Abstract
Skeletal muscle dysfunction, functional exercise capacity impairment and reduced physical activity are characteristic features in patients with chronic obstructive pulmonary disease (COPD). Assessments addressing muscle strength of the upper limb, such as measurement of handgrip strength (HGS), are rarely performed and reported. We aimed to analyze the course of HGS and possible predictors of changes in HGS over time in COPD. Yearly assessments of various disease markers were performed for a follow-up of up to seven years in a cohort of COPD patients to assess the longitudinal disease process. Data of 194 patients with at least one follow-up measurement were analyzed. HGS decreased significantly by B = −0.86 (95% CI −1.09/−0.62, p < 0.001) over time. The multivariate mixed effects model showed an independent association between greater annual declines in HGS and lower numbers of steps per day by B = 0.11 (95% CI 0.03/0.18, p = 0.006) and an enhanced change in COPD Assessment Test scores by B = −0.01 (95% CI −0.01/−0.00, p = 0.034). No evidence for an independent association between annual decline in HGS and FEV1% pred. by B = −0.01 (95% CI −0.03/0.01, p = 0.297) was shown. Patients who died during follow-up did not exhibit greater declines in HGS compared to survivors (p = 0.884). Although HGS significantly decreased over time, no pathophysiological link with COPD disease progression could be demonstrated. Previous cross-sectional associations between HGS and mortality could not be confirmed in this longitudinal setting. Our data suggests that repeated monitoring of HGS in clinical settings seems not to be helpful to predict COPD specific disease progression.
Background
Skeletal muscle dysfunction, functional exercise capacity impairment and reduced physical activity (PA) are characteristic features in patients with chronic obstructive pulmonary disease (COPD) [Citation1–3]. A low functional exercise capacity (e.g., walking, sit-to-stand) is associated with impaired health-related quality of life (HrQoL) and increased mortality in COPD [Citation3,Citation4]. Handgrip strength (HGS) as another test representing functional exercise performance showed inconsistent results and a moderate but less strong association with mortality compared to walking tests in cross-sectional settings [Citation2,Citation4–10]. In the general population, HGS predicts all-cause (hazard ratio 1.16 per 5 kg reduction in HGS) and cardiovascular mortality (hazard ratio 1.17 per 5 kg reduction in HGS) [Citation11]. In the COPD population, testing of endurance related components, such as the six-minute walk test (6MWT) and endurance-strength-combined tests, such as the 1-minute sit-to-stand test (1MSTS) in outpatient or rehabilitation settings, is established and highly standardized [Citation2]. In contrast, regular strength testing and its clinical value has not been in the focus of research except single studies on lower limb isometric strength [Citation3,Citation12–14]. Assessments addressing muscle strength of the upper limb, such as measurement of HGS, are rarely performed in clinical practice.
Despite HGS being time-efficient, and relatively inexpensive in contrast to common functional exercise capacity tests, regular assessment remains uncommon and the course of HGS over time and its clinical meaning is underexplored. The longitudinal course and impact of changes of various clinical outcomes on HGS in COPD is unknown. Since COPD patients are followed-up regularly, repeated measurements of HGS would be easily available in clinical practice and changes may provide additional information on a patient’s condition, and possibly add important predictive impact.
Thus, we aimed to analyze the course of HGS and possible predictors of changes in HGS over time in a group of heterogeneous COPD patients.
Materials and methods
Yearly assessments of various disease markers such as exercise capacity, daily PA and lung function were performed for a follow-up of up to seven years in a heterogeneous group of COPD patients to assess the longitudinal disease process.
Subjects
An a posteriori analysis of the prospective, non-interventional data collected as part of the cohort project “The Obstructive Pulmonary Disease Outcomes Cohort Study (TOPDOCS)” was performed. Patients with mild to very severe COPD from seven pulmonary outpatient clinics in Switzerland were included. Recruitment took place from October 2010 to April 2016 during outpatient visits or hospital stays. Patients between 40 and 75 years of age at inclusion with confirmed COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) [Citation15] were assessed for eligibility. Patients with diagnosed mental or physical disability precluding informed consent or compliance with the protocol were not included. In case of a COPD exacerbation, study inclusion or annual follow-up was delayed for at least 6 weeks.
