Abstract
Six-minute walk test (6MWT) measures walking distance (6MWD) and desaturation status in chronic obstructive pulmonary disease (COPD) patients. This study aimed to examine whether change in 6MWD and desaturation in 1 year were risk factors for later mortality, lung function decline and number of exacerbations. A total of 295 COPD patients performed 6MWT at baseline and 1 year later in the Bergen COPD cohort study 2006–2011. They were clinically examined and interviewed at annual visits. Mortality information was collected from the Norwegian Cause of Death Registry in 2015. We performed cox regression for mortality outcomes, linear mixed effect models for lung function, and negative binomial regression for exacerbations. Patients who desaturated in both 6MWTs had increased risk of all-cause and respiratory mortality, hazard ratio (HR) 2.7 (95% confidence interval [CI] 1.5–5.0) and 3.6 (95% CI 1.7–7.6), respectively, compared to non-desaturators. Patients who desaturated only at second 6MWT were at risk for all-cause mortality (HR 2.0, 95% CI 1.0–3.8). There were no apparent association between 6MWD and mortality. Desaturation in second 6MWT was associated with later increased rate of decline in forced vital capacity (FVC) % predicted (after 1 year predicted mean 4.2% above non-desaturators, after 5 years 0.7% below). Decline in 6MWD ≥ 30m was borderline (p = 0.06) associated with later decline in forced expiratory volume in 1 second % predicted, and with exacerbations (p = 0.07). Repeated desaturation in the 6MWT over time in COPD patients is a risk factor for all-cause and respiratory mortality, while onset of desaturation is associated with future FVC decline.
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of mortality and morbidity worldwide [Citation1]. Diagnosis and disease progression are mainly classified by lung function, commonly expressed by forced expiratory volume in 1 second (FEV1) [Citation2].
The six-minute walk test (6MWT) measures functional exercise capacity in COPD patients. The main outcome is six-minute walk distance (6MWD), a major independent risk factor for mortality [Citation3–5]. The 6MWD can also identify subsets of COPD patients at higher risk of exacerbation-related admission [Citation6]. Another 6MWT outcome is desaturation which is associated with increased mortality [Citation7,Citation8], lung function decline [Citation9], and increased number of exacerbations [Citation10].
Also change in 6MWD over time has been associated with mortality [Citation11–13]. Change in 6MWD has further been examined in association with change in lung function within the same time period, but existing results are inconclusive. Karanth et al. [Citation11] measured both 6MWD and lung function at baseline and 1 year later and found no correlation between change in 6MWD and change in lung function. Pinto-Plata et al. [Citation12] followed COPD patients 2 years, and measured 6MWD and lung function at baseline and follow-up. They found no correlation between yearly change in 6MWD and yearly change in FEV1 within the same time period. Casanova et al. [Citation14] measured 6MWD and lung function at baseline and annually for 5 years, and found that decline in 6MWD increased with increasing COPD severity. However, these studies measured 6MWD and lung function at the same time points. No studies have to our knowledge examined change in 6MWD as a risk factor for future longitudinal change in lung function.
Associations between change in 6MWD and later occurrence of exacerbations have not been studied before either, as far as we know. The same applies to associations between change in desaturation and later longitudinal change in lung function and exacerbations.
If changes in outcomes from repeated 6MWTs affect future COPD disease progression, such knowledge could help tailoring disease management for individual patients. Thus, the aim of this study was to examine whether change in 6MWD and desaturation in 6MWT during 1 year are risk factors for later mortality, lung function decline, and number of exacerbations.
Methods
Design and participants
The Bergen COPD Cohort Study is a longitudinal prospective observation study with 433 COPD patients, aged 40–76 years, at baseline in 2006/2007. All COPD patients were ever-smokers with >10 pack-years, post bronchodilator (post BD) FEV1/forced vital capacity (FVC) ratio <0.7, and post BD FEV1% predicted <80%. Selection of study participants have been described in detail previously [Citation15]. A total of 370 patients completed 6MWT without using supplemental oxygen at baseline. Of those, 295 completed a new 6MWT 1 year later. Informed written consent was obtained from the study participants. The Bergen COPD Cohort Study was approved by the regional committee of medical research ethics (REK Vest, case number 165.08, addition of mortality data case number 2014/2153). This study complies with the STROBE guidelines [Citation16].
