Abstract
The frequency, characteristics and outcomes of acute myocardial infarction (AMI) during exacerbation of chronic obstructive pulmonary disease (COPD) are unknown. Adult patients hospitalized with a principle diagnosis of acute COPD exacerbation were identified using retrospective analysis of the Nationwide Inpatient Sample (NIS) from 2003 to 2016. Patients were stratified into 2-groups with and without a secondary diagnosis of AMI. The study’s endpoints were in-hospital morbidity, mortality, and resource utilization. We also assessed the impact of invasive management strategy on the same end-points. We included 6 894 712 hospitalizations, of which 56 515 (0.82%) were complicated with AMIs. Patients with AMI were older, and had higher prevalence of known coronary disease (48.9% vs. 27.4%), atrial fibrillation (23.3% vs. 15.2%), heart failure (47.8% vs. 26.2%), and anemia (20.7% vs. 14.8%) (p < 0.001). Rates of oxygen dependence were similar (16.3% vs. 16.1%, p = 0.24). In 56 486 propensity-matched pairs of patients with and without AMI, mortality was higher in the AMI group (12.1% vs. 2.1%, p < 0.001). Rates of major morbidities, non-home discharge, and cost were all higher in the AMI group. A minority (18.1%) of patients with AMI underwent invasive assessment, and those had lower in-hospital mortality before (4.9% vs. 13.8%) and after (5.0% vs. 10.0%) propensity-score matching (p < 0.001). This lower mortality persisted in a sensitivity analysis accounting for immortal time bias. AMI complicates ∼1% of patients admitted with acute COPD exacerbation, and those have worse outcomes than those without AMI. Invasive management for secondary AMI during acute COPD exacerbation may be associated with improved outcomes but is utilized in <20% of patients.
Introduction
Coronary artery disease (CAD) is a common cause of morbidity and mortality in patients with chronic obstructive pulmonary disease (COPD) [Citation1]. Indeed, cardiovascular disease account for ∼30% of death among COPD patients [Citation2]. The care of CAD among patients with COPD is often challenging due to its atypical presentation. For example, COPD patients with acute coronary syndrome have less frequent typical angina, and lower level of diagnostic cardiac biomarkers [Citation1,Citation3–5]. Hence, investigating the interrelation between CAD and COPD in various setting is key to optimize clinical outcomes. It has recently become more apparent that an acute COPD exacerbation leads to an increased risk of acute myocardial infarction (AMI), by nearly fourfold, within the first 30 days and remains heightened up to one year [Citation6,Citation7]. Prior studies highlight the overlapping period for these co-conditions to exist, yet there remain few details regarding the cardiac management strategies of these co-morbid patients. Several studies have assessed the impact of COPD on the management and the short- and long-term outcomes of AMI [Citation1,Citation3,Citation8–11]. These studies consistently showed worse AMI outcomes in patients with COPD compared with those without COPD. They also documented a higher tendency to avoid invasive management in COPD patients. However, these studies examined the impact of COPD among patients admitted with a primary diagnosis of AMI, and regardless of the COPD status (stable vs. acute exacerbation). Studies investigating the impact of secondary AMI among patients admitted with acute COPD exacerbation are lacking. To augment this gap in clinical knowledge, we sought to assess the frequency of AMI during hospitalization for acute COPD exacerbations, determine predictors of patients receiving invasive coronary management, and estimate the morbidity, mortality, and health care utilization among those patients hospitalized with a primary COPD exacerbation complicated by AMI.
To do this, we utilized a nationwide database to assess the incidence, management patterns, and outcomes.
Methods
Study data
The Nationwide Inpatient Sample (NIS) was used to derive patient relevant information between January 1st, 2003 and December 31st, 2016. The NIS, part of the Healthcare Cost and Utilization Project (HCUP) databases, is the largest publicly available all-payer claim based database in the United States. The NIS contains hospital inpatient stays derived from billing data submitted by hospitals to statewide data organizations across the U.S. Researchers and policymakers use the NIS to make national estimates of health care utilization, access, charges, quality, and outcomes. The NIS sampling frame includes data from 47 statewide organizations, covering >97% of the U.S. population. The annual sample encompasses ∼8 million discharges, which represents 20% of inpatient hospitalizations across different hospital types and geographic regions. The national estimates of the entire hospitalized population are calculated using a standardized sampling and weighting method provided by HCUP [Citation12]. The Institutional Review Board exempted the study from board approval and waived the requirement for informed consent because the NIS is a publicly available de-identified database.
