Abstract
The American Thoracic Society guidelines recommend long-acting β2-agonist (LABA)/long-acting muscarinic antagonist (LAMA) dual bronchodilation over LAMA or LABA monotherapy as maintenance therapy for patients with chronic obstructive pulmonary disease suffering from dyspnea or exercise intolerance. Previous studies, which included patients receiving background inhaled corticosteroids (ICS), have shown the benefits of dual bronchodilation over monotherapy. This analysis aimed to confirm the benefits of LAMA/LABA over LAMA alone, without any confounding effects from ICS use. This pooled post hoc analysis compared the efficacy of tiotropium/olodaterol with tiotropium alone in patients from the TONADO® and OTEMTO® clinical trials who were not receiving ICS at study entry or during the studies. We analyzed change from baseline in trough forced expiratory volume in 1 s (FEV1), St. George’s Respiratory Questionnaire (SGRQ) score and Transition Dyspnea Index (TDI) score in all patients, by Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage, baseline SGRQ score, and Baseline Dyspnea Index score. In this analysis of 1596 patients, tiotropium/olodaterol improved trough FEV1, SGRQ and TDI compared with tiotropium alone. The observed mean differences were: trough FEV1, 0.054 L (95% confidence interval [CI] 0.036, 0.073; p < 0.001); SGRQ, −1.918 (95% CI −2.994, −0.843; p < 0.001); and TDI, 0.575 (95% CI 0.301, 0.848; p < 0.001). Similar improvements were seen in each of the subgroup analyses. Tiotropium/olodaterol therapy significantly improved lung function, symptoms and health status compared with tiotropium alone. In a population free from ICS treatment, these data confirm the benefits of dual bronchodilation versus monotherapy.
Plain language summary
It is recommended that most people with COPD begin treatment with two types of inhaled medicine. These medicines are known as LAMAs and LABAs, and they help to make breathing easier. Doctors can prescribe these treatments alone or together (LAMA/LABA). In the past, several studies have shown that combining LAMAs with LABAs is better than giving either treatment alone. However, many patients in these studies also received an inhaled steroid treatment in addition to their LAMA and/or LABA therapy, which can make it harder to interpret the study results. To help doctors decide whether combined LAMA/LABA treatment is better than single treatment, we looked at people with COPD who took LAMA or combined LAMA/LABA treatment – without inhaled steroids – as part of two large studies (TONADO® and OTEMTO®). We measured changes in how well the lungs worked, patients’ health status, or their ability to breathe. After 12 weeks, each of these outcomes was better in patients who received LAMA/LABA than in patients who received LAMA alone. These findings are similar to those from studies that included patients receiving steroids in addition to the other treatments. These results support recommendations to doctors to prescribe combined LAMA/LABA treatment to their patients with COPD, rather than LAMA alone.
A graphical abstract to accompany this manuscript is included in the online supplement.
Introduction
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy report recommends that patients be treated with long-acting β2-agonists (LABA) or long-acting muscarinic antagonists (LAMA) therapy as first-line treatment, with dual bronchodilation recommended for more symptomatic patients. Inhaled corticosteroids (ICS) should be reserved for patients with high eosinophil counts (>300 cells/µL) and with a history of frequent exacerbations (≥2 moderate exacerbations or >1 severe exacerbation in the previous year) [Citation1]. Furthermore, the American Thoracic Society (ATS) recommends dual bronchodilation in patients with COPD who experience dyspnea or exercise intolerance [Citation2]. The National Institute for Health and Care Excellence (NICE) recommends dual LAMA/LABA therapy in patients with COPD with no indication of asthmatic features or corticosteroid responsiveness who remain breathless or have exacerbations despite optimized non-pharmacologic management and use of short-acting bronchodilators [Citation3].
These recommendations are largely based on studies that included patients on a range of background therapies [Citation4–7], including ICS use in a proportion of patients [Citation4, Citation7]. Patients who are prescribed ICS differ from those not given these treatments and report more exacerbations of disease [Citation8], which can impact their health status. Hence, it cannot be presumed that treatments are equally effective in patients requiring and not requiring regular ICS.
