Abstract
Non-invasive ventilation (NIV) treatment decisions are poorly understood for patients with COPD exacerbation complicated by acute hypercapnic respiratory failure and respiratory acidaemia (ECOPD-RA). We identified 420 NIV-eligible patients from the DECAF study cohorts admitted with an ECOPD-RA. Using bivariate and multivariate analyses, we examined which indices were associated with clinicians’ decisions to start NIV, including whether the presence of pneumonia was a deterrent. Admitting hospital, admission from institutional care, partial pressure of oxygen, cerebrovascular disease, pH, systolic blood pressure and white cell count were all associated with the provision of NIV. Of these indices, only pH was also a predictor of inpatient death. Those not treated with NIV included those with milder acidaemia and higher (and sometimes excessive) oxygen levels, and a frailer population with higher Extended Medical Research Council Dyspnoea scores, presumably deemed not suitable for NIV. Pneumonia was not associated with NIV treatment; 34 of 111 (30.6%) NIV-untreated patients had pneumonia, whilst 107 of 309 (34.6%) NIV-treated patients had pneumonia (p = 0.483). In our study, one in four NIV-eligible patients were not treated with NIV. Clinicians’ NIV treatment decisions are not based on those indices most strongly associated with mortality risk. One of the strongest predictors of whether a patient received a life-saving treatment is which hospital they attended. Further research is required to aid in the risk stratification of this patient group which may help standardise and improve care.
Background
Non-invasive ventilation (NIV) is standard care for patients with COPD (chronic obstructive pulmonary disease) exacerbation complicated by acute hypercapnic respiratory failure (type two respiratory failure) and respiratory acidaemia (ECOPD-RA) [Citation1]. National UK audit data show that approximately one-half of patients who meet these criteria are not treated with NIV [Citation2,Citation3]. However, the median pH of those treated with NIV has dropped in successive audits suggesting that clinicians’ are treating more severely unwell patients.
Little is known about the factors that influence clinicians’ decisions not to ventilate NIV-eligible patients. Important patient factors may include indices related to adverse mortality, co-existent pneumonia, quality of life assessments, and/or the view that the patient will either improve without NIV or may not respond to treatment. Clinician factors include specialty, with respiratory clinicians more likely to report initiating NIV than geriatricians or palliative care doctors [Citation4]. Clinicians may be unduly pessimistic in their assessment of patients with COPD [Citation5], and whilst prognostic scores exist for ECOPD [Citation6–12], further prognostic research is required in those with ECOPD-RA [Citation13]. Clinicians may make their decisions based on a judgement of the patients’ quality of life, though most patients report acceptable quality of life following NIV [Citation14,Citation15]. Amongst survivors of admission to ITU with COPD or asthma, including those requiring NIV or invasive ventilation, 96% would choose similar treatment again [Citation16].
In current clinical practice, community-acquired pneumonia (CAP) and pneumonic exacerbation of COPD (pECOPD) are not exclusive diagnoses. Twenty-three percent of patients in the 2015 UK CAP audit had co-existent COPD [Citation17], and 18% of patients with ECOPD had co-existent pneumonia in the 2014 UK COPD audit [Citation18]. More than one of four COPD deaths at post-mortem are attributed to pneumonia [Citation19].
The UK national NIV guidelines state that NIV is not indicated in pneumonia complicated by respiratory failure [Citation20]. It is unclear whether this statement was intended to apply only to isolated CAP, or also when pneumonia complicates another condition associated with a favourable response, such as an ECOPD-RA. The UK national pneumonia guidelines report that NIV is not routinely indicated in the management of patients with respiratory failure due to CAP, and if NIV is provided, patients should be treated in a critical care unit. Therefore, the presence of pneumonia may deter some clinicians from providing NIV to patients with ECOPD and respiratory acidaemia.
In the seminal randomised controlled trial (RCT) of NIV in COPD (n = 236) [Citation21], the presence of pneumonia was not associated with treatment failure [Citation21,Citation22]. In an RCT of NIV in pneumonia, NIV was associated with lower intubation and two-month mortality rates only in the subgroup with COPD [Citation23]. Current UK practice favours the use of NIV in this setting; in the 2013 and 2019 NIV audits, most patients had COPD, 36%–40% had chest X-ray consolidation and NIV was predominantly administered outside of critical care units [Citation3]. In the 2017 National Confidential Enquiry into Patient Outcome and Death for NIV [Citation24], pneumonia was the primary diagnosis in 12% and present in 50% of reviewed cases; expert reviewers considered NIV appropriate in three in four patients.