Data collection
Data was collected from clinical examinations, physician-led interviews and self-administered questionnaires at the patients’ yearly visits until 2011. Information on all-cause and respiratory mortality was obtained from the Norwegian Cause of Death Registry in 2015 and merged onto the dataset using Norwegian unique personal identification numbers.
Outcomes
Main and underlying respiratory mortality was defined using the International Classification of Disease 10 (ICD 10) codes J40–J47. FEV1 and FVC were measured using a Viasys-Jaeger Masterscope (Viasys, Hoechberg, Germany) before and after inhalation of 0.4 mg salbutamol. Exacerbations were defined as worsening of respiratory symptoms that required treatment with oral steroids and/or antibiotics. The study physician recorded each patient’s exacerbations since last visit using medical records and information from the patient.
Main exposures
The 6MWT was performed at baseline and at 1-year follow-up and has been described in detail previously [Citation17]. 6MWD (in meters) was registered after the subject had walked as far as possible for 6 min. The 6MWT was performed once at each time point without a practice test. Those who stopped before 6 min or used oxygen supplementation were excluded from the analysis. Change in 6MWD in 1 year was defined as ≥30 m decrease between the two 6MWTs, in accordance with the minimal clinical important difference [Citation13,Citation18].
Oxygen saturation (SpO2) was measured before and after the 6MWT using a wrist-worn Nonin 3100 pulse oximeter with a finger sensor (Nonin Medical Inc., Plymouth, MN). Desaturation was defined according to the Royal College of Physicians’ guidelines as ≥4% reduction between arterial oxygen saturation measured by pulse oximetry pre- and post-test and post-test SpO2 <90% [Citation19]. Change in desaturation in 1 year was defined using three categories: no desaturation in both tests (non-desaturators), desaturation only at second test (new desaturators) and desaturation at both tests (repeated desaturators).
Covariates
Patients were defined as ex-smokers or daily smokers based on baseline smoking history. Charlson comorbidity score was calculated from the physician interview [Citation20,Citation21]. Modified Medical Research Council (mMRC) breathlessness score was registered using a self-administered questionnaire. Arterial oxygen tension was measured in 1.0 mL blood drawn from the radial artery using a radiometer PICO 70 arterial sampler and immediately analyzed using an ABL 520 blood gas analyzer (Radiometer, Copenhagen, Denmark). Residual volume/total lung capacity (RV/TLC) was measured using a Viasys Healthcare Vmax Encore 22D (VIASYS Healthcare/Cardinal Health, CA). Participation in pulmonary rehabilitation during the first 2 years of the study was registered.
Statistical analyses
All analyses were complete-case-analysis performed using Stata 15 (StataCorp LP, College Station, TX) for Windows. p Values were two-sided and values <0.05 were considered statistically significant.
To examine associations of 1-year change in 6MWD and change in desaturation with repeated measures of FEV1 and FVC, we used linear mixed effects models for continuous outcomes. All models included change in 6MWD or change in desaturation as categorical exposures, time in years as a continuous covariate, and the interaction between the main exposure and time to examine whether changes in outcomes over time differed across exposure groups. To account for both correlation and heteroscedasticity in the outcome over time, we specified a random intercept and slope for the individual and time, assuming unstructured correlation between effects. We used a similar model for the repeated numbers of exacerbations. However, as this outcome was a count variable with substantial overdispersion, we used a negative binomial regression model with the log-link function.
To examine associations of 1-year change in 6MWD and desaturation with all-cause mortality, we used Kaplan-Meier survival functions and Cox proportional hazard regression models. Timing of death and cause of death was recorded for all individuals. Individuals who were still alive were censored after 9 years. To examine time to death due to respiratory causes, we performed cause-specific hazard regression for respiratory mortality using Cox proportional hazard regression models. We treated death of non-respiratory causes as a competing event by censoring these individuals at their timing of occurrence of non-respiratory mortality. We report the associations with all-cause mortality as hazard ratios (HRs) and the associations with respiratory mortality as cause specific HR with 95% confidence intervals (CIs). Both crude and multivariate analyses were performed and we assumed independency of the competing events. A test based on Schoenfeld residuals, verified that the proportional-hazards assumption was fulfilled for all variables in the final multivariate analyses.
