups were significant different (p = 0.030 for 25 ppb as the cutoff, p = 0.041 for 30 ppb as the cutoff, respectively). Patients in the low FeNO group showed higher proportion of moderate or severe exacerbations in the preceding year, regardless of blood eosinophil levels.
Figure 2. Receiver operating characteristic analysis for blood eosinophil prediction of FeNO.
Notes: A: Correct prediction of FeNO less than 25 ppb or more. AUC = 0.565 (95% CI 0.506–0.623, P = 0.028); B: Correct prediction of FeNO less than 30 ppb or more AUC = 0.560 (95% CI 0.495–0.625, P = 0.060).
Abbreviations: AUC, area under the curve.
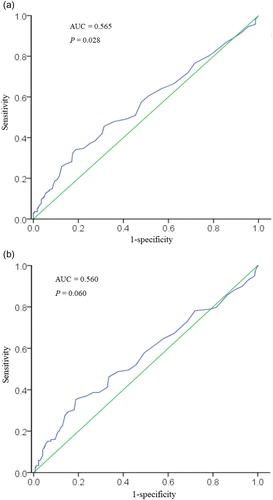
Table 3. Lung function and exacerbations for patients in groups stratified by FeNO and blood eosinophil counts.
In a regression model to examine the influence of groups stratified by FeNO on moderate or severe exacerbation for COPD in preceding 12 months, FeNO level, ICS use, COPD stage were the variables with significance in the model, after adjusting for demographics, smoking status, smoking pack-year (). High FeNO level was associated with a lower moderate or severe exacerbation in the preceding 12 months (RR 0.541, [95%CI 0.319–0.917], p = 0.023).
Table 4. Adjusted relative impact of groups stratified by FeNO on moderate or severe exacerbation in preceding 12 months.
Discussion
In the present study, we stratified a large cohort of COPD patients according to their FeNO values. The data revealed that the low FeNO was significantly associated with the poor lung function, quality of life and moderate or severe exacerbations in the preceding 12 months. In contrast, blood eosinophil counts did not associate with exacerbations of COPD, and did not, generally, associate with other phenotypic markers either. These findings suggest that compared to the blood eosinophil counts, the value of FeNO is more reliable for identifying subgroups with poor quality of life or more exacerbations in COPD patients.
Although all patients enrolled in the present study met GOLD criteria [Citation13] for COPD, who had a mean age of 65 years, had fixed airflow limitation, had a history of heavy smoking (approximately 40 pack-years), and ever physician-diagnosed asthma was excluded, we observed some of patients recruited in the present study had some asthma-like traits, such as high blood eosinophils, high FeNO level, high IgE. These phenomena were observed in other previous study. For example, Colak Y and colleagues showed that there were 16.8% in COPD patients with blood eosinophils ≥ 300 cells/μL [Citation23], which was similar with our present report of 15.1% (See supplementary). Other several reports from COPD clinical trials also showed there was higher proportion of COPD with blood eosinophils ≥ 300 cells/μL from 20 to 27% [Citation6,Citation24].
In the present study, we used the 25 ppb as the cutoff of FeNO based on the mean level of FeNO in the recruited patients with COPD, which was a little higher than the cutoff of 20 ppb used in the study conducted by Alcázar-Navarrete [Citation19]. Based on this 25 ppb as cutoff of FeNO, our data showed that there were more patients with GOLD stage III–IV in low FeNO groups than that of the high group. In the meanwhile, poor quality of life assessed by SGRQ score was also observed in the low FeNO group, but not in any other groups categorized by blood eosinophil count. The results suggest that the FeNO and blood eosinophil counts might not be substituted each other in COPD patients, as shown in asthmatics [Citation25].
It is noted that patients with high FeNO had significantly increased positive proportion of sIgE. Correspondingly, Donohue and colleagues [Citation26] also found that the FeNO of asthma-COPD overlap (ACO) patients was significantly higher than that of COPD patients with or without emphysema. Since the increased level of sIgE is a feature of atopic subjects [Citation27], our data suggest that FeNO is a better indicator of allergic airway inflammation in COPD patients than blood eosinophil count. These observations also imply that FeNO might be a better biomarker for identifying COPD patients who might benefit from inhaled corticosteroid therapy.
Exacerbations of COPD are important events in the management of COPD, and frequent exacerbation has been identified as a phenotype of COPD [Citation28]. Many studies have been tried to find biomarkers that could predict future exacerbations of COPD. For example, an analysis of SPIROMICS cohort shows that eosinophil count in induced sputum, but not in blood could be used as a biomarker to identify the subgroup with more exacerbation of COPD [Citation6]. The ECLIPSE study also reveals that the numbers of exacerbations seemed to be similar in the previous year of patients with COPD stratified by blood eosinophil counts [Citation29]. In fact, our data show that patients of the low FeNO group, but not of the low blood eosinophil group, have not only the higher proportions of current smokers, with more severe COPD (GOLD III–IV), and poorer quality of life assessed by SGRQ scores, but also have the more exacerbations compared with the high FeNO group in the preceding 12 months.