This study aimed to:
Establish whether the presence of pneumonia deters clinicians’ provision of NIV to patients with ECOPD and respiratory acidaemia.
To examine the factors that clinicians use to guide NIV treatment.
Methods
Patients with ECOPD who were eligible for NIV (pH less than 7.35 and partial pressure of carbon dioxide, PaCO2, greater than 6 kPa) from the six sites in both the DECAF derivation (December 2008 to June 2010) and validation (January 2012 to May 2014) studies were identified (n = 420) [Citation6,Citation7]. DECAF comprises five indices, Dyspnoea, Eosinophil, chest X-ray Consolidation, Acidaemia, and atrial Fibrillation, and is a prognostic score developed to risk-stratify patients with ECOPD for inpatient mortality. Evidence of airflow obstruction and clinical diagnosis of both COPD and an acute exacerbation were required for inclusion. Sociodemographic data, markers of disease severity, co-morbidity, admission clinical data and blood sample results were collected. Treatment with NIV was not influenced by the research team. Missing data were imputed using Rubin’s method [Citation25]. Further details of eligibility criteria and data collection are provided elsewhere [Citation6,Citation7].
In the first analysis, the outcome of interest was treatment with NIV. We subdivided those eligible for NIV treatment into those not treated with NIV (“NIV-untreated”), and those that were treated with NIV (“NIV-treated”), and performed bivariate and multivariate analyses across these groups to identify indices associated with NIV treatment, with a focus on pneumonia as a key index. We included indices that are regarded as strong mortality predictors based on the findings from the DECAF studies and from previous literature reviews to see if these indices would also be associated with decisions to treat with NIV. The eMRCD (extended modified research council dyspnoea) score was included which is a measure of breathlessness and frailty, and one of the strongest single predictors of acute mortality in ECOPD [Citation26]. A score of 5a or 5b means that a patient is too breathless to leave house without assistance, and a score of 5b means a patient is additionally unable to wash and dress independently.
In a second analysis, the outcome of interest was inpatient mortality. We performed bivariate and multivariate analyses of NIV-eligible patients to look for indices associated with inpatient mortality, and compared these indices to those associated with NIV treatment decisions.
In the final analysis, we identified all patients with ECOPD irrespective of pH (n = 2,645), grouped them into those deemed not for ventilation (“not for NIV”) and those “for NIV”, and performed bivariate and multivariate analyses to see what factors were associated with “not for NIV” decisions. In the absence of a “not for NIV” decision, we assumed patients were for NIV treatment. We included patients in the “not for NIV” group even if this decision was over-turned later in their admission.
Group comparisons used Fisher’s exact test for proportions, t-test for data that were normally distributed, and Mann–Whitney U test for data that were not normally distributed. Pneumonia (defined as consolidation reported on the admission chest X-ray by a radiologist or the senior clinician) and other indices were assessed in a backwards, stepwise multivariate regression analysis, and were presented as odds ratio (OR) with 95% confidence interval (95% CI). The full list of indices inputted into regression models is shown in .
Table 1. Indices included in multivariate regression models.
Results
Patients with respiratory acidaemia
There were 420 patients identified with respiratory acidaemia, of whom 60% were female. The mean age (standard deviation, SD) was 72.2 (9.8) and forced expiratory volume in 1 s was 37.7 (16.2) % predicted. The median pH was 7.28 (interquartile range [IQR] 7.22–7.32), DECAF score was 2 (IQR 2–3) and the Charlson index was 2 (IQR 1–3). All sites included patients with pneumonic ECOPD and respiratory acidaemia who were treated with NIV. Rates of missing data were extremely low and are detailed elsewhere [Citation6,Citation7]. Almost three in four NIV-eligible patients (309 of 420) were treated with NIV. Six patients were immediately treated with invasive ventilation and 17 had NIV and invasive ventilation. Twenty-three patients had invasive ventilation as well as NIV. Overall mortality in this group was 16.7% (compared to 7.1% in the 2,225 patients who did not have respiratory acidaemia at admission).
Association between pneumonia and NIV treatment
Pneumonia was not associated with NIV treatment. On bivariate analysis, 34 of 111 (30.6%) NIV-untreated patients had pneumonia, whilst 107 of 309 NIV-treated patients (34.6%) had pneumonia (p = 0.483). In the subsequent regression analysis, the adjusted OR between pneumonia and NIV treatment was 1.20 (95% CI 0.75–1.91, p = 0.45).