We a priori considered several covariates for inclusion in the multivariate models, and identified the minimal sufficient adjustment set for the multivariate models using directed acyclic graphs (DAGs) [Citation22]. Analyses of change in FEV1 and change in FVC were adjusted for sex, age, baseline 6MWT distance, baseline mMRC dyspnea, Charlson comorbidity score, baseline smoking, and participation in pulmonary rehabilitation during the first 2 years of study (yes/no). Analysis of exacerbations and of mortality (all-cause and respiratory) were adjusted for the same covariates, but the exacerbation and mortality analyses were in addition adjusted for exacerbations 12 months before baseline. Additional sensitivity analysis were done for change in FVC and change in desaturation with adjustment for baseline values of FEV1, FVC and blood gas, and for mortality and change in desaturation, with adjustment for baseline values of FEV1 and blood gas. Additional analyses were also done for change in FVC and change in desaturation with adjustment of RV/TLC. Univariate analysis of association between participating in rehabilitation and change in 6MWD were done using logistic regression analysis. Univariate analyses of association between participating in pulmonary rehabilitation and change in desaturation status, and RV/TLC and change in desaturation status were done using multinomial logistic regression.
Results
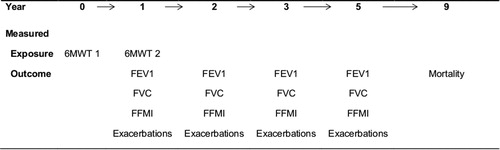
The study design is explained in the flow chart () and a timeline (). The median follow-up time was 5.3 years (95% CI 5.3–5.4). The characteristics of the 295 patients who completed the two 6MWTs are shown in . Mean 6MWD was 446 m in the first test and 454 m 1 year later. Sixty-nine (23%) patients decreased ≥30 m 6MWD. In the first test, 20% desaturated, and 30% in the second test. Of those, 13% desaturated only in the second test, and 17% in both tests.
Figure 1. Flow chart of the study design and the COPD patients who performed 6MWT at baseline and at follow-up 1 year later in the Bergen COPD Cohort Study.
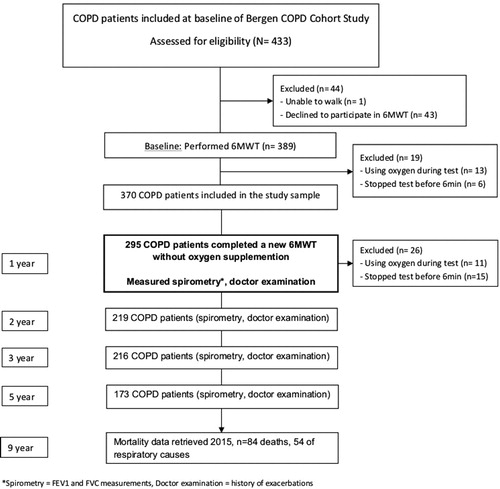
Table 1. Characteristics of 295 COPD patients in the Bergen COPD Cohort Study.
Mortality
Eighty-four (29%) patients had died by the end of 2015, 54 (18%) of respiratory causes. The crude and adjusted survival curves with all-cause mortality according to desaturation status in the two 6MWT are shown in . The adjusted HR (95% CI) for all-cause mortality among new desaturators or repeated desaturators compared to non-desaturators were 2.0 (1.0–3.8) and 2.7 (1.5–5.0), respectively (). The adjusted HR (95% CI) for all-cause mortality among patients who decreased ≥30 m 6MWD in 1 year compared to those who decreased <30 m was 1.4 (0.9–2.3) (, supporting information e-Figure 1).
Figure 3. Associations of change in desaturation status in 6MWT in 1 year and all-cause and respiratory mortality after 9 years in 295 COPD patients, Kaplan-Meier for crude analyses and Cox proportional hazard regression for adjusted analyses (graph created using the posthoc stcurve syntax in Stata which plots mortality by desaturation category while setting each covariate to its mean values). (a) Unadjusted all-cause mortality. (b) After adjustment for sex, age, 6MWD, mMRC dyspnea scale, Charlson comorbidity index, smoking status, exacerbations 12 month prior inclusion and participation in pulmonary rehabilitation. (c) Unadjusted respiratory mortality. (d) After adjustments for sex, age, 6MWD, mMRC dyspnea scale, Charlson comorbidity index, smoking status and exacerbations 12 month prior inclusion and participation in pulmonary rehabilitation.