The study was conducted in accordance with the declaration of Helsinki and all subjects provided written informed consent. The Ethics Committee of the Canton of Zurich approved the study (EK-ZH-NR: 1734 and 2011-0106), and the study is registered at www.ClinicalTrials.gov, NCT01527773.
Measurements
Functional exercise capacity
HGS was assessed using digital dynamometry (EH101 hand dynamometer, Camry Electronic Co. Ltd., Guangdong, China). Performance, as well as instructions were standardized according to published recommendations [Citation16]. Patients were seated with the elbow flexed at 90°, shoulders adducted, and neutral position of the forearm. They were instructed to squeeze the dynamometer as hard as possible. The observer stopped the measurement when no further rise on the display was present. Out of three repeatable measurements (i.e. variability between the measurements being ≤ 5.0 kg) for each hand the maximal HGS was reported [Citation17]. HGS results were obtained in kilogram (kg) for both hands, and are presented for the measurement of the dominant side in this publication. Predicted values were calculated according to published reference equations [Citation18].
The 6MWT was performed according to technical standards set up by the American Thoracic Society and the European Respiratory Society (ATS/ERS) [Citation19]. In brief, the test was performed on a marked 75 m indoor track, and subjects were told to walk as far as possible within the 6 min. The walking distance (in meter) was registered at the end of the test. Subjects were allowed to take brakes during the test if needed, however, time was not interrupted. Standardized instructions and phrases of encouragement were given each minute. Oxygen supplementation was installed if required, and subjects carried their own oxygen device during the test.
The 1MSTS was performed using a standardized protocol [Citation20–22] on a conventional chair without armrest and a seat height of 46 cm. For safety reasons, the chair was positioned against a wall. The subjects were instructed to standup and sit-down as often as possible at a self-chosen speed during 1 min and the number of sit-to-stand repetitions was counted. Verbal encouragement was not provided during the test, but subjects were informed when 15 s were remaining of the 60 s test [Citation20–23]. Subjects were allowed to stop at any time during the test. For a counting repetition, the legs had to be completely straight at the end of the standup phase, and the buttocks had to have clear contact with the chair when sitting down. The subjects were told to place their hands at the hips and were not allowed to use their hands or arms to assist movement.
Daily physical activity
The number of steps per day, an indicator for PA, was measured through a validated, triaxial accelerometer of a multisensory activity monitor (SenseWear Pro™; Bodymedia Inc., Pittsburgh, PA, USA) [Citation24]. The monitor was worn on the upper left arm for seven consecutive days once a year. The threshold for valid data from the armband was set at a usage time of 4 days with a minimum of 22.5 h per day [Citation25]. Seasonality was considered in the analysis. For interpretable reporting of step data we divided the values by 1000.
Respiratory and hematological variables
All patients underwent pulmonary function testing according to ATS/ERS standards to measure forced expiratory volume in one second (FEV1), forced vital capacity (FVC), residual volume to total lung capacity (RV/TLC), and diffusing capacity of the lung for carbon monoxide (TLco) [Citation26–28]. All reported values were obtained after short-acting bronchodilating agent application.
Native, daytime partial pressure of oxygen in arterial blood (PaO2) and partial pressure of carbon dioxide in arterial blood (PaCO2) were measured by arterial blood gas analysis (ABL 700 series, Radiometer Copenhagen) after 5 min of rest.
Exacerbations
Acute exacerbations of COPD (AECOPD) were defined as increases in dyspnea, cough and sputum production leading to the prescription of antibiotics and/or corticosteroids. AECOPD requiring hospital admission were defined as severe acute exacerbations of COPD (SAECOPD). Annual AECOPD count was recorded through patient’s medical history and confirmed through medical documentation of the patient’s general practitioner, pulmonologist, and hospital. Patients were categorized into infrequent exacerbators (0 to 1 annual AECOPD), and frequent exacerbators (≥2 annual AECOPD).