Predictors of NIV treatment on bivariate analysis
shows the 420 NIV-eligible patients divided into (A) the 111 patients not treated with NIV (NIV-untreated) and (B) the 309 patients treated with NIV (NIV-treated).
Table 2. Characteristics of patients with COPD exacerbation and respiratory acidaemia grouped into those (A) not treated with NIV and (B) treated with NIV.
The NIV-untreated group had higher proportions of institutional care (p = 0.002) and cerebrovascular disease (p = 0.046), and a higher median eMRCD score (p = 0.008) compared to the treated group. The higher median eMRCD scores were due to the larger group of eMRCD 5b patients in the untreated group. However, the NIV-untreated group had lower median DECAF scores (p < 0.001).
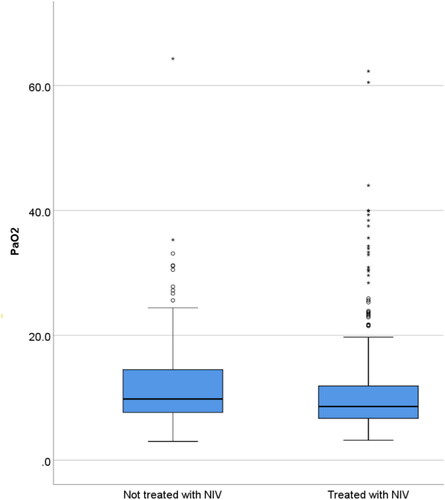
The NIV-untreated group also had higher blood pressure (BP) (p = 0.008), pH (p < 0.001) and PaO2 (p = 0.028). These, as well as the lower DECAF score, are features typically associated with a better outcome: overall, they had a non-significantly lower percentage of inpatient deaths (13.5% versus 17.8%, p = 0.373). More patients in the NIV-untreated group had excess oxygenation as defined by a PaO2 of 13 kPa or more (34.2% versus 21.0%, p = 0.007), though excess oxygenation occurred commonly in both groups (). There was a trend towards greater rates of pre-admission cognitive impairment in the NIV-untreated group (p = 0.088), though the converse was seen with confusion which was higher in the treated group (p = 0.059). There were no statistically significant differences for the other indices shown in .
Figure 1. Boxplot showing the distribution of the partial pressure of oxygen measured in kilopascals for the group not treated with NIV and those treated with NIV. The median value and interquartile range for those not treated with NIV was 9.8 (7.6–14.7) and for those treated with NIV was 8.6 (6.7–11.9).
Multivariate predictors of NIV treatment in patients with respiratory acidaemia
Indices included in the multivariate analysis are shown in . Indices associated with NIV treatment were admission hospital, institutional care (nursing or residential home), PaO2, cerebrovascular disease, pH, systolic BP and white cell count (OR and 95% CI are shown in ).
Table 3. Multivariate regression analysis showing predictors of NIV treatment in patients that meet the criteria for NIV treatment at admission.
Institutional care, cerebrovascular disease and higher white cell counts are typically associated with worse outcome, and in this study were less common in those treated with NIV. A lower pH, PaO2 and systolic BP are typically associated with worse outcome, and we found that lower scores were associated with NIV treatment.
Mortality outcome in patients with respiratory acidaemia
On bivariate analysis, predictors of death in those with respiratory acidaemia were age, DECAF score, eMRCD score, atrial fibrillation, left ventricular failure, cognitive impairment, Charlson index, confusion, chest X-ray consolidation, systolic BP, NEWS2 score, pH and C-reactive protein (online Supplementary Table S1). Institutional care showed a trend towards worse mortality. Partial pressure of oxygen was similar in those that died and survived, as were the proportions with cerebrovascular disease. The strongest predictors of mortality in multivariate regression are shown in . Only pH was a predictor of mortality and also a predictor of NIV treatment.
Table 4. Predictors of mortality on multivariate analysis in patients with respiratory acidaemia.
We forced admission from institutional care into the regression model, but it did not add any additional prognostic information (OR = 0.88, 95% CI 0.32–2.41, p = 0.795).
Patients not for NIV during admission
From the DECAF cohort, there were 112 patients from 2,645 patients with ECOPD who were deemed unsuitable for NIV. This group is shown in and comprises those who the clinician judged had an unacceptably poor outcome, that NIV would not work, and/or that their quality of life was such that NIV was inappropriate.
Table 5. Characteristics of the 112 patients with COPD exacerbation that were deemed “not for NIV” at admission from all ECOPD population.
