Abstract
The isolation of Pseudomonas aeruginosa (PA) in patients with chronic obstructive pulmonary disease (COPD) is associated with increased mortality. Yet, factors associated with first PA sputum isolation, and PA persistence have not been investigated before. The objective of the present study was to investigate risk factors for new acquisition and persistence of PA infection and their relationship with all-cause mortality in patients with COPD. Post-hoc analysis of prospectively collected cohort of 170 COPD patients (GOLD II-IV) who were free of previous PA isolation and followed up every 3-6 months for 85 [50.25-110.25] months. PA was isolated for the first time in 41 patients (24.1%) after 36 [12-60] months of follow-up. Risk factor for first PA isolation were high cumulative smoking exposure, severe airflow limitation, previous severe exacerbations, high fibrinogen levels and previous isolation of Haemophilus Influenzae. PA was isolated again one or more times during follow-up in 58.5% of these patients. This was significantly associated with the presence of CT bronchiectasis and persistence of severe exacerbations, whereas the use of inhaled antibiotic treatment after the first PA isolation (at the discretion of the attending physician) reduced PA persistence. During follow-up, 79 patients (46.4%) died. A single PA isolation did not increase mortality, but PA persistence did (HR 3.06 [1.8-5.2], p = 0.001). We conclude that PA occurs frequently in clinically stable COPD patients, risk factors for a first PA isolation and PA persistence are different, and the latter (but not the former) is associated with increased all-cause mortality.
Introduction
Potentially pathogenic microorganisms (PPM) can be isolated from airway samples in a proportion of clinically stable patients with chronic obstructive pulmonary disease (COPD) [Citation1–3]. This is associated with increased bronchial and systemic inflammation, poorer clinical outcomes, including more frequent exacerbations and worse health status [Citation4–6] and, in the case of Pseudomonas aeruginosa (PA) [Citation7–12], increased mortality [Citation13–15]. Further, treatment with inhaled antibiotics appears to reduce the number and severity of exacerbations in COPD patients in whom PA was isolated [Citation16–18]. Surprisingly, however, practice recommendations pay little attention to airway infection by PA in the management of COPD [Citation19–23]. This is in sharp contrast with other chronic airway diseases, such as bronchiectasis and cystic fibrosis, where the diagnosis and treatment of both a first PA isolation and its persistence over time form a cornerstone of their management [Citation24–28]. In fact, to our knowledge, no previous study has attempted to dissect the risk factors associated with a first PA isolation from those that modulate its persistence in the airways of patients with clinically stable COPD. Here, we investigated these factors and their relationship with all-cause mortality in a cohort of patients with COPD followed up regularly for more than a decade [Citation29–31].
Methods
Study design and ethics
Post-hoc analysis of a long-term observational cohort of consecutive COPD patients recruited between January 2004 and February 2007 in two tertiary COPD outpatient clinics in Spain followed-up every 3–6 months, depending on the severity of their clinical condition, until the end of 2015. We have used this same cohort in other previous studies that addressed other scientific questions [Citation29–31]. All participants signed their informed consent, and the study was approved by the Ethics Committee of the Hospital General Universitario de Valencia; approval number: 2003-0089).
Participants
As detailed elsewhere [Citation29–31], we included in this cohort 201 patients with GOLD II-IV COPD according to the Global Initiative for Chronic Obstructive Lung Disease criteria [Citation21], who were able to provide spontaneous valid sputum samples for bacterial culture. Importantly, for the current analysis, patients in whom PA had been isolated before recruitment were excluded (). Likewise, patients with a primary diagnosis of bronchiectasis or asthma were excluded too.