Study population
Adult patients (≥18 years) who were admitted with a principle diagnosis of acute COPD exacerbation were identified using International Classification of Diseases-9th and 10th Revision-Clinical Modification codes (491.21, J44.1, J44.0) with inclusion of exacerbation of bronchiectasis (J47.0, J47.1). Patients were then further classified into 2 groups; those with AMI (COPD-AMI group), and those without AMI (COPD- no-AMI group) based on ICD-9-CM and ICD-10-CM codes (Supplementary material, Table 1).
Study Outcomes: Our study investigated the following endpoints: (1) Incidence of AMI among patients admitted with acute COPD exacerbation, (2) In-hospital morbidity, mortality, and resource utilization among acute COPD exacerbation patients with and without AMI, (3) The frequency and predictors of undergoing invasive coronary assessment among AMI patients, and the impact of this invasive strategy on in-hospital outcomes.
Statistical analysis
Weighted data were used for all statistical analyses. For trend analysis, we used Cochrane-Armitage test for categorical variables and linear regression for continuous variables. The trend weight files were merged onto the original NIS files by Year and HOSPID. For years prior to 2012, the trend weight (TRENDWT) was used to create national estimated for trend analysis. For 2012 and after, no trend weight is needed, and the regular discharge weight (DISCWT) was used consistent with the redesigned NIS trend analysis [Citation12]. Descriptive statistics were presented as frequencies with percentages for categorical variables. Mean and standard deviation were used to report continuous measures.
To account for potential confounding factors and reduce the effect of selection bias, multivariable logistic regression derived propensity scores were matched 1:1 to attain comparable groups of AMI vs no-AMI with a caliper of 0.01 to ensure perfect matching. Same approach was also performed to obtain comparable groups of invasive vs noninvasive groups in the AMI cohort. Matched categorical variables were presented as frequencies with percentages and compared using McNamara’s test. Matched continuous variables were presented as means with standard deviations and compared using a paired-samples t-test. Predictors of undergoing invasive coronary assessment among AMI patients were assessed in univariate logistic regression analysis. Those with a p value of <0.1 were then further assessed in a multivariate logistic regression analysis. Variables included in the regression models included demographics (age, sex, race), socioeconomic factors (primary expected payer, median household income), and clinically relevant comorbidities.
To account for immortal time bias among patients who underwent invasive strategy and those who did not survive long enough to undergo the invasive strategy, we constructed a Cox regression model using the invasive procedure day as a time-dependent covariate for the primary end-point of death [Citation13,Citation14]. Methodological standards in research using the national-inpatient-sample were met as recommended [Citation15]. A Type-I error of <0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 24 (IBM Corporation, Armonk, NY).
Results
A total of 6 ,894 ,712 hospitalizations with a primary diagnosis of acute COPD exacerbation were identified in the NIS between 2003 and 2016 (Supplementary material, Figure 1). Of those, 56,,515 (0.82%) suffered an AMI during the hospitalization. There was a temporal increase in the incidence of AMI in this population from 0.74% in 2003 to 1.05% in 2016 (Ptrend < 0.001) (, Supplementary material, Table 5). The majority of AMIs were NSTEMIs (86.3%) vs. STEMIs (13.7%). Compared with those without AMI, patients who had AMI were older (73 ± 10 vs. 69 ± 12 years, p < 0.001), but less likely to be White (83.2% vs. 81.3%), or females (53.9% vs. 55.8%, p < 0.001). Patients who suffered AMI had a distinctive risk profile characterized by the higher prevalence of CAD (48.9% vs. 27.4%), atrial fibrillation (23.3% vs. 15.2%), congestive heart failure (47.8% vs. 26.2%), and anemia (20.7% vs. 14.8%) (p < 0.001 for all). They also had higher rates of non-coronary atherosclerosis, and chronic kidney and liver disease (). However, both patients with or without AMI had similar prevalence of severe COPD (i.e. home oxygen dependence; 16.3% vs. 16.1%, p = 0.24).