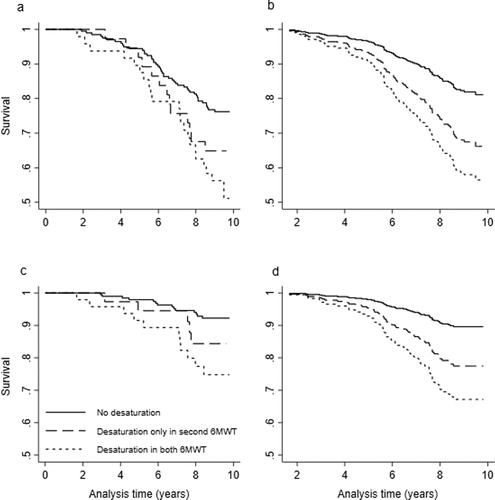
Table 2. Associations of 1 year change in walking distance and desaturation status in two 6MWTs with all-cause mortality and respiratory mortality after 9-year follow-up obtained from the Norwegian Cause of Death Registry, among 295 COPD patients in the Bergen COPD Cohort Study, using cox proportional hazard regression analyses.
The crude and adjusted survival curves with respiratory mortality for desaturation status are shown in . The adjusted cause-specific HR (95% CI) for respiratory mortality among new desaturators or repeated desaturators compared to non-desaturators was 2.3 (1.0–5.2) and 3.6 (1.7–7.6), respectively (). The adjusted HR (95% CI) for respiratory mortality among patients who decreased ≥30 m 6MWD between baseline and follow-up compared to those who decreased <30 m was 1.4 (0.7–2.6) (, supporting information e-Figure 2).
The sensitivity analyses of mortality and change in desaturation with additional adjustments of FEV1% predicted and blood gas showed that the association between repeated desaturators and respiratory mortality remained significant (HR 2.8; 95% CI 1.2–6.5). There were borderline significant associations between all-cause mortality and repeated desaturators (p = 0.06) and between new desaturators and respiratory mortality (p = 0.068) ().
Table 3. Associations of desaturation status in two 6MWTs with all-cause mortality and respiratory mortality after 9-year follow-up obtained from the Norwegian Cause of Death Registry, among 295 COPD patients in the Bergen COPD Cohort Study, using cox proportional hazard regression analyses, with additional adjustment for baseline values of FEV1% predicted and blood gas values.
Lung function
The estimated decline in FVC % predicted among new desaturators and repeated desaturators compared to non-desaturators are shown in and . The observed annual FVC decline in patients who did not desaturate or desaturated in both tests was 0.2% predicted and 0.6% predicted respectively, while the annual decline in FVC for new desaturators was 1.6% predicted. In adjusted analyses, after 1 year % predicted mean FVC for new desaturators was 4.2% above non-desaturators, but after 5 years they had declined steeper than the non-desaturators and were 0.7% below them (p = 0.04).
Figure 4. Estimated decline in FVC % predicted with 95% CI from year 1 through 5 according to desaturation status in two 6MWTs in year 0 and 1 in 295 COPD patients, from linear mixed effects models.
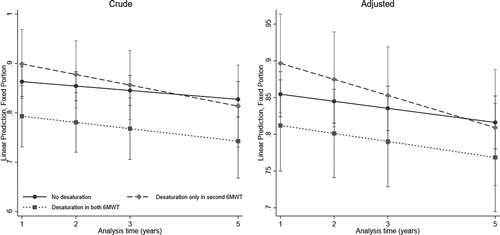
Table 4. One year change in desaturation status in two 6MWTs and association with decline in FVC % predicted over 5 years in 295 COPD patients from the Bergen COPD Cohort Study.[AQ3]
The sensitivity analyses with additional adjustments of baseline values of FEV1% predicted, FVC % predicted and blood gas showed that new desaturators was 0.9% above non-desaturators after 1 year, but after 5 years they were 4.5% below them (p = 0.03) (supporting information e-Table 1).
There were no apparent associations between decline in FVC % predicted and decrease in 6MWD, or between decline in FEV1% predicted and change in desaturation (supporting information e-Tables 2 and 3, e-Figures 3 and 4). In both crude and adjusted analyses of decrease in 6MWD, there was a tendency for larger decline in FEV1 for patients who decreased ≥30 m compared to those who did not (p = 0.06) (, supporting information e-Figure 5). The observed annual decline in FEV1% predicted in patients who decreased ≥30 m in 6MWD in 1 year was 0.8%, compared to 0.6% for those who decreased <30 m. In the adjusted analysis, mean predicted FEV1 among those who declined ≥30 m was 1.6% below the mean predicted FEV1 in those who declined <30 m after 1 year, while after 5 years they had mean predicted FEV1 of 4.0% below those with <30 m decline.