To be confident that the guidance to use dual bronchodilation in many COPD patients is applicable to those naïve to ICS, we sought to determine if dual bronchodilation with LAMAs/LABAs is more effective than a single bronchodilator in patients with COPD unconfounded by ICS use. We performed a pooled post hoc analysis of patients with COPD from the TONADO® 1&2 [Citation4] and OTEMTO® 1&2 [Citation9] studies excluding around 44.5% of patients who were receiving ICS from the original trial populations who were randomized to receive tiotropium 5 µg or tiotropium/olodaterol 5/5 µg.
We assessed the impact of dual bronchodilation with tiotropium/olodaterol on lung function, health status and symptoms versus tiotropium monotherapy, using a range of endpoints including trough forced expiratory volume in 1 s (FEV1), St. George’s Respiratory Questionnaire (SGRQ) total score and Transition Dyspnea Index (TDI) focal score. We also evaluated whether the baseline status of these endpoints impacted patients’ responses to treatment.
Methods
The study designs and inclusion criteria of the 52-week TONADO 1&2 (NCT01431274 and NCT01431287) and 12-week OTEMTO 1&2 (NCT01964352 and NCT02006732) studies have previously been described [Citation4, Citation9]. These replicate, double-blind, parallel-group, active-controlled, multicenter, randomized, Phase III studies assessed patients with moderate-to-severe (OTEMTO) or moderate-to-very-severe (TONADO) COPD, with a focus on lung function, health status, and dyspnea severity [Citation4, Citation9]. While OTEMTO included placebo-controlled and comparator-controlled arms, TONADO did not include a placebo-controlled arm [Citation4, Citation9].
Patients
The current analysis includes patients with moderate-to-very-severe COPD (GOLD stage 1–4) from the TONADO and OTEMTO studies. At baseline, patients included in this analysis were receiving either no maintenance therapy, LABA monotherapy, LAMA monotherapy or dual LAMA/LABA bronchodilator therapy, and received either tiotropium monotherapy or tiotropium/olodaterol dual bronchodilation therapy during the TONADO and OTEMTO clinical trials. Patients who were on ICS were excluded from this analysis.
Treatments were administered once daily with the Respimat® inhaler (Boehringer Ingelheim, Ingelheim am Rhein, Germany). Rescue medication (salbutamol [albuterol]) was provided as required for all study participants.
The studies included in this post hoc analysis were previously approved by the review boards of the relevant national, regional or independent ethics committee or institutional review boards. These studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Patients in these studies provided written informed consent.
Study endpoints and assessments
Change from baseline to Week 12 in trough FEV1, SGRQ total score, and TDI focal score was calculated from pooled data for all patients and subsets of these patients.
A mixed-effects model for repeated measures (MMRM) was used to generate adjusted means of treatment effect, including the fixed effects of treatment, study, planned test day, treatment-by-test day interaction, baseline and baseline-by-test day interaction, plus the random effect of patient. The current analyses were conducted on the whole population, GOLD 2 and 3 subgroups, Baseline Dyspnea Index (BDI) ≤6 and >6 subgroups, and SGRQ < median and ≥ median subgroups. Median SGRQ scores were selected for this analysis to create reasonably sized subgroups.
Responder rates for these endpoints were also analyzed using a logistic regression model that included the covariates study and treatment. Responders for trough FEV1 (a change of >100 mL), SGRQ (a decrease of ≥4.0 points) and TDI (an increase of ≥1.0 point versus baseline at the time of analysis) were defined based on suggested minimum clinically important differences (MCID) [Citation4, Citation10–12]. All p-values included in this analysis are nominal.
Safety endpoints were assessed through the number of adverse events (AEs) reported in the trials for the pooled population.
