 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Asthma patients may have an increased risk for diagnosis of chronic obstructive pulmonary disease (COPD). However, risk factors accelerating time-to-COPD diagnosis are unclear. This study aims to estimate risk factors associated with the incidence of COPD diagnosis in asthma patients. Canada’s Population Data BC (PopData BC) was used to identify asthma patients without prior COPD diagnosis between January 1, 1998, to December 31, 1999. Patients were assessed for time-to-incidence of COPD diagnosis from January 1, 2000, to December 31, 2018. The study estimated the effects of several risk factors in predicting the incidence of COPD in asthma patients during the 18-year follow-up period. Patient factors such as Medication Adherence (MA) were assessed by the proportion of days covered (PDC) and the medication possession ratio (MPR). The log-logistic mixed-effects accelerated failure time model was used to estimate the adjusted failure time ratios (aFTR) and 95% Confidence Interval (95% CI) for factors predicting time-to-COPD diagnosis among asthma patients. We identified 68,211 asthma patients with a mean age of 48.2 years included in the analysis. Risk factors accelerating time-to-COPD diagnosis included: male sex (aFTR: 0.62, 95% CI:0.56–0.68), older adults (age > 40 years) [aFTR: 0.03, 95% CI: 0.02–0.04], history of tobacco smoking (aFTR: 0.29, 95% CI: 0.13–0.68), asthma exacerbations (aFTR: 0.81, 95%CI: 0.70, 0.94), frequent emergency admissions (aFTR:0.21, 95% CI: 0.17–0.25), longer hospital stay (aFTR:0.07, 95% CI: 0.06–0.09), patients with increased burden of comorbidities (aFTR:0.28, 95% CI: 0.22–0.34), obese male sex (aFTR:0.38, 95% CI: 0.15–0.99), SABA overuse (aFTR: 0.61, 95% CI: 0.44–0.84), moderate (aFTR:0.23, 95% CI: 0.21–0.26), and severe asthma (aFTR:0.10, 95% CI: 0.08–0.12). After adjustment, MA ≥0.80 was significantly associated with 83% delayed time-to-COPD diagnosis [i.e. aFTR =1.83, 95%CI: 1.54–2.17 for PDC]. However, asthma severity significantly modifies the effect of MA independent of tobacco smoking history. The targeted intervention aimed to mitigate early diagnosis of COPD may prioritize enhancing medication adherence among asthma patients to prevent frequent exacerbation during follow-up.
Introduction
Asthma affects millions of people worldwide and it is associated with significant morbidity, resulting in high health care utilization [Citation1, Citation2]. Recent epidemiological studies have speculated patients with asthma may have increased risk for COPD development regardless of tobacco smoking history [Citation3–8]. In a recent meta-analysis [Citation9] patients with prior history of asthma were 7.87 times more likely to develop COPD in later life after controlling for relevant confounders.
Despite the strong association between prior history of asthma and subsequent COPD development, patient factors accelerating or delaying the progressive lung function decline in asthma patients and subsequent risk of COPD are unclear. Very few related studies have attempted to explain the factors progressing asthma to COPD incidence over time. For instance, in a study by To et al. (2016) [Citation10], air pollution was a strong predictor for the development of COPD in asthma patients or asthma-COPD overlap (ACO). ACO has shown a strong association with increased disease severity and poorer quality of life compared with patients with asthma or COPD alone [Citation11]. Other individual risk factors associated with increased risk of COPD among women with asthma included low education, high body mass index, rurality, and cigarette smoking, while air pollution (fine particulate matter) was not a predictor [Citation11]. While there exists mixed evidence on the risk factors responsible for COPD development in asthma patients, the existing literature on the subject matter is very limited.