COPD-specific health status
Subjective health status capturing COPD symptom burden was recorded by the COPD Assessment Test (CAT), a valid and reliable questionnaire in the COPD population [Citation29]. The CAT consists of eight questions, each describing a COPD specific symptom. Patients were asked to rate the severity of each symptom on a numeric rating scale ranging from 0 to 5 and summed up to a total score.
Comorbidities
Comorbidities were recorded annually out of the patient’s medical history and were confirmed through medical documentation of the patient’s general practitioner, pulmonologist, and hospital. Classification of the conditions was carried out according to the International Classification of Diseases, tenth revision [Citation30]. We defined stroke, pulmonary cachexia, peripheral arterial disease, Parkinson’s disease, osteoporosis, neuropathies, gout, diabetes, degenerative joint disease, connective tissue disorders and adipositas as relevant musculoskeletal comorbidities, possibly confounding HGS [Citation31]. The number of (musculoskeletal) comorbidities affects PA and was therefore counted in each patient [Citation31].
Data analysis and statistics
All results are shown as mean (SD) or median (25%, 75% quartiles) unless otherwise stated. Statistical analysis was performed with STATA 15.1 (StataCorp, Texas, TX, USA).
Univariate mixed effect models were used to investigate the annual change in HGS and possible predictors (input variables) of change in HGS such as Body-Mass-Index (BMI), spirometry, exacerbations, and daily step count. Furthermore, the association between annual changes in possible predictors (input variables) and the course in HGS was assessed by univariate mixed effect models. Linear relationship was tested by visualization with locally weighted scatterplot smoothing (LOWESS). In case of non-linearity, the independent variable was transformed to reach linearity. For multivariate mixed effect modeling, all variables with a p-value of <0.05 in univariate regression were included in the analysis. All models were adjusted for baseline values in HGS.
A two-sided p-value of <0.05 was considered to be statistically significant.
Results
Study participants
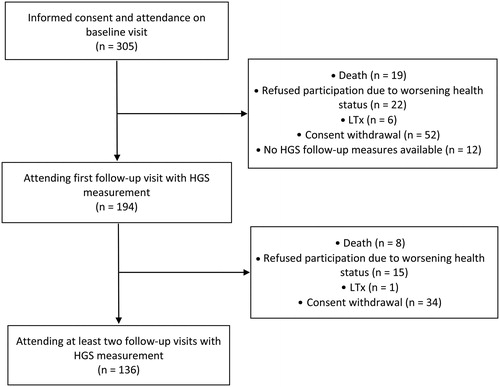
Of the 305 patients taking part in the cohort study, data of 194 patients with at least one follow-up measurement of HGS were analyzed in this publication. Median (quartiles) follow-up time was 2.1 (1.54/3.01) years and HGS of the dominant hand at baseline was 35.3 (28.2/44.4) kg, detailed patient characteristics are presented in . At second follow-up 136 patients were available. Reasons for missing first or second follow-up visits were consent withdrawal, inability to participate due to worsening health status, lung transplantation, and death. Details are shown in the study flow (). An additional third follow-up was performed in 46 patients.
Table 1. Patient characteristics.
Longitudinal course of HGS
HGS significantly decreased by B = −0.86 (95% CI −1.09/−0.62, p < 0.001) kg over time in our cohort of COPD patients. After adjustment for baseline HGS, a significant annual decrease by B = −1.04 (95% CI −1.35/−0.74, p < 0.001) kg was shown. Predicted HGS decreased by B = −2.80 (95% CI −3.93/−1.68, p < 0.001) %, and after adjustment for baseline HGS by B = −3.40 (95% CI −4.72/−2.10, p < 0.001) %.