This is a group that contained frail, elderly patients. The proportion of patients with CXR consolidation was 34.8%, which is similar to the NIV-treated (34.6%) and NIV-untreated group (30.6%). Once again, the proportion of patients admitted from institutional care was high (17%). In multivariate analysis, institutional care and admission long term oxygen therapy were the strongest predictors of being assigned “not for NIV” in all patients admitted with an ECOPD (online Supplementary Table S2). It is of note that 9.8% of this group were subsequently treated with NIV despite this initial decision, suggesting that respiratory and intensive care specialists do not always concur with the initial NIV decision at admission. In this group, 15 of 112 patients did not have ABGs (arterial blood gases). In those with a pH of less than 7.35 at admission, 14 of 29 died (48.3%) and in those with a pH of 7.35 or more at admission 15 of 68 died (22.1%).
eMRCD and atrial fibrillation were the only indices that were associated with both being “not for NIV” and with acute mortality in the NIV-eligible patients. Paradoxically, atrial fibrillation was protective in terms being made “not for NIV”.
Discussion
In our study, one in four patients eligible for NIV were not treated, but the presence of pneumonia did not substantially deter clinicians from providing NIV. In common with the National COPD Resources and Outcomes project [Citation2], the NIV-untreated population had a non-significantly lower mortality than the NIV-treated group. Whilst they had lower DECAF scores (less severe acute clinical deterioration), their eMRCD scores were higher (poorer baseline performance status). The NIV-untreated group may include two subgroups: the first subgroup comprise those with less severe acidaemia and higher PaO2 whose acidaemia is more likely to resolve with controlled oxygen alone, whilst the second is a frailer population who are deemed to be too unwell/unsuitable for NIV. Given the broad evidence base showing the large benefit of NIV in ECOPD-RA, the milder subgroup likely account for the better than expected mortality in the untreated group: it is recognised that around one in five patients will correct their acidaemia with medical treatment alone [Citation27]. Of interest, patients were less likely to get NIV if they were from institutional care or had a stroke, whereas other variables more strongly associated with adverse mortality outcome – such as eMRCD score – did not show an association on multivariate analysis. Clinicians may over-estimate the chances of a negative outcome for some indices, and fail to recognise when multiple adverse indices are collinear, which may lead to incorrect decisions not to give NIV and to palliate patients.
The admitting hospital was a strong predictor of NIV treatment after adjusting for patient risk factors, which demonstrates that part of the variation in practice is unrelated to patients’ characteristics. Similarly, the CAOS study also showed marked variation in the clinical characteristics of patients between units [Citation16]. RightCare is a national NHS England programme that seeks to improve performance by identifying unwarranted variation between demographically similar populations; unsurprisingly acute NIV has been identified as a target to reduce excess mortality. Furthermore, 1 in 10 patients made “not for NIV” were subsequently given NIV. It is clear that clinicians do not use key predictors of mortality to guide decisions; this and variations in practice underlines the need for further prognostic research in this area. To date, the literature is sparse; one large study of 1,033 patients assessed NIV failure defined as intubation or death, which used prospectively collected data to create a modified APACHE (Acute Physiology and Chronic Health Evaluation) II score stratified by pH, with further subdivision by respiratory rate and Glasgow Coma Scale (which are also components of APACHE II). Two small studies looked at five-year failure [Citation28], or treatment failure [Citation29]. The NIV outcomes study (NIVO) is a 10-centre prospective derivation and validation UK study whose aim is to create a simple prognostic tool that can be used at the bedside (ISRCTN22921168) and aid in ventilation decisions.
Clinicians may be using indices that they believe informs future quality of life rather than mortality, and this could explain the strong association with institutional care. Quality of life is a key factor when considering any potentially burdensome treatment, but it is the patients’ view of both their quality of life and the treatment burden that is key. Many patients following NIV treatment for ECOPD-RA return to a quality of life that is similar to their pre-exacerbation level and/or is acceptable to them, and almost all would undergo the same treatment again [Citation15,Citation16].
Terminology varies with regard to pneumonic ECOPD. National audit data show that some physicians are labelling pECOPD as CAP, though the treatments for pECOPD are more consistent with ECOPD rather than CAP. Given that pneumonia is a predictor of mortality in ECOPD [Citation26], how pECOPD is labelled will influence reported mortality rates and highlights the need for consistency in terminology to allow meaningful comparisons between different hospitals in the national audit. In this study, we have shown that the presence of pneumonia does not appear to be a deterrent to providing NIV, which is consistent with national audit data and expert opinion [Citation3,Citation24], and supports the use of the term pECOPD.