Measurements
All measurements were obtained in clinically stable conditions (at least 6 weeks from an exacerbation, if any). As reported previously [Citation29,Citation31], we used standardized protocols in all patients at all clinical visits to record symptoms, exacerbations and hospitalizations. Moderate COPD exacerbations were defined by the increase in at least 2 cardinal symptoms (dyspnoea, sputum quantity or purulence) requiring oral antibiotic and/or short curse of oral steroids therapy, whereas severe exacerbations were defined as those requiring hospitalization. Spirometry was measured annually according to international guidelines, and reference values were those of Roca et al. [Citation32]. A peripheral venous blood sample for general biochemical measurements and acute phase reactants was obtained in clinic at each visit. A high-resolution computed tomography (HRCT) scan of the chest was obtained at recruitment in all patients. The presence of bronchiectasis was determined following the criteria proposed by Naidich et al. [Citation33]. At each clinical visit, patients were asked to provide at least two sputum samples for microbiological assessment. Briefly, patients were taught to collect sputum samples at home using the most sterile technique possible and were asked to bring these samples to hospital within less than 3 h after collection. Only sputum samples with <25 squamous epithelial cells and >25 leukocytes per high-powered field were processed further [Citation34]. In these samples, sputum was separated from saliva, Gram stained, and homogenized, and diluted secretions were plated on blood, chocolate, McConkey, and Saboreaud agar. A cutoff point of ≥103 CFU was used to identify abnormal positive culture results for PA or other PPM (e.g. Haemophilus influenzae – HI).
Data analysis
Descriptive results are presented as percentages, mean ± standard deviation or median [inter-quartile range]. The Kolgomorov-Smirnov test was used to analyze the normality of their distribution, and groups were compared using the Student T test, Mann-Whitney U test or Chi2 test, as appropriate.
To determine risk factors associated with the first PA isolation, we used a time-dependent Cox proportional hazard regression analysis that includes the statistically significant variables identified by the bivariate analysis between groups (, horizontal arrows). We used a forward stepwise technique (Wald test) that removed variables with p > 0.1 from the final model. Hazard ratios (HRs) and 95% confidence intervals [95%CI] were calculated. We used the same statistical approach to explore risk factors associated with PA persistence (more than one isolation) during follow-up (, horizontal arrows). All Cox models fulfilled the proportional hazard assumption.
To get a more comprehensive understanding of the potential relationships between factors related, directly and indirectly, to first PA isolation or PA persistence, we built association networks for each of these two circumstances and plotted them using Cytoscape v. 3.7.1 [Citation35]. As reported in previous studies by our group [Citation31,Citation36,Citation37], to this end we: (1) selected for analysis all variables that, pairwise, had a p value < 0.1 (Student T test, Mann-Whitney U test or Chi2 test) for first PA isolation or PA persistence; (2) these variables were then dichotomized following the criteria shown in , respectively; and, (3) we built the association network independently for first PA isolation (, panel A) or PA persistence (, panel B) using all dichotomized variables that showed a significant bivariate relationship with other ones (p < 0.01 by Chi2 test). In these networks, each node represents one dichotomized variable, its size is proportional to the prevalence of abnormal values (as defined in ) and its color indicates the variable category. Edges linking two nodes denote the existence of a significant (p < 0.01) bivariate relationship between them, edge thickness being proportional to their Odd Ratio (OR) as determined by a Chi2 test, and edge color indicating an OR significantly higher (black) or lower (green) than 1.
Figure 2. Association network for first PA isolation (Panel A) and PA persistence (Panel B). For network description and further information, see text.
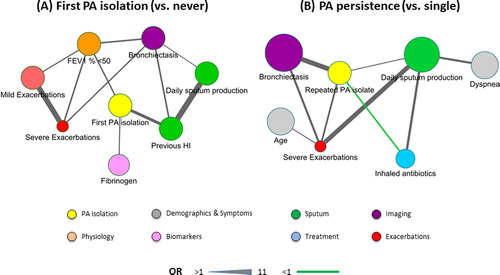
Table 1. Baseline and follow-up characteristics of patients with a first isolation of PA vs. those without any during follow-up.
Table 2. Factors associated with first isolation of PA identified by the Cox multivariate analysis, ordered by Hazard Ratio (HR) values.