Figure 1. Temporal trends in AMI among patients with acute COPD exacerbation in the United States between 2003 and 2016. AMI; acute myocardial infarction, COPD; chronic obstructive pulmonary disease.
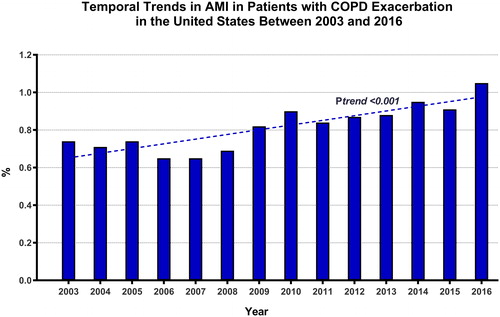
Table 1. Baseline characteristics of the study cohort.
Unmatched Outcomes: The utilization of invasive coronary assessment ± revascularization was low in the AMI group, with only 18.1% of patients undergoing coronary angiography and <5% of patients undergoing percutaneous (4.1%) or surgical (0.3%) revascularization (). Patients with AMI had an 8-fold increase in in-hospital mortality compared with those without AMI (12.2% vs. 1.5%, p < 0.001). Patients with AMI also had higher rates of major complications and non-home discharge, longer hospitalizations and higher total cost ().
Table 2. Outcomes of patients admitted with acute COPD exacerbation stratified by the occurrence of acute myocardial infarction.
Propensity-Matched Outcomes: after propensity score matching (PSM), 56 ,486 pairs of COPD patients with and without AMI were included in the comparative analysis. Baseline characteristics after PSM were well balanced between the two groups. In-hospital mortality remained substantially higher in the AMI group (12.1% vs. 2.1%, p < 0.001). Rates of stroke, acute kidney injury, new dialysis requirement, vascular complications, blood transfusion, invasive and noninvasive mechanical ventilation, prolonged ventilation, tracheostomy, and gastrostomy were all significantly higher in the AMI group (). Patients who suffered an AMI during an admission for acute COPD exacerbation had 100% increase in the rate of non-home discharge, 50% increase in length of stay, and accrued a 100% higher cost compared with PSM patients who did not have an AMI.
Outcomes of Invasive vs. Noninvasive Management Strategy: The minority of patients with AMI (18.1%) underwent an invasive management strategy, but with a slight upward trend in the utilization of invasive angiography over time (ptrend −0.003) (Supplementary material, Figure 2). Compared with those who managed noninvasively, patients who underwent invasive management were younger, less likely to be of White race, and to have Medicare/Medicaid insurance. They were also more likely to have prior coronary artery disease (71.1% vs. 43.9%), but less likely to have congestive heart failure or another chronic organ disease (p < 0.001 for all) (Supplementary material, Table 2). The type of MI also differed between those undergoing invasive assessment vs. not, with STEMI being less frequent in the former group (7.0% vs. 15.2%, p < 0.001). In-hospital mortality was lower in patients who underwent an invasive assessment strategy (4.9% vs. 13.8%, p < 0.001). The remainder of unmatched outcomes between patients who underwent invasive vs. noninvasive management strategy are listed in .
Table 3. Outcomes of patients admitted with AMI stratified by management strategy.
To account for differences in baseline characteristics, PSM identified 9,991 well-balanced pairs of AMI patients with or without subsequent invasive assessment (Supplementary material, Table 3). In this PSM cohort, patients who underwent invasive assessment ± coronary revascularization have significantly lower in-hospital mortality (5.0% vs. 10.0%, p < 0.001). This observed reduction in mortality in the invasive strategy group remained significant after accounting for potential immortal time bias using Cox regression model with a time-dependent (Hazard Ratio 0.31; 95% C.I [confidence interval] 0.196–0.505) (Supplementary material, Table 4). In-hospital morbidities were similar in both groups, with the exception of vascular complications, which were more common in the invasive group, and prolonged ventilation, which was more frequent in the noninvasive group (). Patients in the invasive group had longer and costlier hospitalizations, but were more likely to be discharged home (vs. to an intermediate care facility) compared with those in the noninvasive strategy group.
Table 4. Univariate and multivariate logistic regression analysis for predictors of undergoing invasive coronary assessment strategy.