This study has several strengths. The DECAF populations were well defined, the COPD diagnosis was reviewed by clinicians, consecutive patients were recruited and the small amount of missing data was treated with multiple imputation, which is the gold standard.
Our study has several limitations. We have assumed that a positive association with an index means clinicians are using this as part of their decision-making process, which may be incorrect. Semi-structured interviews of clinicians and patients may have provided clarity, particularly with regard to the relevance of institutional care and the level of involvement of the patient in decision-making. It is unclear how generalisable our results will be, as the centres included in the study may differ from others. There should be some caution comparing different regression models as there may be chance variation in models. Comparisons between the NIV-untreated group and “not for NIV” group are complicated by the fact that the NIV-untreated group includes both patients who responded to medical management as well as those deemed too well for NIV treatment. Lastly, the time frame for the DECAF derivation and validation studies is wide, and clinical practice may have changed over this time.
In conclusion, clinicians’ NIV treatment decisions do not seem to be based on those indices most strongly associated with mortality risk, and co-existent pneumonia is not a deterrent to the provision of NIV. One of the strongest predictors of whether a patient receives a life-saving treatment is which hospital they attend. Further research is required to understand why these differences exist, and to provide clinicians with a risk stratification tool that can be used at the bedside which may help standardise care. This could help inform discussions about potential benefits and/or risks of treatment (or non-treatment) and improve shared decision-making between clinicians and their patients. Currently, clinicians may put too much emphasis on certain indices, such as institutional care, and overlook more important indices which better capture risk such as the eMRCD score.
Authors’ contribution
C.E., S.C.B. and J.S. contributed to trial design. C.E. performed the statistical analysis and drafted the manuscript. All authors contributed to data analysis and interpretation and redrafted and approved the final manuscript.
Declaration of interest
John Steer, Nicholas Lane and Tom Hartley have no conflicts of interest to declare. Carlos Echevarria reports grants from National Institute of Health Research, outside of the submitted work. Stephen C. Bourke reports grants from National Institute of Health Research, Philips Respironics and from Pfizer Open Air, personal fees from Pfizer, AstraZeneca and ResMed, and non-financial support from Boehringer Ingelheim and GlaxoSmithKline outside the submitted work. No author has financial relationships with any organisation that might have an interest in the submitted work.
Supplemental Material
Download PDF (180.8 KB)References
- Rochwerg B , Brochard L , Elliott MW , et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426. DOI:10.1183/13993003.02426-2016
- Roberts CM , Stone RA , Buckingham RJ , et al. Acidosis, non-invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66(1):43–48. DOI:10.1136/thx.2010.153114
- Davies M . British Thoracic Society NIV audit. 2013 (national audit period 1 February–31 March 2013). Available from: www.brit-thoracic.org.uk/document-library/audit-andquality-improvement/audit-reports/bts-adult-niv-auditreport-2013/
- Gabler M , Ohrenberger G , Funk GC . Treatment decisions in end-stage COPD: who decides how? A cross-sectional survey of different medical specialties. ERJ Open Res. 2019;5(3):00163-2018. DOI:10.1183/23120541.00163-2018
- Wildman MJ , Sanderson C , Groves J , et al. Implications of prognostic pessimism in patients with chronic obstructive pulmonary disease (COPD) or asthma admitted to intensive care in the UK within the COPD and Asthma Outcome Study (CAOS): multicentre observational cohort study. BMJ. 2007;335(7630):1132. DOI:10.1136/bmj.39371.524271.55
- Steer J , Gibson J , Bourke SC . The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax. 2012;67(11):970–976. DOI:10.1136/thoraxjnl-2012-202103
- Echevarria C , Steer J , Heslop-Marshall K , et al. Validation of the DECAF score to predict hospital mortality in acute exacerbations of COPD. Thorax. 2016;71(2):133–140. DOI:10.1136/thoraxjnl-2015-207775