It seems to be inconsistent with a report of Alcázar-Navarrete et al. [Citation19], who observed that stable COPD patients with persistent high FeNO levels (≥20 ppb) had higher risk of acute exacerbation in a prospective 1-year observational study. There are several possible explanations for this inconsistence. First, our present study was a cross-sectional study, and the frequency of acute exacerbation in the previous 12 months was self-reported in different groups of FeNO levels with the 25 ppb or 30 ppb at baseline as cutoff value. However, the study of Alcázar-Navarrete et al. was a prospective study, and category using FeNO ≥ 20 ppb at least twice persistent in 3 visits at recruitment, 6 months and 12 months. Second, the diversity in baseline treatment make also it is difficult to simply compare our results with other. For example, the approximate 66% of patients who received inhaled therapy with ICS or long acting bronchodilators in the present study was much lower than those with above 90% of patients in the study of Alcázar-Navarrete et al.
ICS was recommended for patients with severe COPD by GOLD 2019 [Citation13], and it has been shown to cause the decline of FeNO level [Citation30]. So we adjusted the potential confounders by a regression model with such as demographics, smoking status, smoking pack-year, ICS use, and COPD stage, the results still showed FeNO level was associated with the severity of COPD, irrespective of previous ICS use and smoking status.
Because the present study is a cross-sectional study, patients recruited in this study were from a real world, and we only recorded the moderate or severe exacerbation defined as exacerbation required treated with antibiotics and/or oral corticosteroids, or required hospitalization or visited emergency room in the preceding 12 months. The mild exacerbation is not recorded in this present study considered the inaccurate memory in a retrospective study. For mild acute exacerbation of COPD, patients often inhaled ICS/LABA, especially inhaled the formoterol/budesonide on-need, which is easy available in China to relieve symptoms by themselves, whereas patients with lower FeNO were less effective to inhale ICS/LABA. In addition, current smoking could reduce the FeNO level, which can lead to more serious COPD status. All above might explained, at least partly, that low FeNO were associated with more severe COPD, including more exacerbations in the preceding 12 months.
There are several limitations need to be noted. First, this is a single center study with relative small sample size, we used 25 ppb for FeNO or 200 cells/μL of blood eosinophils as cutoff based on the mean levels of FeNO or blood eosinophils in the present study is a little arbitrary. Second, the present there are more male than female patients involved in this study, thus, we cannot rule out whether sex of subjects affects the data. Third, considering the reliability and traceability of the data, we only recorded moderate and severe exacerbations in the present study. Finally, it was the single measurement of FeNO and blood eosinophils in the cross-sectional study, whereas the previous studies showed that levels of FeNO and blood eosinophils were variable in longitudinal observational studies [Citation12,Citation19].
Conclusion
In stable COPD patients, stratification by FeNO might be better for identifying a subgroup of patients with more severe disease, more exacerbations in the preceding 12 months, which may also be helpful to identify those of COPD patients with respiratory allergic disease. However, whether or not FeNO is superior to peripheral blood eosinophils in guiding the personalized treatment of COPD should be further investigated in prospective studies.
| Abbreviations | ||
| ACO | = | asthma-COPD overlap |
| AUC | = | area under the curve |
| BWT | = | bronchial wall thickening |
| CAT | = | COPD assessment test |
| COPD | = | chronic obstructive pulmonary disease |
| FeNO | = | fractional exhaled nitric oxide |
| FEV1 | = | forced expiratory volume in 1 second |
| FVC | = | forced vital capacity |
| GOLD | = | global initiative for chronic obstructive lung disease |
| ICS | = | inhaled corticosteroid |
| LABA | = | long-acting beta-agonist |
| LAMA | = | long-acting muscarinic antagonist |
| LLN | = | lower limits of normal |
| PFT | = | pulmonary function test |
| ROC | = | receiver operating characteristic |
| RR | = | relative risk |
| SGRQ | = | St George respiratory questionnaire |
| sIgE | = | specific immunoglobulin E |
| tIgE | = | total immunoglobulin E |
Disclosure statement
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Collaborators GCRD. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017;5(9):691–706.
- Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9.
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. 2013-02-15. doi:10.1164/rccm.201204-0596PP.