Predictors of Undergoing and Invasive Coronary Assessment Strategy: In a multi-logistic regression analysis, the strongest predictors of undergoing an invasive strategy were known CAD (Odds Ratio [OR] 3.45, 95% CI 3.27–3.63), cardiogenic shock (OR 1.84, 95% CI 1.52–2.23), and carotid artery disease (OR 1.72, 95% CI 1.46–2.03). Patients hospitalized at rural or non-teaching hospitals, those with liver cirrhosis, were significantly less likely to undergo an invasive strategy. Other positive and negative predictors of undergoing an invasive strategy are listed in .
Discussion
The major findings of this study are: (1) AMI complicates a minority (∼0.8%) of hospitalization for acute COPD exacerbation, (2) AMI during an acute COPD exacerbation is associated with a substantial increase in morbidity, mortality, and cost, (3) less than 1 in 5 patients with AMI in the setting of acute COPD exacerbation undergo an invasive coronary management strategy. Patients who underwent an invasive strategy had superior outcomes to those who underwent a noninvasive management strategy.
Data on in-hospital incidence of AMI during acute COPD exacerbation are scarce. In a study of 242 patients with acute COPD exacerbation, 8% fulfilled the 2007 universal definition of AMI [Citation5]. Other studies reported the prevalence of COPD among patients admitted with an AMI but not vice versa [Citation1,Citation3,Citation8–11]. To our knowledge, this is the first nationwide assessment of the incidence and outcomes of AMI among patients hospitalized with acute COPD exacerbation. We found an incidence of 0.82% overall but a temporal increase from 0.74% in 2003 to 1.05% in 2016. Moreover, this reported incidence corresponds to the occurrence of an AMI during the same hospitalization as the acute COPD exacerbation. This should be underscored as there is an increased risk of AMI within the first 30 days following an acute COPD exacerbation and continues on for one year [Citation6,Citation7]. This means the reported incidence of AMI after acute COPD exacerbation is likely under represented by only including AMI during the same hospitalization and the true incidence is likely higher than reported here within for those with AMI occurring one year from COPD exacerbation onset. Additionally, this is lower than what was reported by McAllister et al. This is likely because contrary to the routine AMI survey implemented by McAllister et al. the diagnosis of AMI in our study was made in selected patients who were ‘tested’ for AMI. It is likely that the true incidence of AMI would have been higher, had a systematic survey with cardiac biomarkers and serial electrocardiograms been applied in all patients. The majority of AMI in our cohort were NSTEMIs vs. STEMIs. The evolving definition of AMI, its classification, and the lack of laboratory and electrocardiographic data in this study make it difficult to discern the exact mechanism of AMI. However, it is plausible that most of these patients suffered a type-II AMI (demand ischemia) in the setting of acute respiratory distress. Indeed, even STEMI can often be related to demand ischemia in patients with acute COPD exacerbation, due to severe tachycardia leading to exacerbation of underlying coronary disease, or rate-related ST-elevations in patients with underlying bundle branch block, cardiomyopathy, or ventricular hypertrophy [Citation16].
We found a substantial negative impact of COPD exacerbation-associated AMI. One in 12 patients who developed an AMI in this context died in the hospital, a 6-fold higher rate than propensity matched patients without AMI. These concerning findings deserve further scrutiny: the magnitude of the excess mortality in the COPD group is much higher than what was previously reported [Citation1,Citation3,Citation8–11]. However, prior studies compared AMI outcomes between COPD patients and non-COPD patients regardless of COPD acuity (patients with primary diagnosis with AMI with and without COPD of any severity and acuity). Our study provides unique insights into the outcomes of AMI complicating hospitalizations for a primary diagnosis of acute COPD exacerbation. This study also documented significantly higher rates of renal, neurological, and pulmonary complications, and higher resource utilization in the AMI cohort, highlighting the need for further studies to understand the etiology, predictors, and optimal management strategy of AMI in these patients.