Results
Baseline characteristics
Baseline characteristics were collected from 1596 patients with moderate-to-very-severe COPD (GOLD stage 2–4) who were not treated with ICS at baseline or during the study period. The majority of patients (92.4%) were GOLD stage 2 or 3 (). At baseline, 1078 patients (67.5%) were maintenance naïve (not receiving LAMA, LABA or ICS), 299 (18.7%) were receiving LAMA monotherapy, 127 patients (7.9%) were receiving LAMA/LABA therapy, and 92 (5.7%) were receiving LABA monotherapy. Post-randomization, 825 patients (51.7%) received tiotropium and 771 patients (48.3%) received tiotropium/olodaterol. Baseline characteristics were similar between patients randomized to tiotropium or tiotropium/olodaterol ().
Table 1. This is currently in the middle of the introduction - please could it be moved to the next page, closer to where it's first cited.
Efficacy at week 12
Trough FEV1
An increase of 0.141 ± 0.007 L in trough FEV1 from baseline to 12 weeks of treatment was noted for patients treated with tiotropium/olodaterol; with an increase of 0.086 ± 0.007 L for patients receiving tiotropium monotherapy. The adjusted mean difference between tiotropium/olodaterol and tiotropium was 0.054 ± 0.010 L (95% confidence interval [CI] 0.036, 0.073; p < 0.001) (), with similar results irrespective of baseline GOLD stage, SGRQ score or BDI score (). For GOLD 4 patients, not included in due to small sample size, an increase of 0.115 ± 0.016 L was noted for patients treated with tiotropium/olodaterol, with an increase of 0.057 ± 0.015 L for patients receiving tiotropium monotherapy.
Figure 1. (a) Change in trough FEV1 at Week 12 from baseline; all patients and by GOLD status. (b) Change in trough FEV1 at Week 12 from baseline; by baseline SGRQ and BDI status.
BDI, Baseline Dyspnea Index; CI, confidence interval; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SE, standard error; SGRQ, St. George’s Respiratory Questionnaire; T/O, tiotropium/olodaterol; Tio, tiotropium.
N numbers below bars, adjusted mean response within bars.
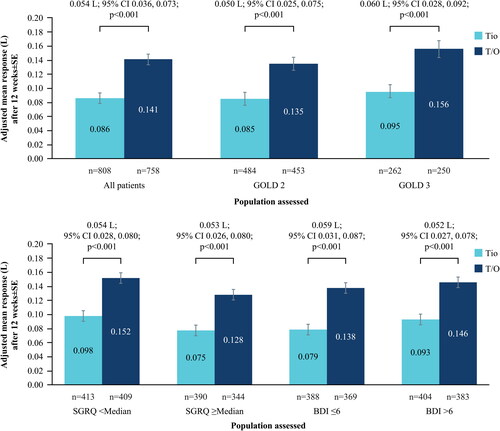
In terms of responders, of the 763 tiotropium/olodaterol-treated patients, 451 (59.1%) achieved the MCID of >100 mL change in trough FEV1; of the 818 tiotropium-treated patients, 342 (41.8%) achieved the MCID; odds ratio 2.012 (95% CI 1.647, 2.458; p < 0.001).
St. George’s Respiratory Questionnaire
A decrease (improvement) from baseline in SGRQ total score was noted after 12 weeks of treatment for patients treated with tiotropium/olodaterol (–6.317 ± 0.403 points) and tiotropium monotherapy (–4.399 ± 0.396 points). The adjusted mean difference between tiotropium/olodaterol and tiotropium was −1.918 ± 0.548 points (95% CI −2.994, −0.843; p < 0.001) (). Similar results were shown in the subgroup analysis for GOLD 2 patients () and when stratified by baseline median SGRQ (43.1 points) and baseline BDI status (p < 0.05) (). In the GOLD 3 subset of patients, the improvement in SGRQ score did not reach statistical significance (p = 0.054). For GOLD 4 patients, a change of −10.74 ± 1.756 points was noted for patients treated with tiotropium/olodaterol, with a change of −7.948 ± 1.692 points for patients receiving tiotropium monotherapy.