Table 5. One year change in walking distance in 6MWT and association with decline in FEV1% predicted over 5 years in 295 COPD patients from the Bergen COPD Cohort Study.
Exacerbation
The mean number of exacerbations during the 5-year follow-up period was 0.7. The crude and adjusted estimated numbers of moderate and/or severe exacerbations during follow-up are shown in the online supplement (supporting information e-Tables 4 and 5, e-Figures 6 and 7). In crude analysis there were no significant associations with change in 6MWD, while in the adjusted analysis there was a borderline significant association suggesting an increased risk of exacerbations for patients with <30 m decrease in 6MWD. There were no significant associations between change in desaturation and exacerbations.
Discussion
This study of 295 COPD patients, who completed two 6MWTs 1 year apart, shows that desaturation status in the 6MWT over time is an important risk factor for later disease prognosis. Repeated desaturation in two 6MWTs doubles the risk of all-cause mortality, and triples the risk for respiratory mortality even after adjustments for known confounders. Onset of desaturation is significantly associated with a greater decline in FVC % predicted, and we observed a tendency that also ≥30 m decrease in 6MWD was associated with future decline in FEV1% predicted. This is the first study to examine associations between change in risk factors over time and future change in important COPD outcomes such as lung function decline, exacerbations and mortality.
There are some limitations to this study. First, we did not have information on the lowest (nadir) SpO2 during the test. This could have identified more desaturators according to a study who found that patients who desaturated early in the 6MWT had a higher probability to desaturate during daily activities and to develop severe hypoxemia [Citation23]. However, having fewer patients classified as desaturators in our analyses would likely result in underestimation rather than overestimation of the associations.
Second, a healthy participant effect could be a potential selection bias as we excluded patients who stopped before 6 min and used oxygen supplementation in both 6MWTs. Additionally, some participants (94) performed 6MWT only at baseline, and are therefore not included in our study population. Their mean 6MWD (353 m) was lower than the mean 6MWD (446 m) in our study population.
Also, 12 patients desaturated only at the first 6MWT. We chose to exclude them in our analyses because of the number of patients was too small to be a reference category in the analyses, and because our main aim was to examine those who experienced a worsening of either 6MWD or desaturation.
Third, we did not have information of when the patients participated in pulmonary rehabilitation during the first 2 years of the study, making it plausible that for several patients the participation actually occurred after performing both 6MWTs.
Our study shows that repeated desaturation during exercise is a major risk factor for mortality, and especially respiratory mortality. Together with the result that onset of desaturation is associated with greater decline in FVC % predicted, we could hypothesize that onset of desaturation catches a threshold in the disease where lung function starts to decline increasingly—while when repeated desaturation occurs, this represents a more severe phase where risk of mortality increases. These results were only observed for decline in FVC % predicted, and not in FEV1% predicted. One plausible explanation for this could be air trapping. We did additional analysis assessing the relationship between baseline values of RV/TLC and desaturation status, as well as additional analyses of decline in FVC with RV/TLC added as a covariate. The results showed that RV/TLC is significantly associated with repeated desaturation, but not with new desaturation. New desaturation also remained a significant risk factor for decline in FVC even after this additional adjustment (supporting information e-Tables 6 and 7). At this point, we are unfortunately not able to explain the mechanism causing this result, however, it seems unlikely it is caused by confounding. Further studies are needed to shed more light on this, but if true, measuring desaturation status during activity in COPD patients could be an important tool for monitoring disease progression.
A question is whether desaturation could be prevented with use of oxygen supplementation. The Long-Term Oxygen Treatment Trial Research Group investigated this and found that long-term oxygen supplementation did not result in longer time to death or first hospitalization than not using supplementation [Citation24].
We did additional mortality analyses for desaturation with adjustments of baseline FEV1% predicted and blood gas to see whether these data, which is known to associate with mortality in COPD patients, were more important than desaturation status. The association remained significant for repeated desaturators (HR 2.8; 95% CI 1.2–6.5).
Although the results shows that onset of desaturation is associated with future decline in FVC % predicted, could decline in lung function also affect desaturation status? We assessed this by doing additional analysis for desaturation status and change in FVC % predicted with extra adjustments of baseline values of FEV1% predicted and FVC % predicted. We included baseline blood gas values to see if resting hypoxemia could be the underlying cause. The results shows that even after these additional adjustments, new desaturators is significantly associated with a greater decline in FVC % predicted compared to non-desaturators.