Possible predictors for longitudinal course of HGS
The decline in HGS was reinforced with a decreased FEV1% pred. by B = 0.02 (95% CI 0.00/0.03), p = 0.015), decreased BMI by B = 2.20 (95% CI −0.09/0.01, p = 0.028), and with a decreased number of steps per day by B = 0.07 (95% CI 0.01/0.13, p = 0.031). Patients who died before first or second follow-up did not show a greater decline in HGS compared to survivors (p = 0.884). Furthermore, survival time was not associated with the decline in HGS over time by B = 0.01 (95% CI −0.01/0.02, p = 0.343). HGS decline over time was not associated with exacerbation history, blood gases and other functional exercise capacity tests. All outcomes of the univariate mixed effect model are displayed in .
Table 2. Univariate mixed effect analysis of possible predictors for handgrip strength (kg) decline over time, dominant side.
A greater increase in BMI was significantly associated with a smaller decline in HGS over time by B = 0.08 (95% CI 0.01/0.16, p = 0.034). Patients with a greater annual increase in CAT score showed a significantly greater decline in HGS over time by B = −0.01 (95% CI −0.01/−0.00, p = 0.010). Disease progression expressed by changes in lung function and blood gases were not associated with a greater annual decline in HGS. Neither annual changes in 6MWT nor 1MSTS nor number of steps per day were associated with the decline in HGS over time. All outcomes of the univariate mixed effect model of changes are displayed in .
Table 3. Univariate mixed effect analysis of changes in possible predictors for handgrip strength (kg) decline over time, dominant side.
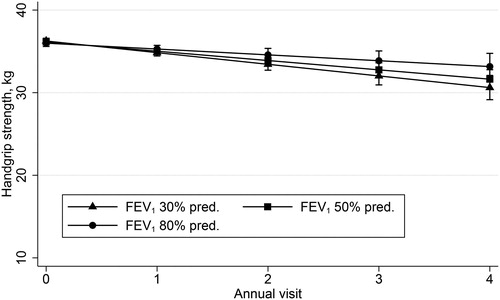
The multivariate mixed effects model showed an independent association between a greater annual decline in HGS and a lower number of steps per day by B = 0.11 (95% CI 0.03/0.18, p = 0.006) and an enhanced change in CAT score by B = −0.01 (95% CI −0.01/−0.00, p = 0.034). No evidence for an independent association between the annual decline in HGS and FEV1% pred. by B = −0.01 (95% CI −0.03/0.01, p = 0.297), see , as well as BMI by B = −0.06 (95% CI −0.13/0.00, p = 0.052) and change in BMI by B = 0.02 (95% CI −0.06/0.10, p = 0.604), respectively was shown, see .
Table 4. Multivariate mixed effect model of possible predictors for handgrip decline (kg) over time, dominant side.
Discussion
Our analysis aimed to assess the longitudinal course of HGS and its possible predictors in a cohort of COPD patients. We found a significant decrease in HGS over time and patients with a more pronounced worsening in COPD symptoms and a reduced PA demonstrated a greater annual decline in HGS. However, no direct markers of COPD disease progression such as lung function and exacerbation history determined HGS decline. Furthermore, the longitudinal course of HGS did not predict mortality, as compared to previous studies using cross-sectional designs [Citation4,Citation5,Citation7].
The importance and impact of muscle dysfunction in COPD is emerging, and functional exercise capacity testing were used to address this in clinical practice. The lower limb musculature is known to be affected by structural changes, such as fiber-shift and increased anaerobic glycolysis, in COPD [Citation3,Citation32]. Potential structural changes in the upper limbs in patients with COPD have not been thoroughly investigated. So far, results reported no differences in muscle fiber type, cross-sectional area, and only slight differences in muscle metabolism in COPD patients compared to healthy controls [Citation32]. Therefore, prevention of lower limb muscle dysfunction and its impact on health related outcomes was the main focus of recent research, mainly in pulmonary rehabilitation settings [Citation2].