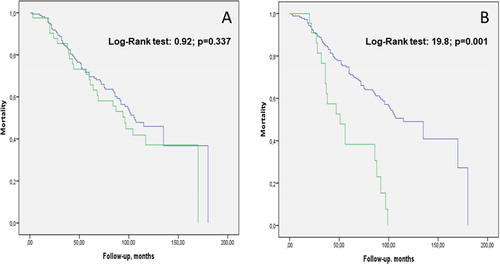
Finally, to investigate the relationship of PA isolation with all-cause mortality we compared Kaplan Meier survival curves between patients with a first and single isolation of PA vs. those with no PA isolation ever, as well as between patients with PA persistence vs. those with no isolation ever, using the log-rank test. Likewise, we used Cox regression analysis to explore the relationship between number of PA isolations during follow-up and the risk of mortality. Analyses were performed using SPSS version 20 (SPSS, Chicago, IL).
Results
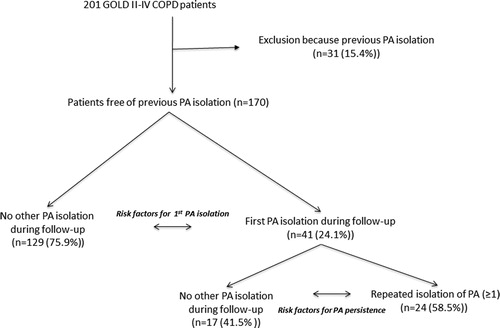
presents the consort diagram of the study. We excluded 31 patients from the 201 participants initially included in the cohort (15.4%) because they had previous PA isolation(s) before recruitment. Thus, analysis here includes 170 patients with no previous PA isolation. These patients were followed-up for 85 [50.25-110.25] months.
First PA isolation during follow-up
PA was isolated for the first time in 41 patients (24.1%) after 36 [12-60] months of follow-up (). compares the characteristics of these two groups of patients. At recruitment, patients with a first PA isolation during follow-up reported a higher cumulative smoking exposure, more frequent daily sputum production, more use of oral corticosteroids and had more severe airflow limitation, more moderate and severe exacerbations during follow-up, and higher circulating fibrinogen levels. No patients had received inhaled antibiotics and only 1 patient has received macrolides before first PA isolation. During follow-up, patients with a first PA isolation during follow-up suffer more frequent and severe exacerbations; of note, HI was isolated (before PA) more frequently whereas other PPM frequently found in COPD patients, such as Streptococcus pneumoniae or Moraxella catarrhalis were not. lists the independent risk factors associated with a first PA isolation identified by the Cox multivariate analysis. A previous isolation of HI was the one with the highest, HR 2.85 [1.51-5.5], followed by previous severe exacerbations, fibrinogen levels, cumulative smoking exposure and airflow limitation severity. (panel A) illustrates the relationships between factors related directly or indirectly to first PA isolation.
Persistence of PA during follow-up
After the first PA isolation, at the discretion of the attending physician, many patients were treated with inhaled antibiotics (70.6%) and/or macrolides (88.2%). Yet, PA was isolated again one or more times during follow-up in 58.5% of all patients with a first PA isolation (). compares the characteristics of patients with a single and only PA isolation vs. those with PA persistence. The latter were slightly older, had more symptoms, had evidence of CT bronchiectasis more often, more use of anticholinergics, and suffered more often moderate and severe exacerbations during follow-up. shows that the presence of CT bronchiectasis and a history of severe exacerbations were independently associated with PA persistence during follow-up, whereas the use of inhaled antibiotic treatment after a first PA isolation was associated with a reduced risk of PA persistence (). (panel B) presents an association network that illustrates graphically the relationships between factors directly or indirectly related to PA persistence in the patients studied here. Of note, the green edge linking use of inhaled antibiotics and persistence of PA indicates a protective effect of the former.
Table 3. Baseline and follow-up characteristics of patients with single PA isolate vs. PA persistence (≥2 isolations).
Table 4. Factors associated with persistence of PA identified by the Cox multivariate analysis, ordered by Hazard Ratio (HR) values.
