Abstract
The purposes of this study were to: (1) study the prevalence of pain in patients with mild-to-very severe chronic obstructive pulmonary disease (COPD) in China; (2) compare the differences in pain characteristics between stable COPD and acute exacerbation of COPD (AECOPD); (3) explore the clinical associations with pain in those with COPD. This cross-sectional study was conducted in China from October 24, 2017, to January 11, 2019. A face-to-face interview was conducted to collect data. The Chinese version of the brief pain inventory (BPI-C) was applied to investigate the pain characteristics in patients with COPD. Of the 901 patients in this study, 226 (25.1%) patients reported pain problems. The prevalence of pain in patients with mild to very severe COPD was 32.9%, 23.9%, 25.2%, and 23.5%, respectively (p = 0.447). According to the BPI-C results, 31.3% (31/99) of patients reported pain of AECOPD, compared to 24.3% (195/802) of stable COPD (p = 0.13). Reported pain intensity and pain interference evaluated by the BPI-C were significantly higher in AECOPD than stable COPD (p < 0.001, p < 0.05, respectively). Those with body mass index (BMI) ≥ 24kg/m2 or COPD assessment test (CAT) score > 20 were significantly more likely to have pain problems than BMI < 24kg/m2 (aOR = 1.568, a95IC = 1.132–2.170, p = 0.007) or CAT ≤ 20 (aOR= 1.754, a95IC = 1.213–2.536, p = 0.003). Pain was common in patients with both stable COPD and AECOPD. AECOPD patients had a significantly higher pain intensity than stable COPD. Overweight and CAT > 20 were significantly related to higher prevalence of pain.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive disease characterized by persistent respiratory symptoms and airway obstruction caused by toxic particles or gases [Citation1]. The newest epidemiological data shows that about 174.5 million (2.4%) people suffer from COPD around the world [Citation2], and the prevalence among Chinese patients aged 40 years or older was 13.7% [Citation3]. COPD is also the third-leading cause of death worldwide [Citation4]. With the progress of the disease, the burden of symptoms increases and the quality of life declines. The treatments for COPD focus on symptoms relief. Symptom burden is an important determinant of disease-specific health status, so it is of great significance to realize and further optimize symptom management in COPD patients.
Patients with COPD report multiple symptoms, not limited to common symptoms such as dyspnea, cough, and wheezing but also including other symptoms such as fatigue, insomnia, depression, and pain [Citation5, Citation6]. Compared with healthy control subjects, pain prevalence was greater in COPD [Citation7]. Recent literature have reported that the prevalence of pain ranges from 21% to 96% in stable COPD patients, with severity scores in the moderate to very severe range [Citation5–13]. A systematic review showed the pooled prevalence of pain was 66%. The heterogeneity of I2 of 93% and Q = 73 suggests strong variability in the prevalence of pain between studies, depending on the different setting, sample and measurements of pain used [Citation7]. There was only one study of pain in China which found more than one third (37%) had pain problems in patients with mild and moderate stable COPD in the community settings of Shanghai [Citation10]. COPD phenotypes in Asia may be somewhat different from those in Western countries [Citation14]. In Asian cities, the characteristics of COPD patients vary and the history of exposure to biomass fuels or dusty jobs was related to frequency of symptoms and severe airflow limitation [Citation15]. Current findings suggest that pain prevalence was related with symptoms (such as dyspnea, fatigue, anxiety, depression and insomnia), COPD assessment test (CAT) score, Clinical COPD questionnaire (CCQ) score, body mass index (BMI), comorbidity, nutritional status and HRQOL, and pain intensity was associated with breathlessness, BMI, specific comorbidities [Citation7, Citation10, Citation12, Citation16–18]. Acute exacerbations represent a major burden for COPD patients, which often leading to rehospitalizations, further decline in health status, and high mortality [Citation16]. Recently, only two studies have shown that the prevalence of pain in patients with acute exacerbation of COPD (AECOPD) is 39.6% and 92%, respectively [Citation16, Citation17]. The prevalence of pain in patients with AECOPD is quite heterogeneous due to the differences in the selected study population, such as inpatient or outpatient. Less is known about the characteristics and influencing factors of pain in Chinese COPD patients at all stages(mild-to-very severe) including the stable and unstable COPD. Therefore, the purposes of this study were to: (1) study the prevalence of pain in patients with mild-to-very severe COPD in China; (2) compare the differences in pain characteristics between stable COPD and AECOPD; (3) explore the clinical associations with pain in those with COPD.
Methods
Study participants and procedures
A cross-sectional study with both inpatient and outpatient subjects was carried out in the Department of Respiratory and Critical Care Medicine, at the Second Xiangya Hospital, Central South University, Changsha, Hunan, China, from October 24, 2017, to January 11, 2019. A researcher explained the purpose of the study at enrollment, and written informed consent was obtained from all study participants. The inclusion criteria for participants in this study were that they: (1) met the diagnosis criterion of COPD defined by the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations (spirometry with a ratio of the forced expiratory volume in 1 s to the forced vital capacity (FEV1/FVC) lower than 0.70 after bronchodilator administration) [Citation1]; (2) were over 40 years of age; and (3) had no mental disorders that limited their ability to provide informed consent, complete the questionnaires, and finish spirometry. Exclusion criteria were: (1) patients with other chronic respiratory diseases, such as bronchiectasis (based on high-resolution computed tomography), asthma (clinically diagnosed and reversibility > 12%), Interstitial lung disease, pulmonary hypertension, sarcoidosis and concurrent malignancy (including lung cancer), (2) patients with other serious or unstable conditions, such as acute cardiovascular diseases, acute cerebrovascular diseases, acute soft tissue injury or musculoskeletal injury in the last four weeks [Citation11]; (3) patients unable to complete the questionnaires.