- Singh D, Edwards L, Tal-Singer R, et al. Sputum neutrophils as a biomarker in COPD: findings from the ECLIPSE study. Respir Res. 2010;11(1):77. 2010-06-15. doi:10.1186/1465-9921-11-77.
- Sethi S, Mahler DA, Marcus P, et al. Inflammation in COPD: implications for management. Am J Med. 2012;125(12):1162–1170. doi:10.1016/j.amjmed.2012.06.024.
- Hastie AT, Martinez FJ, Curtis JL, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967. doi:10.1016/S2213-2600(17)30432-0.
- Tworek D, Majewski S, Szewczyk K, et al. The association between airway eosinophilic inflammation and IL-33 in stable non-atopic COPD. Respir Res. 2018;19(1):108. doi:10.1186/s12931-018-0807-y.
- Negewo NA, McDonald VM, Baines KJ, et al. Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. COPD. 2016;11:1495–1504. 2016-01-20. doi:10.2147/COPD.S100338.
- Couillard S, Larivée P, Courteau J, et al. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. 2017;151(2):366–373. doi:10.1016/j.chest.2016.10.003.
- Hardin M, Silverman EK, Barr RG, et al. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12(1):1–8. doi:10.1186/1465-9921-12-127.
- Zeiger RS, Tran TN, Butler RK, et al. Relationship of blood eosinophil count to exacerbations in chronic obstructive pulmonary disease. J Allergy Clin Immunol Pract. 2018;6(3):944–954. 2018-05-01. doi:10.1016/j.jaip.2017.10.004.
- Casanova C, Celli BR, De-Torres JP, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J. 2017;50(5):1701162. doi:10.1183/13993003.01162-2017.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for prevention, diagnosis and management of chronic obstructive pulmonary disease, 2019.
- Yun JH, Lamb A, Chase R, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141(6):2037–2047.
- Crespo A, Giner J, Torrejón M, et al. Clinical and inflammatory features of asthma with dissociation between fractional exhaled nitric oxide and eosinophils in induced sputum. J Asthma. 2016;53(5):459–464. doi:10.3109/02770903.2015.1116086.
- Alcázarnavarrete B, Romeropalacios PJ, Ruizsancho A, et al. Diagnostic performance of the measurement of nitric oxide in exhaled air in the diagnosis of COPD phenotypes. Nitric Oxide. 2016;54:67–72.
- Arif AA, Mitchell C. Use of exhaled nitric oxide as a biomarker in diagnosis and management of chronic obstructive pulmonary disease. J Prim Care Community Health. 2016;7(2):102–106. doi:10.1177/2150131915624922.
- Bazeghi N, Gerds TA, Budtz-Jorgensen E, et al. Exhaled nitric oxide measure using multiple flows in clinically relevant subgroups of COPD. Respir Med. 2011;105(9):1338–1344. 2011-09-01. doi:10.1016/j.rmed.2011.03.015.
- Alcazar-Navarrete B, Ruiz RO, Conde BP, et al. Persistently elevated exhaled nitric oxide fraction is associated with increased risk of exacerbation in COPD. Eur Respir J. 2018;51(1):1701457.
- Jian W, Gao Y, Hao C, et al. Reference values for spirometry in Chinese aged 4–80 years. J Thorac Dis. 2017;9(11):4538–4549. doi:10.21037/jtd.2017.10.110.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005; 2005-08-0126(2):319–338. doi:10.1183/09031936.05.00034805.
- American Thoracic Society ERS. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med. 2005;171(8):912–930.
- Colak Y, Afzal S, Nordestgaard BG, et al. Combined value of exhaled nitric oxide and blood eosinophils in chronic airway disease: the Copenhagen General Population Study. Eur Respir J. 2018;52(2):1800616. doi:10.1183/13993003.00616-2018.
- Watz H, Tetzlaff K, Wouters EF, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390–398. doi:10.1016/S2213-2600(16)00100-4.
- Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009; 360(10):973–984. doi:10.1056/NEJMoa0808991.
- Donohue JF, Herje N, Crater G, et al. Characterization of airway inflammation in patients with COPD using fractional exhaled nitric oxide levels: a pilot study. Int J Chron Obstr Pulm Dis. 2014;9:745–751.
- Chang M, Shao B, Liu Y, et al. Analysis of allergens in 5 473 patients with allergic diseases in Harbin, China. Biomed Environ Sci. 2013;26(11):886–893. doi:10.3967/bes2013.017.
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883.
- Singh D, Kolsum U, Brightling CE, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–1700. doi:10.1183/09031936.00162414.
- Scott M, Raza A, Karmaus W, et al. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax. 2010;65(3):258–262. doi:10.1136/thx.2009.125443.