- Echevarria C , Brewin K , Horobin H , et al. Early supported discharge/hospital at home for acute exacerbation of chronic obstructive pulmonary disease: a review and meta-analysis. COPD. 2016;13(4):523–533. DOI:10.3109/15412555.2015.1067885
- Tabet R , Ardo C , Makrlouf P , et al. Application of Bap-65: a new score for risk stratification in acute exacerbation of chronic obstructive pulmonary disease. J Clin Respir Dis Care. 2016;2(1). DOI:10.4172/2472-1247.1000110
- Tabak YP , Sun X , Johannes RS , et al. Mortality and need for mechanical ventilation in acute exacerbations of chronic obstructive pulmonary disease: development and validation of a simple risk score. Arch Intern Med. 2009;169(17):1595–1602. DOI:10.1001/archinternmed.2009.270
- Shorr AF , Sun X , Johannes RS , et al. Validation of a novel risk score for severity of illness in acute exacerbations of COPD. Chest. 2011;140(5):1177–1183. DOI:10.1378/chest.10-3035
- Roche N , Zureik M , Soussan D , et al. Predictors of outcomes in COPD exacerbation cases presenting to the emergency department. Eur Respir J. 2008;32(4):953–961. DOI:10.1183/09031936.00129507
- Bourke SC , Piraino T , Pisani L , et al. Beyond the guidelines for non-invasive ventilation in acute respiratory failure: implications for practice. Lancet Respir Med. 2018;6(12):935–947. DOI:10.1016/S2213-2600(18)30388-6
- Vilaca M , Aragao I , Cardoso T , et al. The role of noninvasive ventilation in patients with “do not intubate” order in the emergency setting. PLoS One. 2016;11(2):e0149649. DOI:10.1371/journal.pone.0149649
- Steer J , Gibson GJ , Bourke SC . Longitudinal change in quality of life following hospitalisation for acute exacerbations of COPD. BMJ Open Respir Res. 2015;2(1):e000069. DOI:10.1136/bmjresp-2014-000069
- Wildman MJ , Sanderson C , Groves J , et al. Predicting mortality for patients with exacerbations of COPD and Asthma in the COPD and Asthma Outcome Study (CAOS). QJM. 2009;102(6):389–399. DOI:10.1093/qjmed/hcp036
- Daniel P , Bewick T , Welham S , et al. Adults miscoded and misdiagnosed as having pneumonia: results from the British Thoracic Society pneumonia audit. Thorax. 2017;72(4):376–379. DOI:10.1136/thoraxjnl-2016-209405
- Stone RA , Holzhauer-Barrie J , Lowe D , et al. COPD: who cares matters. National Chronic Obstructive Pulmonary Disease (COPD) Audit Programme: clinical audit of COPD exacerbations admitted to acute units in England and Wales 2014. London: RCP; 2015.
- Zvezdin B , Milutinov S , Kojicic M , et al. A postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbation. Chest. 2009;136(2):376–380. DOI:10.1378/chest.08-2918
- Davidson AC , Banham S , Elliott M , et al. BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax. 2016;71(Suppl 2):ii1–35. DOI:10.1136/thoraxjnl-2015-208209
- Plant PK , Owen JL , Elliott MW . Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355(9219):1931–1935. DOI:10.1016/S0140-6736(00)02323-0
- Plant PK , Owen JL , Elliott MW . Non-invasive ventilation in acute exacerbations of chronic obstructive pulmonary disease: long term survival and predictors of in-hospital outcome. Thorax. 2001;56(9):708–712. DOI:10.1136/thorax.56.9.708
- Confalonieri M , Potena A , Carbone G , et al. Acute respiratory failure in patients with severe community-acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1585–1591. DOI:10.1164/ajrccm.160.5.9903015
- The National Confidential Enquiry into Patient Outcome and Death . Inspiring change. London: NCEPOD; 2017.
- Rubin DB . Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91(434):473–489. DOI:10.1080/01621459.1996.10476908
- Steer J , Norman EM , Afolabi OA , et al. Dyspnoea severity and pneumonia as predictors of in-hospital mortality and early readmission in acute exacerbations of COPD. Thorax. 2012;67(2):117–121. DOI:10.1136/thoraxjnl-2011-200332
- Plant PK , Owen JL , Elliott MW . One year period prevalence study of respiratory acidosis in acute exacerbations of COPD: implications for the provision of non-invasive ventilation and oxygen administration. Thorax. 2000;55(7):550–554. DOI:10.1136/thorax.55.7.550
- Chung LP , Winship P , Phung S , et al. Five-year outcome in COPD patients after their first episode of acute exacerbation treated with non-invasive ventilation. Respirology. 2010;15(7):1084–1091. DOI:10.1111/j.1440-1843.2010.01795.x
- Anton A , Guell R , Gomez J , et al. Predicting the result of noninvasive ventilation in severe acute exacerbations of patients with chronic airflow limitation. Chest. 2000;117(3):828–833.