We confirmed that the study was conducted in accordance with the Declaration of Helsinki. The research protocol was approved by the local Ethics Committee of the Second Xiangya Hospital of Central South University. And our study was registered in the Chinese Clinical Trial Registry (ChiCTR-POC-17010431).
Instruments
A face-to-face interview was conducted to collect data on personal characteristics and health conditions. Patients completed all of the self-report questionnaires by paper.
Outcomes and measurements
Outpatient patients included stable COPD and moderate AECOPD were recruited at the office on the day of admission and inpatient patients included severe AECOPD were recruited at the bedside on the second day of admission. At enrollment, patients provided information on age, sex, height, weight, and education. We collected data on the diagnosis: stable COPD or AECOPD, smoking status, and exacerbation in the past 12 months. AECOPD is an acute worsening of respiratory symptoms that results in additional therapy in patients with COPD [Citation1]. Pack-year smoking was calculated as the average number of cigarettes smoked per day divided by 20 and multiplied by the total number of years smoking [Citation19]. Education level was regrouped into four categories: did not complete primary middle school (nine years, free and compulsory education warranted by the Chinese government), completed primary middle school, completed high school, and completed college or above. According to the recommendations of the Working Group on Obesity in China, the BMI cutoff points for Chinese were 24 kg/m2 for overweight [Citation20]. Dyspnea was measured by using the Modified Medical Research Council Dyspnea Scale (mMRC) (scores ranged from 0 to 4); mild dyspnea was defined as having a score less than 2; moderate to severe dyspnea was defined as having a score of no less than 2 (1). The COPD assessment test (CAT) consists of eight items, including cough, expectoration, dyspnea, chest tightness, confidence, limitation of daily activities, quality of sleep, and levels of energy with scores ranging from 0 to 5 (0 = no impairment, 5 = greatest impairment). An overall score is calculated by simply adding the points of the eight questions, with total scores ranging from 0 to 40— higher scores indicating more severe health status impairment of COPD. Scores of 0–10, 11–20, 21–30, and 31–40 represent low, medium, high, and very high impact level, respectively [Citation21]. In recent years, the CAT has been proven to be very useful in evaluating the health status of patients with stable COPD and AECOPD [Citation22]. The ABCD group was classified based on the patient's symptoms and exacerbation history in the last 12 months. They were divided into four groups: group A (mMRC 0–1 or CAT < 10 and 0 or 1 exacerbation that not leading to hospital admission), group B (mMRC ≥ 2 or CAT ≥ 10 and 0 or 1 exacerbation that not leading to hospital admission), group C (mMRC 0–1 or CAT < 10 and ≥2 or ≥1 exacerbation that leading to hospital admission), or group D (mMRC ≥ 2 or CAT ≥ 10 and ≥2 or ≥1 exacerbation that leading to hospital admission) [Citation1].
Pain
Although all relevant measurement properties of each instrument specific to pain have not been established, the construct validity of the BPI is the most comprehensive, suggesting this tool may be the optimal choice in clinical practice until further research is completed [Citation7].The BPI is a valid, reliable, comprehensive, and widely used pain questionnaire in COPD studies and clinical practice [Citation6, Citation7, Citation18, Citation23–25]. The Chinese version of the brief pain inventory (BPI-C) was applied to investigate the pain characteristics [Citation26, Citation27]. At first, our participants were asked to answer this question: “Throughout our lives most of us have had pain from time to time (such as minor headaches, sprains, and toothaches). Have you had pain other than these everyday kinds of pain today?” (yes/no). If they were generally bothered by pain in the past 24 h, they should completed the full BPI-C, which consisted of nine items subdivided into three components: [Citation1] a body diagram that is used to indicate pain locations (participants were asked to shade the areas where they felt pain and place an “X” on the area that hurt they most); [Citation2] a magnitude domain consisting of four items that asked about pain magnitude—now, worst level, least level, and on average, respectively—with each item then rated using a numeric rating scale anchored by 0 (no pain) to 10 (pain as bad as you can imagine); and [Citation3] an interference domain that contained seven items querying how pain interfered with seven aspects of daily living, with each item then rated using a numeric rating scale anchored by 0 (does not interfere) to 10 (completely interferes). In addition, two items addressed pain treatment and pain relief by treatment, ranging from 0% (no relief) to 100% (complete relief). In this study, the pain severity and interference items were rated as mild if scored 0–4, moderate if scored 5–6, and severe if scored 7–10, as in previous studies [Citation28].