Approximately 30% of the patients who either experienced new or repeated desaturation in the 6MWTs, participated in pulmonary rehabilitation in the first 2 years of the study. In our analyses we included participation in rehabilitation as a covariate. The impact of pulmonary rehabilitation on desaturation in the 6MWT, however, is not known, and we performed an additional analysis assessing this relationship. We found no significant association between participating in pulmonary rehabilitation and either new or repeated desaturation (supporting information e-Table 6).
We found no significant association in the multivariate analyses between ≥30 m 6MWD and mortality. Our results differ from other studies where change in 6MWD has been shown to be an important risk factor for mortality [Citation11–13]. There are plausible causes for this discrepancy. First, mean 6MWD increased between the tests, and this may have affected our results. Other studies have shown that 6MWD increases after rehabilitation [Citation25], and in our study, 74 patients participated in pulmonary rehabilitation during the first 2 years of the study. Thirteen of these patients had a ≥ 30 m decline in 6MWD. In our main analyses we adjusted for participation in rehabilitation. We also performed additional analyses examining the relationship between rehabilitation and ≥30 m decline in 6MWD, and found no significant association (supporting information e-Table 8). However, as we do not have information on when the participants underwent rehabilitation during the first 2 years, we are cautious to conclude based on this analysis. Second, we could hypothesize that patients who decrease ≥30 m enters a more severe disease stage somewhere between the first and second test, while those who decrease <30 m are in a severe stage from the beginning. In that case, those who decreased ≤30 m would likely be more at risk for mortality during our follow-up period because they were severely ill already from baseline. An inspection of our data revealed that subjects who decreased <30 m had in fact a shorter mean 6MWD at baseline than those who decreased more (441 m versus 461 m), rendering this explanation plausible.
Two studies have examined change in 6MWD and association with change in lung function. The study by Karanth et al. [Citation11] included 39 COPD patients, and found no significant association between change in 6MWD and change in lung function within 1 year. The study by Pinto-Plata et al. [Citation12] included 198 COPD patients, and found no association between the change in 6MWD per year and the change in FEV1 per year within the same time period. A study by Casanova et al. [Citation14] including 294 COPD patients examined change in 6MWD over 5 years. They found that decline in 6MWD increases with disease severity while decline in FEV1 was smaller in more severe patients. While existing studies have investigated change in 6MWD and change in lung function within the same time frame, our study examined change in 6MWD and its association with later longitudinal change in FEV1% predicted. The results showed a tendency to an association between ≥30 m decrease in 6MWD and later decline in FEV1% predicted. This could mean that, although the decline in 6MWD and FEV1 are perhaps in general independent of one another, the disease enters a more severe stage when the patients decrease ≥30 m 6MWD, and that this disease progression coincides with increased decline in FEV1% predicted.
Our study also found a tendency for an association between change in 6MWD and number of exacerbations. The result actually means that decreasing ≥30 m 6MWD in 1 year may be associated with fewer exacerbations over time. This was an unexpected result, and would need verification from other populations. However, one speculative possibility could be that patients with decreasing 6MWD are more limited in their daily activities; thus indirectly avoiding potential contact with risk for contracting respiratory infections for instance.
We analyzed change in exposures, 6MWD and desaturation, prior in time to the changes in outcomes. This is a major strength in our analyses, eliminating the risk of reverse causation. Another strength is the selection of covariates. Using DAGs, we were able to identify the minimal sufficient adjustment set for our analyses—ensuring that confounders affecting both exposures and outcomes are accounted for, as well as minimizing the risk of over-adjustment. A third strength is taking competing risk into account in the analyses of respiratory mortality; taking into account that the subjects may die from other causes than respiratory diseases. This methodological framework ensures more correct estimates than methods not accounting for competing risks.
Conclusion
This is the first study to show that repeated desaturation in the 6MWT over time in COPD patients is a risk factor for mortality, both all-cause and respiratory, and that onset of desaturation is associated with future decline in FVC % predicted.
The results add important information on COPD disease progression, and measuring desaturation status in the routine evaluation of patients with the disease should be considered.