Figure 2. (a) Change in SGRQ at Week 12 from baseline; all patients and by GOLD status. (b) Change in SGRQ at Week 12 from baseline; by baseline SGRQ and BDI status.
BDI, Baseline Dyspnea Index; CI, confidence interval; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SE, standard error; SGRQ, St. George’s Respiratory Questionnaire; T/O, tiotropium/olodaterol; Tio, tiotropium.
N numbers below bars, adjusted mean response within bars.
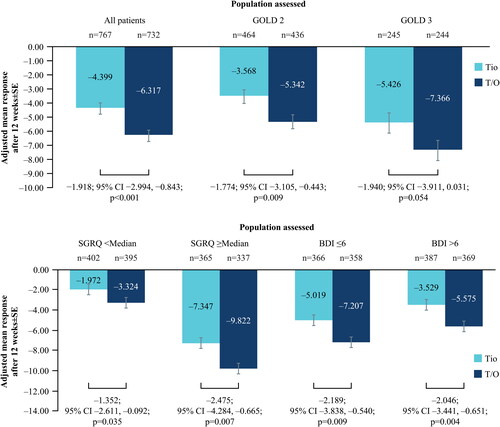
For the responder analysis, of the 734 tiotropium/olodaterol-treated patients, 424 (57.8%) achieved the MCID of ≥4.0-point decrease in SGRQ score; of the 772 tiotropium-treated patients, 377 (48.8%) achieved the MCID; odds ratio 1.439 (95% CI 1.173, 1.765; p < 0.001).
Transition Dyspnea Index
After 12 weeks of treatment, the mean TDI score improved by 2.154 ± 0.100 points for patients treated with tiotropium/olodaterol and 1.579 ± 0.098 points with tiotropium monotherapy. The improvement was significantly greater with tiotropium/olodaterol compared with tiotropium (treatment difference: 0.575 ± 0.140 points; 95% CI 0.301, 0.848; p < 0.001) (). Similar results were seen in the subgroup analyses by GOLD 2/3, SGRQ and BDI () (p < 0.05). For GOLD 4 patients, an increase of 2.378 ± 0.384 points was noted for patients treated with tiotropium/olodaterol, with an increase of 1.234 ± 0.371 points for patients receiving tiotropium monotherapy.
Figure 3. (a) Change in TDI at Week 12 from baseline; all patients and by GOLD status. (b) Change in TDI at Week 12 from baseline; by baseline SGRQ and BDI status.
BDI, Baseline Dyspnea Index; CI, confidence interval; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SE, standard error; SGRQ, St. George’s Respiratory Questionnaire; T/O, tiotropium/olodaterol; TDI, Transition Dyspnea Index; Tio, tiotropium.
N numbers below bars, adjusted mean response within bars.
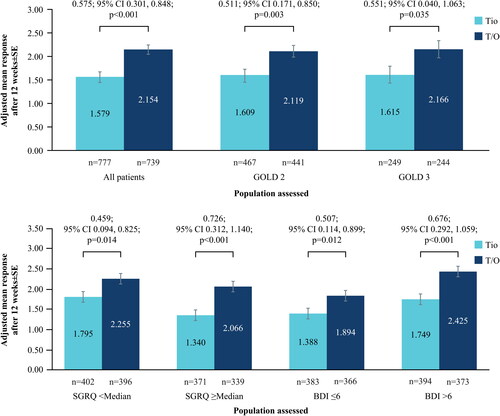
In terms of responders, of the 741 tiotropium/olodaterol-treated patients, 449 (60.6%) achieved the MCID of ≥1.0 point; of the 777 tiotropium-treated patients, 379 (48.8%) achieved the MCID; odds ratio 1.642 (95% CI 1.337, 2.018; p < 0.001).
Safety
shows a summary of AEs for the combined steroid-free population. The proportion of patients with any AE was similar between the two treatment arms and most AEs were mild or moderate. In the tiotropium arm, 61.8% of patients reported at least one AE versus 61.0% in the tiotropium/olodaterol arm. The proportion of patients with an investigator-defined drug-related AE was also similar between the two treatment arms: 46 (5.6%) in the tiotropium arm and 49 (6.4%) in the tiotropium/olodaterol arm.