Quality of life (QOL)
The CCQ is a validated health-related QOL questionnaire for individuals with COPD [Citation6, Citation16]. It consists of 10 items in three domains (symptoms, functional state, and mental state). Each question scores 0–6 points. The total points from all 10 items are divided by 10 to calculate the CCQ total score; those from items 1, 2, 5, and 6 are divided by 4 to calculate the symptom score; those from 7 to 10 are divided by 4 for the functional score; and those from 3 and 4 are divided by 2 for the mental score. The CCQ questionnaire can be interpreted in this way: CCQ < 1 means acceptable, 1 ≤ CCQ < 2 means acceptable for moderate disease, 2 ≤ CCQ < 3 means unstable–severe limited, CCQ ≤ 3 means very unstable–very severe limited, and even higher CCQ scores indicate worse quality of life [Citation21, Citation29].
Lung function
Spirometry was performed to obtain FEV1 and forced vital capacity (FVC), and predicted values were calculated according to the guidelines of the European Respiratory Society [Citation30]. COPD disease severity was classified using the GOLD guidelines and was divided into four stages: mild (stage I, FEV1 ≥80% predicted), moderate (stage II, FEV1 50%–79% predicted), severe (stage III, FEV1 30%–49% predicted), or very severe (stage IV, FEV1 < 30% predicted) [Citation1].
Sample size estimation
The sample size was calculated by using PASS 15.0 in the part of confidence intervals for one proportion. We used the pain prevalence rate (28.7%) obtained from the pre-experiment as the assumed sample proportion, set the interval type as two-sided, and entered the confidence level (1 - Alpha) as 0.95 and confidence interval width (two-sided) as 0.06. Finally, the sample we acquired was 905 [Citation31, Citation32].
Data analysis
Statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows version 20.0). Descriptive analyses were done to evaluate demographics, clinical features, and pain characteristics. Categorical variables are described as frequencies, while continuous variables were tested for normality and are presented as mean ± standard deviation (SD) or median ± interquartile range (IQR) in case of skewed data. Chi-square or Fisher’s test was used for categorical variables, and Student’s t-test, one-way ANOVA and Mann-Whitney U test were used for continuous variables. Risk factors for pain (with pain/without pain) were identified, and their crude odds ratios (cORs), adjusted odds ratios(aORs), and 95% confidence intervals were estimated using logistic regression analyses. The correlations analyses of pain intensity were examined using Spearman rank correlations when the bivariate did not conform to normal distribution. Simple linear regression analyses were first performed to determine the association. All statistical tests were two-sided at the significance level of 0.05. Multiple comparisons of differences between groups were Bonferroni adjusted.
Results
Patient characteristics
A total of 1044 patients were approached to participate. 128 patients did not meet the study's inclusion criteria and 15 refused to participate in this study. Finally, 901 COPD patients were included and finish the questionnaires. Of the 901 patients in this study, 87.9% were male, with a mean age of 63.1 ± 8.5 (M ± SD) years and a median FEV1 percentage predicted of 45.2 ± 25.4% (M ± IQR). The 901 patients included 802 patients (89.0%) with stable COPD and 99 (11.0%) with AECOPD. Severity of COPD was as follows: 70 participants (7.7%) with mild COPD (stage I), 309 (34.3%) with moderate COPD (stage II), 369 (41.0%) with severe COPD (stage III), and 153 (17.0%) with very severe COPD (stage IV). Characteristics of the participants at enrollment are summarized in .
Table 1. Patient characteristics and prevalence of pain of COPD patients in the study.
Prevalence and characteristics of pain
Overall, 226 (25.1%) patients reported pain problems. As exhibited in and , according to the BPI-C results, 31 (31.3%) patients reported pain in AECOPD compared to 195 (24.3%) in stable COPD (p = 0.13). The prevalence of pain in patients with mild to very severe COPD was 32.9%, 23.9%, 25.2%, and 23.5%, respectively (p = 0.447).
Table 2. Characteristics of pain in patients between stable COPD and AECOPD measured by BPI-C.
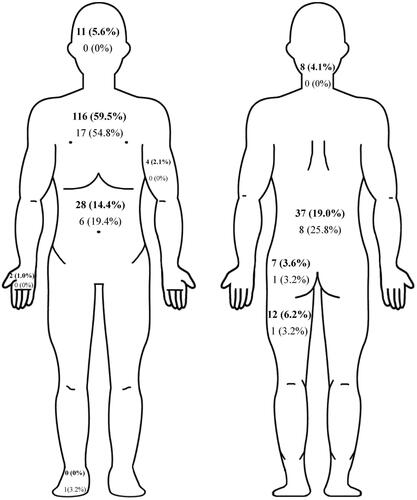
Pain was located in the chest (58.9%), lower back (19.9%), abdomen (15.0%), buttocks (3.5%), upper limbs (2.6%), lower limbs (6.2%), head (4.9%), neck (3.5%), respectively. () The main location of pain was similar between stable COPD and AECOPD which located primarily in the chest (59.5%, 54.8%) and lower back (19.0%, 25.8%) (). Patients with AECOPD mainly had moderate pain intensity, while the pain intensity of patients with stable COPD was mainly mild (p < 0.001). Reported pain intensity and pain interference evaluated by the BPI-C were significantly higher in AECOPD than in stable COPD (4.7 ± 2.0 vs 3.6 ± 1.7; p < 0.001 and 2.6 (3.3) vs 1.1 (2.6), p < 0.005). The majority of COPD patients with pain did not get pain-related treatments. And there was no significant difference in the rate of pain-related treatment between stable COPD and AECOPD ().