In the general population, the risk factors that speed up asthma progression to COPD diagnosis in later life have not been widely explored in the current literature. Understanding and investigating the modifiable risk factors driving the acceleration or deceleration of asthma to COPD diagnosis will enable specific interventions and prevention strategies to be targeted to reduce the burden of COPD diagnosis in asthma patients. This study aims to estimate the risk factors for the diagnosis of COPD in patients with asthma in a 20-year observational cohort study in Canada.
Subjects, materials and methods
Study design, data sources and variable definitions
We used a cohort design that links four health administrative claim databases obtained from the Population Data in the British Columbia (PopData BC) province of Canada. The PopData BC covers health records of all residents of BC in the provinces insurance program and facilitates interdisciplinary research on the risk factors of human health, well-being, and development [Citation12, Citation13]. The dataset includes longitudinal de-identified electronic medical data from the following sources: (a) the Discharge Abstract Databases (DAD) [containing patients’ hospitalization and discharge records] [Citation14], (b) the Medical Service Plan (MSP) capturing data on patients visits to physicians [Citation15], (c) the PharmaNet database (contains records of all dispensed medications) [Citation16], and (d) the demographic and registration database (consolidated file) capturing demographic records of patients [Citation17, Citation18].
Health records captured in all four databases spanned from January 1, 1998, to December 31, 2018. The study cohort was identified during the index period (between January 1, 1998, and December 31, 1999) as patients with physician-diagnosed asthma with no prior history of COPD diagnosis. The two-year index period defined between January 1, 1998, and December 31, 1999, was used as the wash-in period to increase the incident asthma cases at baseline.
Cohort definition
We employed a validated case definition to identify all physician-diagnosed adult asthma patients from three databases (DAD, MSP, and PharmaNet) [Citation12, Citation19]. Patients were included in the source population if they met at least one of the following criteria:
Patients who had at least one record of asthma-related hospitalizations in the DAD database based on the international classification of diseases-9th edition (ICD-9): 493.x, ICD-10th edition: J45, J46 within a calendar year,
Patients having records of at least 2 physician visits for asthma based on diagnostic codes (ICD-9 codes: 493.x) during a calendar year. The identified asthma patients in the MSP and DAD databases were further validated in the PharmaNet database if they filled at least four asthma related-medications within the same calendar year (see attached asthma-related drug lists in the supplementary material in Table S2).
Table 2. Bivariate analysis* of the association between patient factors predicting risk of COPD.
Based on the case definition specified above, adult asthma patients aged 18 years and older with no prior history or diagnosis of COPD between January 1, 1998, and December 31, 1999, were included in the study cohort. The study followed the identified asthma cohort from January 1, 2000, to December 31, 2018, to measure the risk factors and the outcome of interest over time.
COPD outcomes measure
Our primary outcome was time to the first diagnosis of COPD during the study follow-up period. Using a validated case definition by Chen et al., 2017 [Citation20], COPD was defined based on at least one of the following criteria:
Patients with records of at least one hospitalization with COPD as the most responsible diagnosis using ICD-9 diagnostic codes: 491.xx, 492.xx, 493.2x, 496.xx, and ICD-10 diagnostics codes: J43.xx, J44.xx.
Patients having records of at least one outpatient visit with COPD as the main responsible diagnosis based on ICD-9 diagnostic codes: 491.xx, 492.xx, 493.2x, 496.xx, and ICD-10 diagnostic codes J43.xx, J44.xx.
Risk factor measures
Several socio-demographic information captured for each patient included a year of the index (January 01, 1998, to December 31, 1999), patient sex (male, female), obesity (body mass index > 30 kg/m2), patients age (categorized as “<30 years”, “30–40 years”, and “40 years and older”), and lifestyle variables such as tobacco use/nicotine dependence were examined. Asthma medication adherence (MA) was assessed by two proxy variables on a scale of “0 to 1”: defined as the proportion of days covered (PDC) and the medication possession ratio (MPR). We estimated the PDC as the ratio of the sum of days of medication covered to the sum of days between the first and the last refill [Citation21, Citation22]. Further, we estimated the MPR measure as the ratio of the sum of days of medication supplied to the sum of days between the first and the last fill dates [Citation21, Citation22]. We used the 0.80 cutoff value to classify patients into high-adherent (≥0.80) and low-adherent (<0.80) groups as consistently reported in the literature [Citation23, Citation24].