Relationship with all-cause mortality
During follow-up, 79 patients (46.4%) died. (panel A) shows that mortality was not different between patients with a single and only PA isolation during follow-up vs. those without any (HR: 1.25 [0.79-1.97], p = 0.34). By contrast, the isolation of PA on ≥1 occasion(s) during follow-up (on top of the first PA isolation) was associated with a significantly higher risk of mortality (HR 3.06 [1.8-5.2], p = 0.001) (, panel B). Finally, there was a significant relationship between the number of PA isolates during follow-up and the risk of mortality (HR 1.2 [1.1-1.4], p < 0.001)
Discussion
Summary of the principal findings
The main results of this study show that in COPD patients (GOLD II-IV) with frequent sputum production followed-up long-term: (1) PA can be isolated for the first time in about a quarter of them; (2) risk factors for first PA isolation and PA persistence are different; and, (3) a first and single isolation of PA is not associated with increased all-cause mortality but repeated PA isolations during follow-up are.
Previous studies
Airway bacterial colonization is associated with worse outcomes in patients with COPD [Citation2,Citation4–6], particularly when PA is isolated [Citation1,Citation2,Citation7–15]. In fact, some previous studies suggested that treatment with inhaled antibiotics reduces exacerbations in COPD patients with PA airway infection [Citation16–18]. To our knowledge, however, no previous study has explored the risk factors for a first PA isolation vs. those for PA persistence in these patients. This information may be relevant for the proper design of future studies on antibiotic treatment in COPD [Citation15]. Likewise, no previous study has compared the impact of a single PA isolation vs. PA persistence on survival in these patients either. Yet, separating these two situations has proven to be clinically relevant in other chronic airway diseases, such as bronchiectasis and cystic fibrosis [Citation24–26].
Interpretation of findings
In our cohort, PA could be isolated for the first-time during follow-up in about a quarter of patients (). This is in line within the, otherwise wide, range of PA prevalence reported in previous cross-sectional (4-15%) [Citation1,Citation2] and longitudinal (5-40%) [Citation7–12,Citation15] studies in COPD. We acknowledge, however, that our results cannot be directly generalizable to all patients with COPD because we specifically excluded patients with previous PA isolations and we only included patients who were able to provide spontaneous valid sputum samples for analysis. Remarkably, sputum was tabulated as the percentage of patients with daily sputum production as well as muco-purulent or purulent color.
Our results indicate that, in these patients, risk factors for a first PA isolation are different from those associated with its persistence over time. The former includes markers of disease severity such as more severe airflow limitation, frequent and severe exacerbations, systemic inflammation, high smoking exposure and, interestingly, the isolation of HI in sputum. In this setting, recent studies have shown that different PPM frequently isolated in COPD patients, like HI and PA [Citation38], often interact, so future studies should investigate whether treatment of HI in COPD patients may prevent later PA isolation(s). One interesting observation is the independent relationship between the peripheral concentration of fibrinogen and the PA isolation probably reflecting the known capacity of this microorganism to lead systemic inflammation as it has been already published [Citation39]. This increase of systemic inflammation in patient with PA isolation could explain at least in part the negative impact in different outcomes in COPD patients
On the other hand, it is important to note that the presence of CT bronchiectasis was by far the most important risk factor for the persistence of PA isolation overtime as well as the occurrence of severe exacerbations. These observations are in keeping with some recent observations in this same cohort showing that severe exacerbations and chronic bronchial infection (by PA and other PPM) relate to the new development or progression of previous bronchiectasis overtime [Citation29]. Importantly, we also found that the use of inhaled antibiotic treatment after a first PA isolation reduces the risk of PA persistence. This observation is in line with previous studies in bronchiectasis or cystic fibrosis [Citation24–26]. Yet, we acknowledge that this is an observational study and that the initiation of antibiotic treatment or not was at the discretion of the attending physician, so randomized clinical trials are clearly needed in this field [Citation15].