Associated factors for pain in COPD patients
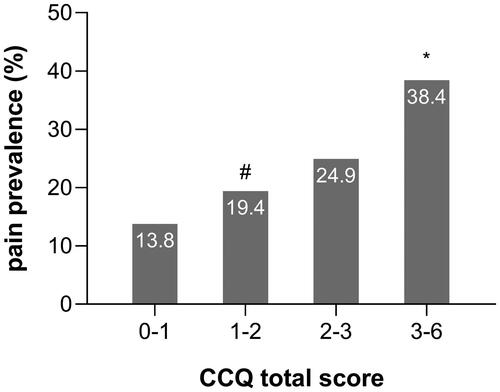
Compared to the without pain group, patients in the with pain group were more likely to have a higher BMI, a higher CAT score, and a higher CCQ total score (which may be on account of the CCQ symptom and CCQ function score) (). As shown in and , patients with 20 < CAT ≤ 30 (35.7%) had a higher prevalence of pain than those with CAT ≤ 10 (21.8%) and 10 < CAT ≤ 20 (22.2%), and patients with a CCQ total score ≥ 3 (38.4%) had a higher prevalence of pain than those with 1 ≤ CCQ total score < 2 (19.4%). (Both of these Bonferroni-adjusted P-values were less than 0.008.) But between these two groups, we found no differences in demographic characteristics—age, sex, education level, smoking status, diagnosis of COPD, exacerbation in the past 12 months, FEV1 percentage predicted, COPD severity, ABCD group, the use of ICS (inhaled corticosteroid) and so on.
Figure 2. Differences in pain prevalence among different CAT groups in patients with COPD. Notes: #p < 0.008 vs CAT 21–30; *p < 0.008 vs CAT 11–20; ▲p < 0.008 vs CAT 0–10. Abbreviations: CAT, COPD assessment test.
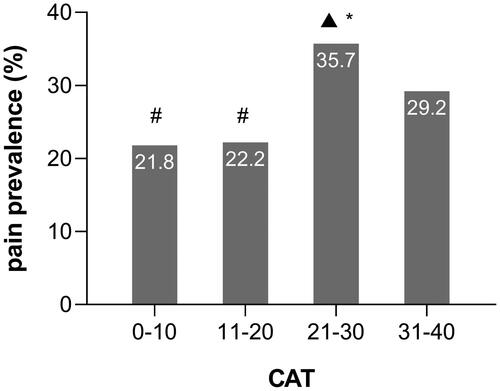
Figure 3. Differences in pain prevalence among different CCQ groups in patients with COPD. Notes: #p < 0.008 vs 3 ≤ CCQ ≤ 6; *p < 0.008 vs 1 < CCQ < 2. Abbreviations: CCQ, Clinical COPD questionnaire.
A logistic regression model showed that COPD patients with BMI ≥ 24kg/m2 were significantly more likely to have pain problems than BMI < 24kg/m2 (cOR = 1.471, c95%CI = 1.070–2.022, p = 0.017). The cOR was 1.891 for the CAT score (>20 vs ≤20, p < 0.001). After adjustment for sex, age, smoking status, BMI, CAT score, mMRC score, CCQ total score, the corresponding odds ratios were 1.568 (a95IC = 1.132–2.170, p = 0.007) and 1.754 (a95IC = 1.213–2.536, p = 0.003) ().
Table 3. Factors correlated with the prevalence of pain.
From the correlation analysis, the worst pain intensity measured by the BPI-C showed mild positive correlations between mMRC score(r = 0.20, p < 0.005) and CCQ function score (r = 0.26, p < 0.001) in patients with COPD, while there was no significant correlation of age, BMI, CAT score, CCQ total score, FEV1 and FEV1 percentage predicted with pain intensity (). As shown in , patients with mMRC score = 4 had higher pain intensity than patients with mMRC score less than 4. And there were significant differences in pain intensity between diffferent CCQ function score subgroups. But we found no differences in pain intensity between different age, sex, BMI, CAT score, exacerbation in the past 12 months, COPD severity, ABCD groups and the use of ICS.
Figure 4. The correlation between the worst severity of pain in the last 24 h measured on BPI-C and age; FEV1% predicted; FEV1; mMRC score; CAT score; CCQ total score; CCQ function score among COPD patients with pain. Abbreviations: FEV1% predicted, forced expiratory volume in one second as a percentage of the predicted value; mMRC, Modified Medical Research Council Dyspnea Scale; CAT, COPD assessment test; CCQ, Clinical COPD questionnaire; BMI, body mass index.
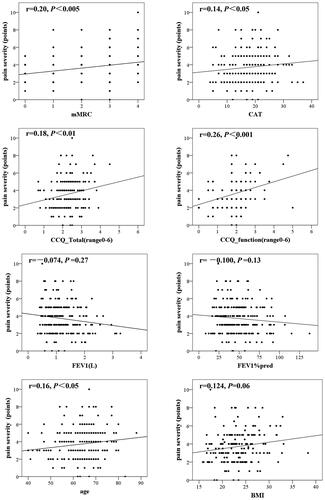
Table 4. Patient intensity in COPD patients with pain between different groups.
Discussion
To our knowledge, this cross-sectional study is the first to describe and compare the characteristics of pain in patients with stable COPD and AECOPD, and it is also the first study conducted on mild to very severe severity of COPD patients in China.