Another factor considered in this study was the Charlson comorbidity index (CCI), responsible for measuring the burden of comorbid conditions among the identified asthma patients after excluding asthma from the score. The CCI scores were grouped into three classes (CCI score 0; CCI score 1; CCI score ≥ 2) as documented in a study by Nunez et al., (2004) [Citation25]. Additionally, we extracted some specific asthma-related comorbidities using ICD codes at baseline (namely sinusitis and upper respiratory diseases). The authors included asthma-related comorbidities because they have been widely documented as a contributing factor for asthma exacerbation and subsequent persistent airflow limitations [Citation26, Citation27].
Healthcare utilization variables including all emergency hospital admissions, specifically intensive care unit [ICU] (asthma and non-asthma related), asthma hospitalizations (yes = 1, vs no = 0), emergency department visits (yes = 1, vs no = 0), and length of hospital stay (categorized as “0”, “1 day”, “2 days”, and “≥3 days”) were also considered. Asthma exacerbation was defined as the occurrence of one or more asthma-related hospitalizations, asthma-related emergency department visits, and episodes of asthma that required a prescription of oral corticosteroids (OCS) [Citation28, Citation29].
Asthma severity levels (categorized as mild, moderate, and severe) were also considered. Patients who had records of prescribed dosages of inhaled corticosteroids (ICS)[such as budesonide, ciclesonide, fluticasone furoate and belomethasone dipropriate] of “0–500 µg/day” without the additional intake of other asthma controller drugs were classified as “mild”. Patients having records of prescribed ICS doses of “0–250 µg/day” and taking additional controller therapies were also defined as mild asthmatic patients. In addition, mild asthma patients must not have a marker of moderate to severe asthma exacerbation or should not have used at least 3 doses of SABA every week within a 12 month period. Moderate asthma patients had records of at least “500 µg/day” doses of ICS with no additional intake of other controller therapies or having a prescription of more than “250 µg/day” doses of ICS plus additional inhalation of other controller therapy. Severe asthma patients were identified as having records of more than 1000 µg/day of ICS doses plus inhalation of more than 10 doses of short-acting beta-2 agonist (SABA) per week [Citation30].
Lastly, the SABA overuse variable was extracted from the PharmaNet database using drug identification numbers (DINs). SABA overuse was defined as patients having a prescription of at least 3 SABA canisters within a year (12 month period) [Citation28]. The SABA overuse variable was dichotomized as overuse (>2 SABA canisters) =1, and appropriate (≤2 SABA canisters).
Statistical methods and analysis
We used the “SAS version 9.4” and “STATA version 16” softwares for performing data analyses. Standard descriptive statistics (specifically frequency table, measures of central tendencies, and measures of dispersion) were used to present baseline characteristics.
Further, we performed bivariate analyses between the individual covariates and the risk of COPD. For the bivariate and multivariate analyses, the study used the mixed-effects log-logistic accelerated failure time (AFT) regression to model the correlated (multiple observations recorded on the same individual) survival data with possible censoring. Thus, traditional methods of estimation that consider observations as independent are inappropriate for this database [Citation31]. The AFT model was considered over the cox proportional hazard (PH) model since the data violated the proportional hazard assumption. The violation of the PH assumption in a Cox PH model can lead to misinterpretation of the estimation results and as well decrease the power of the statistical tests [Citation32]. The AFT regression models survival times directly and assumes a multiplicative effect of covariates on survival time [Citation31, Citation33]. The mixed-effects log-logistic AFT model incorporates random effects with dependence structures to account for within-cluster association [Citation31]. We used the AFT regression methods to estimate an unadjusted and adjusted failure time ratio (FTR) and 95% confidence interval (2-sided p-values) and to check for model assumptions.