Other interesting and clinically relevant finding of our study was that the persistence of PA isolations during follow-up in spite of specific treatment (but not a single or no PA isolation) was associated with increased all-cause mortality in our cohort (). The published studies on the relationship between PA isolation and mortality show different conclusions. Two recent studies observed an increase mortality in those patients with PA colonization [Citation10,Citation12]. However, Boutou et al. that concluded that a single PA sputum isolation was not a predictor of long-term mortality in stable COPD [Citation40]. Our results shed light on this conflicting topic and probably could help to explain the different results observed in the current studies since the impact of a single isolation of PA vs persistence of PA isolation during a stable phase of COPD patients could have a different impact on long-term mortality.
Finally, we used network analysis () to complement multivariate analysis because the latter identifies only those factors that are independently associated with the outcome of interest, whereas the former plots bivariate relationships that illustrates and inform the complexity of any biological system, as we [Citation31,Citation36,Citation37] and others [Citation41,Citation42] have done in previous studies. As shown in , there are a number of factors that are directly or indirectly related to first PA isolation and PA persistence that may need to be considered in clinical practice. In this context, PA isolation may be considered as an emergent property of a complex biological system [Citation41,Citation42].
Strengths and limitations
Our study is the first to dissect the risk factors of a first PA isolation from those associated with PA persistence in patients with clinically stable COPD. Among potential limitations we acknowledge, first, that it is an observational study where treatment was decided by the attending physician, so it cannot represent the natural history of PA airway infection in COPD patients. With this caveat in mind, however, our results provide novel information of potential clinical relevance as shown by the observed relationship with all-cause mortality (). Second, sample size is relatively small so, although p values are clearly significant, we cannot exclude a type-2 error. Third, almost all our patients were male, therefore the results cannot be generalized to females. Finally, we did not quantify sputum bacterial load and we lack information on sputum inflammatory markers that may have allowed us to make pathogenic inferences.
Conclusions
This study shows that airway infection by PA occurs frequently in patients with clinically stable COPD, that the risk factors for a first PA isolation in sputum of COPD (more severe disease and HI isolation) are different from those associated with PA persistence (CT-bronchiectasis and severe exacerbations), which can be reduced by inhaled antibiotic therapy. Finally, a first and single PA isolation does not increase all-cause mortality, but PA persistence does.
Implications for the future
The results found in our study confirm the importance of the isolation PA especially as a chronic bronchial infection in COPD patients and pave the way for prospective, well-designed, interventional studies that explore the possibility that treating chronic bronchial infection by PA infection may reduce mortality in COPD patients
Contributions
MAMG, AA and RF participated in the study's conception and design, supervised the study and wrote the manuscript. GO, DRC, MB and JJSC critically revised the manuscript and approved the final version for publication.
Financial support/funding
This work was not supported by any financial entity
Supplemental Material
Download PDF (87.4 KB)Declaration of interest
The authors of the present manuscript have nothing to disclose related to this paper. For other reasons please see the uploaded ICJME documents.
Conflict of interest
None of the authors have any conflicts of interest.