Table 2. Adverse event profile.
Discussion
This post hoc analysis of data from participants in the TONADO and OTEMTO studies assessed whether dual bronchodilation with tiotropium/olodaterol was superior to tiotropium monotherapy, in a population of patients not using ICS. After 12 weeks, tiotropium/olodaterol provided significantly greater improvements in trough FEV1, TDI, and SGRQ versus tiotropium monotherapy in all patients (p < 0.001) and in most subgroups analyzed (p < 0.05). Additionally, more responders showing improvements greater than the MCID were identified in patients receiving tiotropium/olodaterol than patients receiving tiotropium. Taken together, these results support the recommended use of dual bronchodilation with tiotropium/olodaterol over tiotropium monotherapy in stable COPD patients not receiving ICS.
These results are consistent with those of the primary TONADO and OTEMTO studies, which included patients receiving ICS in addition to study treatments. In TONADO and OTEMTO, greater improvements in FEV1, SGRQ, and TDI focal score were identified for patients treated with tiotropium/olodaterol after 24 weeks and sustained to 52 weeks (TONADO), or after 12 weeks (OTEMTO) compared with either agent alone [Citation4, Citation9]. This suggests that the benefits of tiotropium/olodaterol therapy over the monotherapies are seen regardless of ICS use.
The present analyses intended to compare tiotropium versus tiotropium/olodaterol; due to the nature of this pooled analysis, comparisons with olodaterol monotherapy were not conducted.
The benefits of dual LAMA/LABA therapy versus monotherapies have been shown in other recent clinical trials. The EMAX trial, for example, showed that the proportion of responders was greater for all symptom outcomes in patients randomized to receive dual bronchodilator therapy (umeclidinium/vilanterol) versus those treated with umeclidinium or salmeterol [Citation13]. Additionally, in these symptomatic, low exacerbation-risk patients with COPD, dual bronchodilation provided early and sustained improvements in lung function and symptoms, with reduced probability of short-term COPD worsening, compared with monotherapy [Citation13]. In a pooled analysis of 23 clinical studies, where 53.6% of patients were receiving ICS alongside their current therapies, it was reported that combining LAMA/LABA therapies provided numerically better treatment outcomes than LAMA or LABA monotherapies for FEV1, SGRQ, and TDI [Citation14]. Similarly, in the SHINE study, superior improvements in lung function were identified with dual bronchodilation (indacaterol/glycopyrronium) versus patients who received either indacaterol, glycopyrronium, tiotropium, or placebo [Citation15]. These results were not influenced by concurrent use of ICS [Citation15], adding weight to the argument that dual bronchodilation offers greater improvements compared with monotherapies, regardless of ICS use.
The results of the present analysis support the current ATS and NICE guidance regarding bronchodilation of patients with COPD [Citation2, Citation3]. The ATS recommends LAMA/LABA combination therapy over monotherapy for patients who complain of dyspnea or exercise intolerance [Citation2], while NICE recommends dual LAMA/LABA for patients who experience COPD symptoms despite optimized non-pharmacologic management and use of short-acting bronchodilators [Citation3].
Additionally, the current analysis of ICS-free patients supports superior efficacy benefits in patients receiving LAMA/LABA over LAMA monotherapy with comparable safety profiles. Similar efficacy and safety results have recently been published for patients who escalated their treatment from LAMA to LAMA/LABA or in maintenance-naïve patients when comparing LAMA to LAMA/LABA in a pooled population that did not exclude patients taking ICS [Citation16, Citation17].