For comparison to patients with a COPD exacerbation complicated by AMI in this study, we evaluated the literature in patients hospitalized for community- acquired pneumonia (CAP) or an asthma exacerbation to compare the incidence, outcomes, and intervention rates for those who develop an AMI. In patients hospitalized for CAP, the incidence of AMI is 2.4–5.3% (previous reports 0.8–11%) with 11.7 fold increase compared to the general population risk for developing AMI [Citation17–19]. Little is reported surrounding the timing of AMI complicating CAP, but a single study links the occurrence to a few days following admission to the hospital [Citation19]. Furthermore, the development of AMI when hospitalized for CAP led to a longer hospitalization (10 days) and portends a poorer prognosis with an adjusted odds ratio for in-hospital mortality of 3.57 (p = 0.012) [Citation20]. Raita et al. evaluated AMI in patients hospitalized for an acute asthma exacerbation and found a significantly increased risk during the first 7 days following admission (adjusted incidence rate ratio 5.75, p < 0.001) [Citation21]. Moreover, there appears to be a lack of published reports on the incidence and outcomes of patients hospitalized with CAP. Despite the prevalence of hospitalizations for CAP and asthma, there remains a paucity of data surrounding patients with AMI and whether they undergo an invasive versus ischemic coronary management strategy.
The worse outcomes in this cohort are likely multifactorial, but can possibly be related to: (A) the worse risk profile of patients who develop AMI (a non-modifiable factor); (B) the delay in the recognition of AMI due to the confounding effect of the respiratory distress accompanying acute COPD exacerbations (a modifiable factor); or (C) the tendency to withhold invasive coronary assessment ± revascularization compared with AMI patients without an acute COPD exacerbation (a modifiable factor). Although the former reason is plausible, the excess morbidity and mortality associated with AMI appears to be out of proportion to the difference in baseline characteristics. Furthermore, the higher morbidity and mortality persisted after rigorous PSM accounting for demographic, clinical risk factors, and hospital characteristics.
The delay in recognition of AMI in COPD patients has been documented in several studies [Citation1,Citation4,Citation22–24]. These studies also found significant disparities in the provision of invasive AMI care strategy among COPD patients. We similarly found that coronary angiography was undertaken in <20% of patients. Although these angiography rates are substantially lower than the reported rates of invasive assessment in patients presenting with a primary diagnosis of AMI [Citation25], they are comparable to those of patients admitted with a non-cardiac acute illness [e.g. stroke, motor vehicle accident, diabetic ketoacidosis], and secondary AMI [Citation26–28]. Our logistic regression analysis identified several negative predictors of undergoing an invasive strategy. Those included markers of worse clinical risk profile (chronic renal insufficiency, liver cirrhosis, prior stroke, and anemia), and factors suggestive of disparity in care (Non-White race, weekend admission, rural and non-teaching hospital), and factors that may confound the recognition of AMI (diabetes, heart failure).
Another intriguing relevant finding is that only ∼5% of patients underwent coronary revascularization (1 in 4 patients who underwent angiography). Whether this reflects a second layer of disparity in pursuing revascularization following coronary angiography or the absence of amenable coronary targets for revascularization remain unknown given the lack of angiographic information in this dataset. Additional studies are needed to further assess this issue especially with the strong association detected in our study between an invasive coronary strategy and improved outcomes. Even after PSM, patients who underwent an invasive strategy had significantly lower in-hospital mortality compared with those who did not (5.0% vs. 10.0%, p < 0.001). In theory, this finding is susceptible to immortal time bias (patients did not survive long enough to undergo the procedure), the lower mortality in the invasive strategy group was consistent in a sensitivity analysis accounting for immortal time bias.
Our study has a number of limitations. (1) The NIS is a claim-based database that collects data based on ICD-CM codes mainly for billing and quality improvement purposes. Hence, errors related to under-, over-, or erroneous coding might hamper the results of these data. Nonetheless, we limited our cohort to patients with a principle diagnosis of acute COPD exacerbation (not any patients with COPD). Both these codes and codes used to identify AMI have been found to have excellent positive predictive value [Citation29]. Similarly, ICD-9-CM codes used to code for invasive coronary angiography and PCI have been found to have excellent sensitivity and specificity [Citation30,Citation31]. (2) The NIS lacks granular data on the severity of the underlying lung disease (e.g. forced expiratory volumes, extent of emphysema, etc.), the severity of COPD exacerbation, intensity of COPD treatment, laboratory variables, ECG and the angiographic findings among patients who underwent coronary angiography. It also cannot place when certain complications happened, if there were present on admission or developed in the course of the hospital stay or as a complication of a procedure. However, using the influence of a potentially unmeasured confounder, it is unlikely that inclusion of such factors in our analyses would significantly change our primary results. Furthermore, this database is reflective of real-world practice and avoids biases from data originating from a few specialized centers. Hence, our findings have to be interpreted in the context of this limitation. (3) Long-term data beyond hospital discharge are not available in the NIS. Whether the differential impact of AMI on clinical outcomes persisted or accentuated after discharge, of whether patients who were managed conservatively during the index admission underwent subsequent coronary angiography ± revascularization remain unknown. Despite these limitations, this large nationwide study provides important insights into a concerning entity in a vulnerable population, and call for further investigations to address the highly prevalent coronary artery disease in these patients.