Declaration of interest
P. Bakke reports personal fees from AstraZeneca, personal fees from GlaxoSmithKline, personal fees from Chiesi, personal fees from Novartis, and personal fees from Boehringer-Ingelheim, all outside the submitted work. T.M. Eagan reports personal fees from Boehringer, outside the submitted work. The other authors have no conflict of interest to report.
Supplemental Material
Download PDF (543.9 KB)Acknowledgment
The authors thank the patients in the Bergen COPD cohort study for participation in the study, and Lene Svendsen and Eli Nordeide for help with data collection.
Additional information
Funding
References
- WHO. Global surveillance, prevention and control of chronic respiratory diseases. A comprehensive approach. 2007 [updated 2007 Aug 25; cited 2019 Jun 17]. https://www.who.int/respiratory/publications/global_surveillance/en/.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. 2019 [cited 2019 Jun 17]. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf.
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. DOI:10.1056/NEJMoa021322
- Cote CG, Pinto-Plata V, Kasprzyk K, et al. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest. 2007;132(6):1778–1785. DOI:10.1378/chest.07-2050
- Fermont JM, Masconi KL, Jensen MT, et al. Biomarkers and clinical outcomes in COPD: a systematic review and meta-analysis. Thorax. 2019;74(5):439–446. DOI:10.1136/thoraxjnl-2018-211855
- Spruit MA, Polkey MI, Celli B, et al. Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2012;13(3):291–297. DOI:10.1016/j.jamda.2011.06.009
- Casanova C, Cote C, Marin JM, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest. 2008;134(4):746–752. DOI:10.1378/chest.08-0520
- Takigawa N, Tada A, Soda R, et al. Distance and oxygen desaturation in 6-min walk test predict prognosis in COPD patients. Respir Med. 2007;101(3):561–567. DOI:10.1016/j.rmed.2006.06.017
- Kim C, Seo JB, Lee SM, et al. Exertional desaturation as a predictor of rapid lung function decline in COPD. Respiration. 2013;86(2):109–116. DOI:10.1159/000342891
- Waatevik M, Johannessen A, Gomez Real F, et al. Oxygen desaturation in 6-min walk test is a risk factor for adverse outcomes in COPD. Eur Respir J. 2016;48(1):82–91. DOI:10.1183/13993003.00975-2015
- Karanth MS, Awad NT. Six minute walk test: a tool for predicting mortality in chronic pulmonary diseases. J Clin Diagn Res. 2017;11(4):OC34–OC38.
- Pinto-Plata VM, Cote C, Cabral H, et al. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J. 2004;23(1):28–33. DOI:10.1183/09031936.03.00034603
- Polkey MI, Spruit MA, Edwards LD, et al. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med. 2013;187(4):382–386. DOI:10.1164/rccm.201209-1596OC
- Casanova C, Cote CG, Marin JM, et al. The 6-min walking distance: long-term follow up in patients with COPD. Eur Respir J. 2007;29(3):535–540. DOI:10.1183/09031936.00071506
- Eagan TM, Ueland T, Wagner PD, et al. Systemic inflammatory markers in COPD: results from the Bergen COPD Cohort Study. Eur Respir J. 2010;35(3):540–548. DOI:10.1183/09031936.00088209
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med. 2007;45(4):247–251. DOI:10.1016/j.ypmed.2007.08.012
- Waatevik M, Johannessen A, Hardie JA, et al. Different COPD disease characteristics are related to different outcomes in the 6-minute walk test. COPD. 2012;9(3):227–234. DOI:10.3109/15412555.2011.650240
- Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1447–1478. DOI:10.1183/09031936.00150414
- Wedzicha JA. Domiciliary oxygen therapy services: clinical guidelines and advice for prescribers. Summary of a report of the Royal College of Physicians. J R Coll Physicians Lond. 1999;33(5):445–447.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. DOI:10.1016/0021-9681(87)90171-8
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. DOI:10.1016/0895-4356(94)90129-5
- Evans D, Chaix B, Lobbedez T, et al. Combining directed acyclic graphs and the change-in-estimate procedure as a novel approach to adjustment-variable selection in epidemiology. BMC Med Res Methodol. 2012;12:156. DOI:10.1186/1471-2288-12-156
- Garcia-Talavera I, Tauroni A, Trujillo JL, et al. Time to desaturation less than one minute predicts the need for long-term home oxygen therapy. Respir Care. 2011;56(11):1812–1817. DOI:10.4187/respcare.01164
- Albert RK, Au DH, Blackford A, et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617–1627.
- McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.