References
- Matkovic Z, Miravitlles M. Chronic bronchial infection in COPD. Is there an infective phenotype? Respir Med. 2013;107(1):10–22. DOI:10.1016/j.rmed.2012.10.024
- Leung JM, Tiew PY, Mac AM, et al. The role of acute and chronic respiratory colonization and infections in the pathogenesis of COPD. Respirology. 2017;22(4):634–650. Epub 2017/03/28. DOI:10.1111/resp.13032
- Monso E. Look at the wood and not at the tree: the microbiome in chronic obstructive lung disease and cystic fibrosis. Arch Bronconeumol. 2020;56(1):5–6. Epub 2019/06/05. DOI:10.1016/j.arbres.2019.04.017
- Sethi S, Maloney J, Grove L, et al. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(9):991–998. Epub 2006/02/14. DOI:10.1164/rccm.200509-1525OC PubMed PMID: 16474030;
- Zalacain R, Sobradillo V, Amilibia J, et al. Predisposing factors to bacterial colonization in chronic obstructive pulmonary disease. Eur Respir J. 1999;13(2):343–348. Epub 1999/03/05. DOI:10.1034/j.1399-3003.1999.13b21.x
- Zhang M, Li Q, Zhang XY, et al. Relevance of lower airway bacterial colonization, airway inflammation, and pulmonary function in the stable stage of chronic obstructive pulmonary disease. Eur J Clin Microbiol Infect Dis. 2010;29(12):1487–1493. Epub 2010/08/21. DOI:10.1007/s10096-010-1027-7
- Gallego M, Pomares X, Espasa M, et al. Pseudomonas aeruginosa isolates in severe chronic obstructive pulmonary disease: characterization and risk factors. BMC Pulm Med. 2014;14:103 DOI:10.1186/1471-2466-14-103
- Martinez-Solano L, Macia MD, Fajardo A, et al. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin Infect Dis. 2008;47(12):1526–1533. DOI:10.1086/593186
- Murphy TF. The many faces of Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Clin Infect Dis. 2008;47(12):1534–1536. DOI:10.1086/593187
- Murphy TF. Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2009;15(2):138–142. Epub 2009/06/18. DOI:10.1097/MCP.0b013e328321861a
- Murphy TF, Brauer AL, Eschberger K, et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(8):853–860. DOI:10.1164/rccm.200709-1413OC
- Rodrigo-Troyano A, Melo V, Marcos PJ, et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease patients with frequent hospitalized exacerbations: a prospective multicentre study. Respiration. 2018;96(5):417–424. DOI:10.1159/000490190
- Eklof J, Sorensen R, Ingebrigtsen TS, et al. Pseudomonas aeruginosa and risk of death and exacerbations in patients with chronic obstructive pulmonary disease: an observational cohort study of 22 053 patients. Clin Microbiol Infect. 2020;26(2):227–234. Epub 2019/06/27. DOI:10.1016/j.cmi.2019.06.011
- Almagro P, Salvado M, Garcia-Vidal C, et al. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration. 2012;84(1):36–43. DOI:10.1159/000331224
- Jacobs DM, Ochs-Balcom HM, Noyes K, et al. Impact of pseudomonas aeruginosa isolation on mortality and outcomes in an outpatient chronic obstructive pulmonary disease cohort. Open Forum Infect Dis. 2020;7(1):ofz546. Epub 2020/01/30. DOI:10.1093/ofid/ofz546
- Dal Negro R, Micheletto C, Tognella S, et al. Tobramycin nebulizer solution in severe COPD patients colonized with Pseudomonas aeruginosa: effects on bronchial inflammation. Adv Therapy. 2008;25(10):1019–1030. DOI:10.1007/s12325-008-0105-2
- Haidl P, Bargon J, Gessler T, et al. Effect of inhalation of tobramycin for 12 months on reduction of hospitalisation rate in severe COPD. Pneumologie. 2013;67(9):514–519. DOI:10.1055/s-0033-1344344
- Monton C, Prina E, Pomares X, et al. Nebulized colistin and continuous cyclic azithromycin in severe COPD patients with chronic bronchial infection due to pseudomonas aeruginosa: a retrospective cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:2365–2373. DOI:10.2147/COPD.S209513
- Pesola GR, Dogra S. Stopping inhaling corticosteroids in COPD. Am J Respir Crit Care Med. 2003;168(2):256–257. DOI:10.1164/ajrccm.168.2.951
- Miravitlles M, Soler-Cataluna JJ, Calle M, et al. Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2017;53(6):324–335. DOI:10.1016/j.arbres.2017.03.018