When determining pharmacologic treatment plans, we should consider the general distribution of patients with COPD. The majority of patients with COPD initially fall into GOLD groups A or B [Citation18], and most experience symptoms such as breathlessness rather than frequent exacerbations [Citation19, Citation20]. Indeed, only a subgroup of COPD patients are frequent exacerbators and should require treatment with ICS [Citation21, Citation22]. Therefore, studies such as this are important to help determine optimal treatments for those not requiring ICS. Taken together with the results of previous studies, the present analyses confirm that dual LAMA/LABA therapy provides greater improvements compared with monotherapy, irrespective of ICS use.
The current analysis pools data from four clinical trials, representing a large, clinically relevant patient population and allowing analysis of patients with COPD who were not using ICS at baseline. The patients included in this analysis were generally balanced across the two treatment arms. There was a potential imbalance in patients’ smoking status, but we do not believe that this influences the results of the present analysis. However, due to the post hoc nature of the analysis, these results do not conform to the randomization model of statistical inference, limiting the power for statistical comparisons. Another limitation to this analysis is that OTEMTO was only 12 weeks in duration; however, in TONADO, the changes in the primary endpoints were sustained throughout the 52-week treatment period [Citation4]. As no adjustments for multiplicity were conducted in this analysis, the p-values are nominal, not confirmatory.
This analysis confirms the benefits of dual bronchodilation versus a single bronchodilator, in a population of COPD patients unconfounded by ICS use. In this pooled analysis of over 1500 steroid-free patients with COPD, optimizing bronchodilator treatment with tiotropium/olodaterol significantly improved lung function, symptoms and health status compared with tiotropium alone (p < 0.001), with similar results when assessed in GOLD 2 and 3 patients and according to their baseline symptoms (SGRQ and BDI) (p < 0.05). Overall, these data support GOLD recommendations of dual bronchodilator therapy without ICS to improve breathlessness and health status, and are in line with recent guidance from the ATS and NICE regarding use of dual bronchodilator therapy as primary therapy for patients with COPD.
Disclosures
PMAC reports grants and personal fees from GlaxoSmithKline and also personal fees from AstraZeneca, Boehringer Ingelheim, Recipharm, and Zambon and also other fees from Boehringer Ingelheim outside the submitted work. AdlH and WX are both employees of Boehringer Ingelheim. GTF has received grants, personal fees, and non-financial support from Boehringer Ingelheim during the conduct of this study and from Boehringer Ingelheim, Novartis, AstraZeneca, Pearl Therapeutics, Sunovion, Verona, Theravance, and GlaxoSmithKline; grants and personal fees from Sanofi; grants from Altavent; and personal fees from Mylan, Innoviva and Circassia outside the submitted work. MM has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols, and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, Spin Therapeutics, pH Pharma, Novartis, Sanofi, and Grifols, and research grants from GlaxoSmithKline and Grifols outside the submitted work.
Medical writing, editorial, and other assistance
Medical writing assistance, in the form of the preparation and revision of the manuscript, was supported financially by Boehringer Ingelheim and provided by Paul Todd, PhD, of MediTech Media (Manchester, UK), based on a draft provided by the authors, their feedback and under their conceptual direction.
Compliance with ethics guidelines
The studies included in this post hoc analysis were previously approved by the review boards of the relevant national, regional or independent ethics committee or institutional review boards. These studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Patients in these studies provided written informed consent.
Supplemental Material
Download PDF (669.4 KB)Data availability
The data that support the findings of this study are available from the corresponding author, PMAC, upon reasonable request.