Prevalence of pain
Previous study showed than patients with COPD have a higher prevalence of pain than patients of the same age and sex who have chronic illnesses other than COPD [Citation7]. Our study found that the prevalence of pain in patients across the four stages of COPD was 25.1%. The total pain prevalence in our study was within the range of prevalence (21%–96%) reported in previous literature [Citation6, Citation7, Citation9–13, Citation33, Citation34]. Such a heterogeneity in the prevalence of pain between studies was probably due to variances in study sample, participants and pain related questionnaire. The differences in the questionnaires used in the studies including the BPI and the McGill Pain Questionnaire (MPQ) may influence the different findings between the studies. The prevalence of pain in patients with mild to very severe COPD was 32.9%, 23.9%, 25.2%, and 23.5%, respectively. Furthermore, we found no difference in the prevalence of pain between the four stages of COPD patients. This finding is familiar with previous research that no significant differences were observed in the prevalence of pain between moderate to very severe stages [Citation7, Citation34–36]. The only one previous study in China found that patients with moderate COPD were significantly more likely to have pain problems than those with mild COPD [Citation10], which is not consistent with our study. This previous cross-sectional study included 283 COPD patients at mild to moderate severity with a smaller sample size than us, and only involved communities, which may reduces the representativeness of the COPD population. The study used the short form McGill Pain Questionnaire (SF-MPQ) to evaluate pain problems while the locations of pain were not investigated which may lead to an overestimation of the prevalence of pain. All of these factors may lead to different outcomes of pain prevalence in COPD patients between different severity. Some studies even revealed that patients with moderate COPD had a higher prevalence of pain than those at the severe or very severe stage of COPD [Citation12, Citation35], which may be due to the choice of bias or the neglect of pain caused by more severe symptoms such as cough, sputum production, breathlessness, and wheezing that are experienced as more severe and distressing than pain [Citation37].
Comparing our results with individual studies, we found that the participants of these studies all had similar characteristics (age, BMI, and FEV1 percentage predicted) [Citation10–12, Citation38]. Of the 901 patients in our study, 87.9% were men, but there was no significant difference in the prevalence of pain between men (24.2%) and women (31.2%). This differed from previous studies, which found that the prevalence of pain in female patients was higher [Citation33, Citation34]. Explanations for the differences in pain between women and men include neuroimmunological, hormonal, genetic, psychological, and sociological factors [Citation39, Citation40]. This difference between our study and other studies may be related to the low incidence of COPD in Chinese women [Citation3]. However, according to GOLD grade, there was no difference in the prevalence of pain among the four ABCD groups, which is consistent with previous studies [Citation41].
We have found that the prevalence of pain in stable COPD and moderate to severe AECOPD were 24.3% and 31.3%, respectively. But there was no significant difference in prevalence of pain between these two groups. These findings are consistent with the results of a previous that found that 39.6% of severe AECOPD patients reported pain problems [Citation16]. However, the prevalence of pain in AECOPD patients (39.6%) was significantly different from that in another study of patients with AECOPD (92%), which may be related to the varied study participants (patients with COPD hospitalized for an acute exacerbation and indicated for post-acute pulmonary rehabilitation VS AECOPD patients in emergency departments), region (Netherlands VS France and Canada) and sample (149 VS 55) [Citation17]. We found that the pain intensity and pain interference scores of patients with AECOPD were higher than those patients with stable COPD. At present, there have few studies on the pain characteristics of patients with AECOPD. As we all known, AECOPD represent a major symptom burden for patients, and negatively influence HRQoL and functional capacity. Many symptoms are associated with pain, of which dyspnea, anxiety, depression and insomnia are the most frequent. Furthermore, these symptoms cluster and aggravate each other. Pain in patients with AECOPD might be aggravated compared to the stable COPD due to the mentioned vicious circle of symptoms [Citation5, Citation16]. Based on the results of our study, we suggest that equal attention should be paid to the evaluation of pain both in AECOPD and stable COPD. COPD and AECOPD patients were recruited in both inpatients and outpatients during the same period in our research. The small sample size of the comparison between stable COPD and AECOPD patients was a reflection of our real-world population distribution. The sample size of AECOPD patients for comparative analysis was small in our study. However, we cannot completely exclude partial selection bias in patients with AECOPD which may result in a relatively small sample size of AECOPD patients. Patients with severe acute exacerbation who could not reliably answer the questionnaire due to decreased consciousness were not included in the study.
Pain locations and causes
Our results showed that the main pain locations were the chest and lower back in both stable COPD and AECOPD. The main pain locations of patients with stable COPD are consistent with the results of many previous studies [Citation9, Citation11, Citation33, Citation42]. The main pain locations of AECOPD patients were also consistent with a previous prospective cohort study [Citation17]. There are also certain comorbidities such as arthritis and osteoporosis that can cause pain in COPD patients. But study suggest that chronic comorbid illnesses among patients with COPD does not fully explain the higher prevalence of chronic pain in COPD [Citation43]. According to the literature, the causes of chest pain may be related to pulmonary hyperventilation, loss of elasticity of the parietal pleura, pathological bronchial fibrosis, thoracic vertebral deformity, costotransverse, and intervertebral arthropathy [Citation23, Citation44]. It has also been reported that chest pain is related to systemic and local inflammatory reactions [Citation45], and systemic symptoms caused by COPD may be related to systemic inflammatory reactions induced by abnormal activation of cytokines and inflammatory cells, as well as to reduced skeletal muscle weight leading to impaired skeletal muscle function [Citation45, Citation46]. Moreover, IL-1β and cytokine IL-6 both were associated with increased pain and hyperalgesia [Citation47]. TNF-α has been shown to increase mechanical allodynia and thermal hyperalgesia and decrease the mechanical activation threshold in C fibers [Citation48]. Both of these may provide further theoretical support for the mechanism of chest pain. Previous studies have shown that lower back pain is related to chest remodeling, diaphragm fatigue, and lung volume increase in patients with COPD [Citation49, Citation50]. Furthermore, the increase of lumbar kyphosis leads to lumbar disc protrusion after pathological changes such as decreased abdominal pressure, which leads to pain in the lower back [Citation50].