- Halpin DMG, Criner GJ, Papi A, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(1):24–36. DOI:10.1164/rccm.202009-3533SO
- López-Campos JL, Soler-Cataluña JJ, Miravitlles M. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2019 report: future challenges. Arch Bronconeumol. 2020;56(2):65–67. Epub 2019/07/20. DOI:10.1016/j.arbres.2019.06.001
- Miravitlles M, Calle M, Soler CJJ. GesEPOC 2021: one more step towards personalized treatment of COPD. Arch Bronconeumol. 2021;57(1):9–10. Epub 2020/09/28. DOI:10.1016/j.arbres.2020.08.002
- Martínez-García MÁ, Máiz L, Olveira C, et al. Spanish guidelines on the evaluation and diagnosis of bronchiectasis in adults. Arch Bronconeumol. 2018;54(2):79–87. DOI:10.1016/j.arbres.2017.07.015
- Martinez-Garcia MA, Maiz L, Olveira C, et al. Spanish guidelines on treatment of bronchiectasis in adults. Arch Bronconeumol. 2018;54(2):88–98. DOI:10.1016/j.arbres.2017.07.016
- Polverino E, Goeminne PC, McDonnell MJ, et al. European respiratory society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. DOI:10.1183/13993003.00629-2017
- Redondo M, Chalmers JD. Bronchiectasis guidelines-recommendations into practice. Arch Bronconeumol. 2019;55(6):286–288. Epub 2019/04/15. DOI:10.1016/j.arbres.2018.10.012
- Martinez-Garcia MA, de la Rosa D, Canton R, et al. Bronchiectasis: when the published scientific evidence proves insufficient. Arch Bronconeumol. 2019;55(6):283–285. Epub 2019/06/18. DOI:10.1016/j.arbres.2019.05.001
- Martinez-Garcia MA, de la Rosa-Carrillo D, Soler-Cataluna JJ, et al. Bronchial infection and temporal evolution of bronchiectasis in patients with chronic obstructive pulmonary disease. Clin Infect Dis. 2021;72(3):403–410. DOI:10.1093/cid/ciaa069
- Martinez-Garcia MA, de la Rosa Carrillo D, Soler-Cataluna JJ, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(8):823–831. Epub 2013/02/09. DOI:10.1164/rccm.201208-1518OC
- Martinez-Garcia MA, Faner R, Oscullo G, et al. Inhaled steroids, circulating eosinophils, chronic airway infection and pneumonia risk in chronic obstructive pulmonary disease: a network analysis. Am J Respir Crit Care Med. 2020;201(9):1078–1085. Epub 2020/01/11. DOI:10.1164/rccm908-1550OC
- Roca J, Sanchis J, Agusti-Vidal A, et al. Spirometric reference values from a Mediterranean population. Bull Eur Physiopathol Respir. 1986;22(3):217–224.
- Naidich DP, McCauley DI, Khouri NF, et al. Computed tomography of bronchiectasis. J Comput Assist Tomogr. 1982;6(3):437–444. DOI:10.1097/00004728-198206000-00001
- Murray PR, Washington JA. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin Proc. 1975;50(6):339–344. Epub 1975/06/01.
- Cline MS, Smoot M, Cerami E, et al. Integration of biological networks and gene expression data using cytoscape. Nat Protoc. 2007;2(10):2366–2382. DOI:10.1038/nprot.2007.324
- Cruz T, Lopez-Giraldo A, Noell G, et al. Multi-level immune response network in mild-moderate Chronic Obstructive Pulmonary Disease (COPD). Respir Res. 2019;20(1):152. DOI:10.1186/s12931-019-1105-z
- Noell G, Cosio BG, Faner R, et al. Multi-level differential network analysis of COPD exacerbations. Eur Respir J. 2017;50(3):1700075. DOI:10.1183/13993003.00075-2017
- Jacobs DM, Ochs-Balcom HM, Zhao J, et al. Lower airway bacterial colonization patterns and species-specific interactions in chronic obstructive pulmonary disease. J Clin Microbiol. 2018;56(10) DOI:10.1128/JCM.00330-18
- Menéndez R, Méndez R, Amara-Elori I, et al. Systemic inflammation during and after bronchiectasis exacerbations: impact of Pseudomonas aeruginosa. J Clin Med. 2020;9(8):2631. DOI:10.3390/jcm9082631
- Boutou AK, Raste Y, Reid J, et al. Does a single Pseudomonas aeruginosa isolation predict COPD mortality? Eur Respir J. 2014;44(3):794–797. DOI:10.1183/09031936.00023414
- Barabasi AL. Network medicine–from obesity to the "diseasome. N Engl J Med. 2007;357(4):404–407.
- Barabasi AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet. 2011;12(1):56–68. DOI:10.1038/nrg2918