Additional information
Funding
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2020 report) 2019 [cited 2020 June 15]. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf
- Nici L , Mammen MJ , Charbek E , et al . Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69. DOI:10.1164/rccm.202003-0625ST
- National Institute for Health and Care Excellence . Chronic obstructive pulmonary disease in over 16s: diagnosis and management 2019 [cited 2020 June 15]. Available from: https://www.nice.org.uk/guidance/ng115/chapter/Recommendations#ftn.footnote_3
- Buhl R , Maltais F , Abrahams R , et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J. 2015;45(4):969–979. DOI:10.1183/09031936.00136014
- Mahler DA , Decramer M , D'Urzo A , et al. Dual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: the BLAZE study. Eur Respir J. 2014;43(6):1599–1609. DOI:10.1183/09031936.00124013
- Malerba M , Foci V , Patrucco F , et al. Single inhaler LABA/LAMA for COPD. Front Pharmacol. 2019;10:390. DOI:10.3389/fphar.2019.00390
- Vincken W , Aumann J , Chen H , et al. Efficacy and safety of coadministration of once-daily indacaterol and glycopyrronium versus indacaterol alone in COPD patients: the GLOW6 study. Int J Chron Obstruct Pulmon Dis. 2014;9:215–228. DOI:10.2147/COPD.S51592
- Calverley PMA , Anzueto AR , Dusser D , et al. Treatment of exacerbations as a predictor of subsequent outcomes in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:1297–1308. DOI:10.2147/COPD.S153631
- Singh D , Ferguson GT , Bolitschek J , et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109(10):1312–1319. DOI:10.1016/j.rmed.2015.08.002
- Witek TJ, Jr. , Mahler DA . Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J. 2003;21(2):267–272. DOI:10.1183/09031936.03.00068503a
- Wedzicha JA , Decramer M , Ficker JH , et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209. DOI:10.1016/S2213-2600(13)70052-3
- Jones PW , Beeh KM , Chapman KR , et al. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189(3):250–255. DOI:10.1164/rccm.201310-1863PP
- Maltais F , Bjermer L , Kerwin EM , et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the EMAX randomised trial. Respir Res. 2019;20(1):238. DOI:10.1186/s12931-019-1193-9
- Donohue JF , Jones PW , Bartels C , et al. Correlations between FEV1 and patient-reported outcomes: a pooled analysis of 23 clinical trials in patients with chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2018;49:11–19. DOI:10.1016/j.pupt.2017.12.005
- Bateman ED , Ferguson GT , Barnes N , et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42(6):1484–1494. DOI:10.1183/09031936.00200212
- Buhl R , Singh D , de la Hoz A , et al. Benefits of tiotropium/olodaterol compared with tiotropium in patients with COPD receiving only LAMA at baseline: pooled analysis of the TONADO® and OTEMTO® studies. Adv Ther. 2020;37(8):3485–3499. https://link.springer.com/article/10.1007%2Fs12325-020-01373-3
- Buhl R , de la Hoz A , Xue W , et al. Efficacy of tiotropium/olodaterol compared with tiotropium as a first-line maintenance treatment in patients with COPD who are naïve to LAMA, LABA and ICS: pooled analysis of four clinical trials. Adv Ther. 2020;Epub ahead of print. DOI:10.1007/s12325-020-01411-0
- Halpin DMG , de Jong HJI , Carter V , et al. Distribution, temporal stability and appropriateness of therapy of patients with COPD in the UK in relation to GOLD 2019. EClinicalMedicine. 2019;14:32–41. DOI:10.1016/j.eclinm.2019.07.003
- Vogelmeier CF , Román-Rodríguez M , Singh D , et al. Goals of COPD treatment: focus on symptoms and exacerbations. Respir Med. 2020;166:105938. DOI:10.1016/j.rmed.2020.105938
- Izquierdo JL , Miravitlles M , Esquinas C , et al. Characteristics of COPD patients managed in respiratory medicine departments in Spain, according to GOLD groups and GesEPOC clinical phenotypes. Arch Bronconeumol. 2018;54(11):559–567. DOI:10.1016/j.arbres.2018.03.021
- Vogelmeier C , Worth H , Buhl R , et al . “Real-life” inhaled corticosteroid withdrawal in COPD: a subgroup analysis of DACCORD. Int J Chron Obstruct Pulmon Dis. 2017;12:487–494. DOI:10.2147/COPD.S125616
- Koblizek V , Milenkovic B , Barczyk A , et al. Phenotypes of COPD patients with a smoking history in Central and Eastern Europe: the POPE Study. Eur Respir J. 2017;49(5):1601446. DOI:10.1183/13993003.01446-2016