Pain intensity and pain-related treatment
We have shown that pain intensity is positively mild correlated with mMRC score and CCQ function score. The correlation of pain intensity with mMRC score is controversial [Citation8, Citation10, Citation11, Citation35, Citation37]. However, the positive correlation between mMRC and pain also further verifies the correlation between dyspnea and pain [Citation7, Citation37]. This correlation could be explained by similar perception-mechanisms for pain and dyspnea, the sharing of some cortical areas for processing, and dyspnea that may lead to hyperalgesia [Citation49]. Previous study also suggested that controlling dyspnea symptoms may help to relieve pain [Citation51]. No correlation between pain intensity and lung function (FEV1 and FEV1 percentage predicted) has been found in our studies, which finding is consistent with previous studies [Citation5, Citation7, Citation8]. Because of the relatively weak correlation between pain intensity and these factors, its clinical significance may be overestimated. In the future, we may need to conduct a larger sample of cross-sectional or cohort studies on factors related to pain intensity to further verify these results. More than 80% of patients with COPD in our study did not receive pain-related treatment. Undertreatment of pain has been reported before among patients with COPD. One possible explanation for this is that patients with COPD may not actively acquire related treatments for pain because they adapt to their situation or think it is impossible to improve it, and their physicians did not acknowledge these atypical symptoms either [Citation52–54]. Another explanation for undertreatment of pain in COPD is the lack of knowledge concerning causal mechanisms of pain in COPD and corresponding lack of causal treatment [Citation8].
Clinical features and pain
We have found that participants in the with pain group had higher BMI, higher total CCQ score, and higher CAT scores than those in the without pain group. The results relating to BMI and CAT were consistent with previous studies [Citation10, Citation12, Citation18]. The higher CCQ score in the with pain group of our study was mainly attributed to the CCQ symptom score and functional score. Although there are not enough studies investigating the relationship between pain and CCQ score, our results were similar to those of a previous cross-sectional observational study for AECOPD patients in which the results were also mainly attributed to the functional score [Citation16]. In correlation analysis, we found that the total CCQ score, especially the domain of function, was positively correlated with pain intensity. In general, we found that the prevalence of pain was associated with lower health status, which was consistent with previous studies on the negative correlation between pain and the health status of patients with stable COPD [Citation18, Citation55]. In univariate analysis, we found that patients with overweight (BMI ≥ 24kg/m2), moderate to severe dyspnea (mMRC ≥ 2), CAT score over 20, and a CCQ total score no less than 2 may be more likely to report pain problems. Later, in multiple regression analysis, we found that BMI ≥ 24kg/m2 and CAT > 20 were significantly related to the prevalence of pain. The difference between mMRC and CCQ scores may be due to the confounding effect of other factors, which has no obvious clinical significance in multivariate analysis. Some studies have found that dyspnea is also a risk factor for pain that may require us to perform more studies to further explore the risk factors for pain in COPD patients [Citation51].
Our study also has limitations. Firstly, this study was limited to one center, which may reduce the representativeness of the COPD population. Secondly, we have not extensively documented comorbidities such as arthritis or osteoporosis that may effect pain evaluation in patients with COPD. Lastly, as this was a cross-sectional study, there is insufficient evidence for a causal relationship between clinical risk factors and pain. Longitudinal studies are needed to evaluate changes in pain intensity over time, as well as for the specific etiologies for pain in patients with COPD.
Conclusion
Pain was common both in patients with stable COPD and those with AECOPD. This study found a higher intensity and interference of pain during AECOPD compared to stable COPD. However, we found no significant differences in prevalence of pain between stable COPD patients and AECOPD patients, between different stages of COPD, or between ABCD groups. Overweight (BMI ≥ 24kg/m2) and CAT > 20 were significantly related to higher prevalence of pain. COPD patients with higher age, higher mMRC scores, higher CAT score or higher CCQ total scores may have more severe pain. Based on the results of this study, we inferred that losing weight or relieving systemic symptoms may decrease the prevalence or reduce the intensity of pain. Above all, we think pain should be brought into consideration in both stable COPD and AECOPD general assessment, thereby raising our awareness and further improving the quality of life of patients with COPD.
Declaration of interest
The authors report no conflicts of interest in this work.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
We want to thank all the patients and clinicians who contributed to this study, especially the research assistants Zhiwen Wang, Guoguo Zhong, and Qian Li.
Additional information
Funding
References
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP.
- Collaborators G. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1545–1602. doi:10.1016/S0140-6736(16)31678-6.
- Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2095–2128.