In healthy elderly, an association between HGS and FEV1 was demonstrated [Citation33]. The authors hypothesized that HGS and FEV1 may be identically affected by factors such as systemic inflammation and hormonal changes [Citation33], which has been confirmed earlier in obesity [Citation34]. However, publications addressing this topic are not available in COPD patients. Our findings suggest that objective markers of COPD such as airway obstruction, hypoxia and exacerbation history seem not to lead to structural changes of the upper limb muscle. In more detail, patients with a rapid decline in lung function did not show an enhanced weakness of HGS. However, a subjective worsening of COPD symptoms assessed by change in CAT score, showed an independent association with decline in HGS over time. Therefore, the significant annual decline in HGS may rather be the result of less muscle use due to increased symptoms than the consequence of functional muscle impairment caused by structural changes. This hypothesis is supported by the current finding that patients with lower daily PA showed a more pronounced decline in HGS over time than patients with higher activity levels. A previous large-scaled cross-sectional study comparing HGS in COPD patients and healthy controls found no differences between the two groups [Citation7], supporting our findings and leading to the conclusion that there seems to be no direct link between physiological disease characteristics and HGS. However, to verify the postulated difference in functional changes between upper and lower limb strength in COPD, a longitudinal study assessing both muscle groups would be required.
Previous studies reported significant independent associations of two- and four-year-mortality with cross-sectional measurements of HGS in patients with COPD [Citation4,Citation5]. The authors proposed in their investigation to use the threshold of 10% predicted as a cutoff to classify patients as having HGS weakness and accordingly being exposed to an increased risk of mortality [Citation5]. In contrast, this association could not be confirmed in a trial cross-sectionally investigating 405 COPD patients during a follow-up of five years [Citation10]. In line with their finding, we did not show a steeper decline in HGS in patients with shorter survival time, although our cohort was comparable regarding HGS weakness and airflow obstruction to the patients investigated by Burtin et al. [Citation5]. Since the association between mortality and HGS was only evident in patients with very severe HGS impairment (<10% of the predicted value) it seems not to be useful as a valuable predictive marker of mortality risk.
This study has some limitations. The sample became small due to the long follow-up sequences (i.e. four to six years). However, mixed effect models were applied to address this issue. Furthermore, some patients may have undergone pulmonary rehabilitation and therefore have presented an attenuated decline in HGS.
Conclusion
Although HGS significantly decreased over time, no pathophysiological link with COPD disease progression could be demonstrated. Previous cross-sectional associations between HGS and mortality could not be confirmed in this longitudinal setting. Our data suggests that repeated monitoring of HGS in clinical settings seems not to be helpful to predict COPD specific disease progression.
Conflict of interest
M. Kohler reports personal fees from Bayer, personal fees from Astra Zeneca, personal fees from Boehringer Ingelheim, personal fees from Novartis, personal fees from Roche, personal fees from CSL Behring, personal fees from Mundipharma, outside the submitted work.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Butcher SJ, Pikaluk BJ, Chura RL, et al. Associations between isokinetic muscle strength, high-level functional performance, and physiological parameters in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2012;7:537–542. [Database] doi:10.2147/COPD.S34170.
- McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;2:Cd003793.
- Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–62. doi:10.1164/rccm.201402-0373ST.
- Puhan MA, Siebeling L, Zoller M, et al. Simple functional performance tests and mortality in COPD. Eur Respir J. 2013;42(4):956–963. doi:10.1183/09031936.00131612.
- Burtin C, Ter Riet G, Puhan MA, et al. Handgrip weakness and mortality risk in COPD: a multicentre analysis. Thorax 2016;71(1):86–87. doi:10.1136/thoraxjnl-2015-207451.
- Felipe C, Bartolome C, Miguel D, et al. Longitudinal changes in handgrip strength, hyperinflation, and 6-minute walk distance in patients with COPD and a control group. Chest 2015;148(4):986–994. doi:10.1378/chest.14-2878.
- Jeong M, Kang HK, Song P, et al. Hand grip strength in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:2385–2390. doi:10.2147/COPD.S140915.
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa021322.
- Vaidya T, Chambellan A, de Bisschop C. Sit-to-stand tests for COPD: a literature review. Respir Med. 2017;128:70–77. doi:10.1016/j.rmed.2017.05.003.
- Crook S, Frei A, Ter Riet G, et al. Prediction of long-term clinical outcomes using simple functional exercise performance tests in patients with COPD: a 5-year prospective cohort study. Respir Res. 2017;18(1):112. doi:10.1186/s12931-017-0598-6.
- Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015;386(9990):266–273. doi:10.1016/S0140-6736(14)62000-6.
- Vaidya T, Beaumont M, de Bisschop C, et al. Determining the minimally important difference in quadriceps strength in individuals with COPD using a fixed dynamometer. Int J Chron Obstruct Pulmon Dis. 2018; 13:2685–2693. doi:10.2147/COPD.S161342.
- Lopez-Lopez L, Torres-Sanchez I, Romero-Fernandez R, et al. Impact of previous physical activity levels on symptomatology, functionality, and strength during an acute exacerbation in COPD patients. Healthcare (Basel) 2018;6(4):139.
- Xavier RF, Pereira A, Lopes AC, et al. Identification of phenotypes in people with copd: influence of physical activity, sedentary behaviour, body composition and skeletal muscle strength. Lung 2018;197:37–45. doi:10.1007/s00408-018-0177-8.
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2018. Report). 2018.
- Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. doi:10.1093/ageing/afr051.
- Bohannon RW. Minimal clinically important difference for grip strength: a systematic review. J Phys Ther Sci. 2019;31(1):75–78. doi:10.1589/jpts.31.75.
- Spruit MA, Sillen MJH, Groenen MTJ, et al. New normative values for handgrip strength: results from the UK Biobank. J Am Med Dir Assoc. 2013;14(10):775.e5–775.e11. doi:10.1016/j.jamda.2013.06.013.
- A. T. S. Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166(1):111–117.
- Crook S, Busching G, Schultz K, et al. A multicentre validation of the 1-min sit-to-stand test in patients with COPD. Eur Respir J. 2017;49(3):1601871. doi:10.1183/13993003.01871-2016.
- Radtke T, Hebestreit H, Puhan MA, et al. The 1-min sit-to-stand test in cystic fibrosis - Insights into cardiorespiratory responses. J Cyst Fibros. 2017;16(6):744–751. doi:10.1016/j.jcf.2017.01.012.
- Strassmann A, Steurer-Stey C, Lana KD, et al. Population-based reference values for the 1-min sit-to-stand test. Int J Public Health. 2013;58(6):949–953. doi:10.1007/s00038-013-0504-z.
- Radtke T, Puhan MA, Hebestreit H, et al. The 1-min sit-to-stand test–A simple functional capacity test in cystic fibrosis?. J Cyst Fibros. 2016;15(2):223–226. doi:10.1016/j.jcf.2015.08.006.
- Van Remoortel H, Raste Y, Louvaris Z, et al. Validity of six activity monitors in chronic obstructive pulmonary disease: a comparison with indirect calorimetry. PLoS One. 2012;7(6):e39198. doi:10.1371/journal.pone.0039198.
- Watz H, Pitta F, Rochester CL, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–1537. doi:10.1183/09031936.00046814.
- Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi:10.1183/09031936.05.00035005.
- Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–735. doi:10.1183/09031936.05.00034905.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805.
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509.
- World Health Organization. International statistical classification of diseases and related health problem, 10th revision. 5th ed. Geneva: World Health Organization; 2015.
- Sievi NA, Senn O, Brack T, et al. Impact of comorbidities on physical activity in COPD. Respirology 2015;20(3):413–418. doi:10.1111/resp.12456.
- Donaldson AV, Maddocks M, Martolini D, et al. Muscle function in COPD: a complex interplay. Int J Chron Obstruct Pulmon Dis. 2012;7:523–535. doi:10.2147/COPD.S28247.
- Sillanpää E, Stenroth L, Bijlsma AY, et al. Associations between muscle strength, spirometric pulmonary function and mobility in healthy older adults. AGE 2014;36(4):9667. doi:10.1007/s11357-014-9667-7.
- Stenholm S, Rantanen T, Heliovaara M, et al. The mediating role of C-reactive protein and handgrip strength between obesity and walking limitation. J Am Geriatr Soc. 2008;56(3):462–469. doi:10.1111/j.1532-5415.2007.01567.x.