Conclusions
Secondary AMI is rare among patients admitted with an acute COPD exacerbation. However, it is associated with increased morbidity, mortality, and cost. Invasive management may be associated with improved outcomes but is utilized in a minority of patients.
Supplemental Material
Download Zip (556.6 KB)Disclosure statement
None.
Funding
None.
References
- Rothnie KJ, Quint JK. Chronic obstructive pulmonary disease and acute myocardial infarction: effects on presentation, management, and outcomes. Eur Heart J Qual Care Clin Outcomes. 2016;2(2):81–90. doi:10.1093/ehjqcco/qcw005.
- McGarvey LP, John M, Anderson JA, et al. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62(5):411–415. doi:10.1136/thx.2006.072348.
- Enriquez JR, de Lemos JA, Parikh SV, et al. Association of chronic lung disease with treatments and outcomes patients with acute myocardial infarction. Am Heart J. 2013;165(1):43–49. doi:10.1016/j.ahj.2012.09.010.
- Hadi HA, Zubaid M, Al Mahmeed W, et al. Prevalence and prognosis of chronic obstructive pulmonary disease among 8167 Middle Eastern patients with acute coronary syndrome. Clin Cardiol. 2010;33(4):228–235. doi:10.1002/clc.20751.
- McAllister DA, Maclay JD, Mills NL, et al. Diagnosis of myocardial infarction following hospitalisation for exacerbation of COPD. Eur Respir J. 2012;39(5):1097–1103. doi:10.1183/09031936.00124811.
- Reilev M, Pottegard A, Lykkegaard J, et al. Increased risk of major adverse cardiac events following the onset of acute exacerbations of COPD. Respirology. 2019;24(12):1183–1190. doi:10.1111/resp.13620.
- Mkorombindo T, Dransfield MT. COPD exacerbations and MACE: more than a chance encounter? Respirology. 2019;24(12):1129–1130. doi:10.1111/resp.13683.
- Desai R, Patel U, Singh S, et al. The burden and impact of arrhythmia in chronic obstructive pulmonary disease: insights from the National Inpatient Sample. Int J Cardiol. 2019;281:49–55. doi:10.1016/j.ijcard.2019.01.074.
- Su TH, Chang SH, Chen PC, et al. Temporal trends in treatment and outcomes of acute myocardial infarction in patients with chronic obstructive pulmonary disease: a nationwide population-based observational study. J Am Heart Assoc. 2017;6(3).
- Andell P, Koul S, Martinsson A, et al. Impact of chronic obstructive pulmonary disease on morbidity and mortality after myocardial infarction. Open Heart. 2014;1(1):e000002. doi:10.1136/openhrt-2013-000002.
- Stefan MS, Bannuru RR, Lessard D, et al. The impact of COPD on management and outcomes of patients hospitalized with acute myocardial infarction: a 10-year retrospective observational study. Chest. 2012;141(6):1441–1448. doi:10.1378/chest.11-2032.
- Houchens RL, Rd, Elixhauser A. Using the HCUP national inpatient sample to estimate trends. HCUP Methods Series Report. 2016.
- Shariff SZ, Cuerden MS, Jain AK, et al. The secret of immortal time bias in epidemiologic studies. JASN. 2008;19(5):841–843. doi:10.1681/ASN.2007121354.
- Alkhouli M, Zack CJ, Zhao H, et al. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation versus anticoagulation alone in the treatment of inferior vena caval thrombosis. Circ Cardiovasc Interv. 2015;8(2):e001882. doi:10.1161/CIRCINTERVENTIONS.114.001882.