- van Dam Van Isselt EF, Groenewegen-Sipkema KH, Spruit-van Eijk M, et al. Pain in patients with COPD: a systematic review and meta-analysis. BMJ Open. 2014;4(9):e5898. doi:10.1136/bmjopen-2014-005898.
- Chen Y, Camp PG, Coxson HO, et al. A comparison of pain, fatigue, dyspnea and their impact on quality of life in pulmonary rehabilitation participants with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;15(1):65–72. doi:10.1080/15412555.2017.1401990.
- Lee AL, Harrison SL, Goldstein RS, et al. Pain and its clinical associations in individuals with COPD. Chest 2015;147(5):1246–1258. doi:10.1378/chest.14-2690.
- Janssen DJA, Wouters EFM, Parra YL, et al. Prevalence of thoracic pain in patients with chronic obstructive pulmonary disease and relationship with patient characteristics: a cross-sectional observational study. BMC Pulm Med. 2016;16(1):47. doi:10.1186/s12890-016-0210-8.
- Chen YW, Camp PG, Coxson HO, et al. Comorbidities that cause pain and the contributors to pain in individuals with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2017;98(8):1535–1543. doi:10.1016/j.apmr.2016.10.016.
- Xiao T, Zhou X, He Y, et al. Pain problems for patients with mild and moderate chronic obstructive pulmonary disease-a community-based study in Shanghai. J Pain Res. 2017;10:2247–2252. doi:10.2147/JPR.S141940.
- Lee AL, Goldstein RS, Brooks D. Chronic pain in people with chronic obstructive pulmonary disease: prevalence, clinical and psychological implications. Chronic Obstr Pulm Dis. 2017;4(3):194–203. doi:10.15326/jcopdf.4.3.2016.0172.
- Bentsen SB, Miaskowski C, Cooper BA, et al. Distinct pain profiles in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;13:801–811. doi:10.2147/COPD.S150114.
- Lewthwaite H, Williams G, Baldock KL, et al. Systematic review of pain in clinical practice guidelines for management of COPD: a case for including chronic pain? Healthcare 2019;7(1):15. doi:10.3390/healthcare7010015.
- Kim KY, Miravitlles M, Sliwinski P, et al. Comparison of clinical baseline characteristics between Asian and Western COPD patients in a prospective, international, multicenter study. Int J Chron Obstruct Pulmon Dis. 2019;14:1595–1601. doi:10.2147/COPD.S208245.
- Oh YM, Bhome AB, Boonsawat W, et al. Characteristics of stable chronic obstructive pulmonary disease patients in the pulmonology clinics of seven Asian cities. Int J Chronic Obstr. 2013;8:31–39.
- van Dam Van Isselt EF, Groenewegen-Sipkema KH, van Eijk M, et al. Pain in patients with chronic obstructive pulmonary disease indicated for post-acute pulmonary rehabilitation. Chron Respir Dis. 2018;16:1248795377. doi:10.1177/1479972318809456.
- Maignan M, Chauny JM, Daoust R, et al. Pain during exacerbation of chronic obstructive pulmonary disease: A prospective cohort study. PLoS One. 2019;14(5):e0217370. doi:10.1371/journal.pone.0217370.
- HajGhanbari B, Garland SJ, Road JD, et al. Pain and physical performance in people with COPD. Resp Med. 2013;107(11):1692–1699. doi:10.1016/j.rmed.2013.06.010.
- Avci N, Hayar M, Altmisdortoglu O, et al. Smoking habits are an independent prognostic factor in patients with lung cancer. Clin Respir J. 2017;11(5):579–584. doi:10.1111/crj.12386.
- Zhou BF, Meta C. Analysis group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults-study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;11(1):S685–S693.
- Zhou ZJ, Zhou AY, Zhao YY, et al. A comparison of the assessment of health status between CCQ and CAT in a Chinese COPD clinical population: a cross-sectional analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:1675–1682. doi:10.2147/COPD.S161225.
- Zhou AY, Zhou ZJ, Peng YT, et al. The role of CAT in evaluating the response to treatment of patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2849–2858. doi:10.2147/COPD.S175085.
- Chen Y, Coxson HO, Coupal TM, et al. The contribution of thoracic vertebral deformity and arthropathy to trunk pain in patients with chronic obstructive pulmonary disease (COPD). Resp Med. 2018;137:115–122. doi:10.1016/j.rmed.2018.03.007.
- Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin brief pain questionnaire to assess pain in cancer and other diseases. Pain 1983;17(2):197–210. PMID: 6646795 doi:10.1016/0304-3959(83)90143-4,.
- Tan G, Jensen MP, Thornby JI, et al. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi:10.1016/j.jpain.2003.12.005.
- Wang XS, Mendoza TR, Gao S, et al. The Chinese version of the brief pain inventory (BPI-C): its development and use in a study of cancer pain. Pain 1996;67(2):407–416.
- Guo J, Liu C, Wang X, et al. Relationships between depression, pain and sleep quality with doctor visits among community-based adults in north-west China. Public Health. 2017;147:30–38. doi:10.1016/j.puhe.2017.01.031.
- Serlin RC, Mendoza TR, Nakamura Y, et al. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 1995;61(2):277–284. doi:10.1016/0304-3959(94)00178-H.