- Khera R, Angraal S, Couch T, et al. Adherence to methodological standards in research using the national inpatient sample. JAMA. 2017;318(20):2011–2018. doi:10.1001/jama.2017.17653.
- Deshpande A, Birnbaum Y. ST-segment elevation: distinguishing ST elevation myocardial infarction from ST elevation secondary to nonischemic etiologies. WJC. 2014;6(10):1067–1079. doi:10.4330/wjc.v6.i10.1067.
- Nieto Dominguez A, Furmanek S, Ramirez J. 2195. Incidence of acute myocardial infarction in patients with community-acquired pneumonia: a systematic review and meta-analysis. Open Forum Infect Dis. 2019;6(Supplement_2):S747–S748. doi:10.1093/ofid/ofz360.1875.
- Violi F, Cangemi R, Falcone M, et al. Cardiovascular Complications and Short-term Mortality Risk in Community-Acquired Pneumonia. Clin Infect Dis. 2017;64(11):1486–1493. doi:10.1093/cid/cix164.
- Corrales-Medina VF, Suh KN, Rose G, et al. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med. 2011;8(6):e1001048. doi:10.1371/journal.pmed.1001048.
- Aliberti S, Ramirez J, Cosentini R, et al. Acute myocardial infarction versus other cardiovascular events in community-acquired pneumonia. ERJ Open Res. 2015;1(1):00020-2015. doi:10.1183/23120541.00020-2015.
- Raita Y, Camargo CA, Jr., Faridi MK, et al. Risk of acute myocardial infarction and ischemic stroke in patients with asthma exacerbation: a population-based, self-controlled case series study. J Allergy Clin Immunol Pract. 2020;8(1):188–194. doi:10.1016/j.jaip.2019.06.043.
- Rothnie KJ, Smeeth L, Herrett E, et al. Closing the mortality gap after a myocardial infarction in people with and without chronic obstructive pulmonary disease. Heart. 2015;101(14):1103–1110. doi:10.1136/heartjnl-2014-307251.
- Dai X, Bumgarner J, Spangler A, et al. Acute ST-elevation myocardial infarction in patients hospitalized for noncardiac conditions. J Am Heart Assoc. 2013;2(2):e000004.
- Campo G, Guastaroba P, Marzocchi A, et al. Impact of COPD on long-term outcome after ST-segment elevation myocardial infarction receiving primary percutaneous coronary intervention. Chest. 2013;144(3):750–757. doi:10.1378/chest.12-2313.
- Malta Hansen C, Wang TY, Chen AY, et al. Contemporary patterns of early coronary angiography use in patients with non-ST-segment elevation myocardial infarction in the United States: insights from the national cardiovascular data registry acute coronary treatment and intervention outcomes network registry. JACC Cardiovasc Interv. 2018;11(4):369–380. doi:10.1016/j.jcin.2017.12.016.
- Alkhouli M, Alqahtani F. Incidence and outcomes of acute myocardial infarction during motor vehicle accident related hospitalizations. Am J Cardiol. 2019;123(5):725–728. doi:10.1016/j.amjcard.2018.11.050.
- Issa M, Alqahtani F, Ziada KM, et al. Incidence and outcomes of non-ST elevation myocardial infarction in patients hospitalized with decompensated diabetes. Am J Cardiol. 2018;122(8):1297–1302. doi:10.1016/j.amjcard.2018.07.004.
- Alqahtani F, Aljohani S, Tarabishy A, et al. Incidence and outcomes of myocardial infarction in patients admitted with acute ischemic stroke. Stroke. 2017;48(11):2931–2938. doi:10.1161/STROKEAHA.117.018408.
- Ginde AA, Tsai CL, Blanc PG, et al. Positive predictive value of ICD-9-CM codes to detect acute exacerbation of COPD in the emergency department. Jt Comm J Qual Patient Saf. 2008;34(11):678–680. doi:10.1016/S1553-7250(08)34086-0.
- Epstein AJ, Polsky D, Yang F, et al. Coronary revascularization trends in the United States, 2001-2008. JAMA. 2011;305(17):1769–1776. doi:10.1001/jama.2011.551.
- Kiyota Y, Schneeweiss S, Glynn RJ, et al. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99–104. doi:10.1016/j.ahj.2004.02.013.