- van der Molen T, Willemse BW, Schokker S, et al. Development, validity and responsiveness of the clinical COPD questionnaire. Health Qual Life Outcomes. 2003;1(1):13.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40.
- Julious SA. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi:10.1002/sim.2164.
- Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3rd ed. Wiley Series in Probability and Statistics; 2003. doi:10.1002/0471445428.
- Andenaes R, Momyr A, Brekke I. Reporting of pain by people with chronic obstructive pulmonary disease (COPD): comparative results from the HUNT3 population-based survey. BMC Public Health. 2018;18(1):181. doi:10.1186/s12889-018-5094-5.
- Christensen VL, Holm AM, Kongerud J, et al. Occurrence, characteristics, and predictors of pain in patients with chronic obstructive pulmonary disease. Pain Manag Nurs. 2016;17(2):107–118. doi:10.1016/j.pmn.2016.01.002.
- Bentsen SB, Rustoen T, Miaskowski C. Differences in subjective and objective respiratory parameters in patients with chronic obstructive pulmonary disease with and without pain. Int J Chron Obstruct Pulmon Dis. 2012;7:137–143. doi:10.2147/COPD.S28994.
- Bentsen SB, Gundersen D, Assmus J, et al. Multiple symptoms in patients with chronic obstructive pulmonary disease in Norway. Nurs Health Sci. 2013;15(3):292–299. doi:10.1111/nhs.12031.
- Borge CR, Wahl AK, Moum T. Association of breathlessness with multiple symptoms in chronic obstructive pulmonary disease. J Adv Nurs. 2010;66(12):2688–2700.
- Westerik JA, Metting EI, van Boven JF, et al. Associations between chronic comorbidity and exacerbation risk in primary care patients with COPD. Respir Res. 2017;18(1):31. doi:10.1186/s12931-017-0512-2.
- Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–866.
- Rosen S, Ham B, Mogil JS. Sex differences in neuroimmunity and pain. J Neurosci Res. 2017;95:500–508.
- Christensen VL, Holm AM, Cooper B, et al. Differences in symptom burden among patients with moderate, severe, or very severe chronic obstructive pulmonary disease. J Pain Symptom Manag. 2016;51(5):849–859.
- Bentsen SB, Rustoen T, Miaskowski C. Prevalence and characteristics of pain in patients with chronic obstructive pulmonary disease compared to the Norwegian general population. J Pain. 2011;12(5):539–545. doi:10.1016/j.jpain.2010.10.014.
- Roberts MH, Mapel DW, Hartry A, et al. Chronic pain and pain medication use in chronic obstructive pulmonary disease. A cross-sectional study. Ann ATS. 2013; 10(4):290–298. doi:10.1513/AnnalsATS.201303-040OC.
- Bordoni B, Marelli F, Morabito B, et al. Chest pain in patients with COPD: the fascia's subtle silence. Int J Chron Obstruct Pulmon Dis. 2018;13:1157–1165. doi:10.2147/COPD.S156729.
- HajGhanbari B, Holsti L, Road JD, et al. Pain in people with chronic obstructive pulmonary disease (COPD). Resp Med. 2012;106(7):998–1005.
- Zhang Y, Cao J, Chen Y, et al. Intraperitoneal injection of cigarette smoke extract induced emphysema, and injury of cardiac and skeletal muscles in BALB/C mice. Exp Lung Res. 2013;39(1):18–31. doi:10.3109/01902148.2012.745910.
- Lindenlaub T, Sommer C. Cytokines in sural nerve biopsies from inflammatory and non-inflammatory neuropathies. Acta Neuropathol. 2003;105(6):593–602. doi:10.1007/s00401-003-0689-y.
- Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain 2000;85(1):145–151. doi:10.1016/S0304-3959(99)00262-6.
- Bordoni B, Marelli F, Morabito B, et al. Low back pain and gastroesophageal reflux in patients with COPD: the disease in the breath. Int J Chron Obstruct Pulmon Dis. 2018;13:325–334. doi:10.2147/COPD.S150401.
- O'Sullivan PB, Beales DJ. Changes in pelvic floor and diaphragm kinematics and respiratory patterns in subjects with sacroiliac joint pain following a motor learning intervention: a case series. Man Ther. 2007;12(3):209–218. doi:10.1016/j.math.2006.06.006.
- Clark N, Fan VS, Slatore CG, et al. Dyspnea and pain frequently co-occur among Medicare managed care recipients. Ann ATS. 2014;11(6):890–897. doi:10.1513/AnnalsATS.201310-369OC.
- Janssen DJ, Spruit MA, Uszko-Lencer NH, et al. Symptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failure. J Palliat Med. 2011;14(6):735–743. doi:10.1089/jpm.2010.0479.
- Habraken JM, Pols J, Bindels PJ, et al. The silence of patients with end-stage COPD: a qualitative study. Br J Gen Pract. 2008;58(557):844–849. doi:10.3399/bjgp08X376186.
- Harrison S, Lee A, Button H, et al. The role of pain in pulmonary rehabilitation: a qualitative study. Int J Chron Obstruct Pulmon Dis. 2017;12:3289–3299. doi:10.2147/COPD.S145442.
- Lohne V, Heer HC, Andersen M, et al. Qualitative study of pain of patients with chronic obstructive pulmonary disease. Heart Lung J Crit Care. 2010;39(3):226–234.