Mixed effects log-logistic AFT regression model
The AFT model describes a direct linear relationship between the log of the failure time and sets of explanatory variables. The exposure variables or the covariates accelerate or slow down the expected failure time (median failure time). For clustered data, individuals are normally correlated within a cluster. Mixed-effects log-logistic AFT models account for dependencies of repeated responses on one individual over time by incorporating a random component to the model. The equation/model can be written on a log-scale as:
where Ω = z’ijbi is the random component which is distributed across the individual patient clusters. The random component also accounts for the effects of all relevant unmeasured covariates in the model. From the equation, β
is the regression coefficient for the fixed effects of covariate vector X, and bi
represents the vector of random effects with a set of random covariate vector zij
. The coefficients of the random covariates bi
are distributed with zero (0) mean and variance covariance matrix Σ = Σ (θ)
where θ
is an unknown vector parameter [Citation31].
Interpretation of the mixed effects log-logistics AFT model
The effects of the covariates determined by the regression coefficients are interpreted as accelerating or decelerating the time-to-COPD incidence in asthma patients after controlling for random covariates in the model over time. The adjusted Failure Time Ratio (aFTR) is estimated as the acceleration factor. The acceleration factor or the aFTR for a given risk factor is estimated as the exponent of the corresponding regression coefficient. An aFTR >1 implies the effect of the covariate or risk factor increases the survival time and delays their time to COPD onset in asthma patients over time. However, aFTR < 1 signifies that a covariate is at an increased risk of developing COPD in asthma patients over time or the factor is associated with an earlier time to COPD onset. If aFTR = 1, then there exists no change in the effect of covariate on COPD incidence [Citation34].
Sample size calculations
Power and sample size calculation was conducted in STATA using the command (stpower cox). The command was used to compute sample size, power and effect size using the standard Cox proportional hazard model. Assuming a 10% increase in MA for the asthma patient cohort exposed to only short acting beta-2 agonist [SABA] (including those taking very little ICS) and a COPD diagnosis in asthma patient rate of 20%, with power (1-β) of 80% and type 1 error (α) of 5%, a minimum of 13,028 asthma patients are required to detect a 20% relative risk reduction of COPD diagnosis. (i.e. hazard ratio of 0.80) in 20 years.
Ethics approval
Our study protocol was approved by the Health Research Ethics Board (HREB) at Memorial University of Newfoundland (reference number: 2019.216).
Results
reports the baseline characteristics of the 68,211 patients with physician-diagnosed asthma at baseline identified from the four linked databases between January 01, 1998, and December 31, 1999. The incidence of COPD diagnosis was determined in the 18-year follow-period from January 01, 2000, to December 31, 2018. After 1,036,811 years of person-time of follow-up, a total of 10,170 (15% of 68,211) were diagnosed with COPD. By the severity of the disease, the incidence of COPD diagnosed among the mild asthma patients was 0.85 per 1000 person-years (n = 886), among moderate asthma patients was 2.82 per 1000 person-year (n = 2924) and among severe asthma patients was 6.13 per 1000 person-year (n = 6360).
Table 1. Characteristics of physician-diagnosed asthma patients (N = 68,211).
The mean age of the study cohort was 48.20 years with the majority aged within “40 years and older” and male sex (n = 27,756, 40.69%). With regards to the burden of comorbid conditions associated with asthma patients at baseline, 97.88% of the patients constituting the majority had no comorbid condition (CCI score 0), while 1226(1.80%) had “CCI score of 1”, and 219 (0.32) had at least a CCI score of 2, respectively. Additionally, two asthma-related comorbid conditions namely sinusitis (108, 0.16%) and upper respiratory infections (284, 0.42%) were identified at baseline.
A total of 1023(1.50%) patients had records of emergency admissions (both asthma related and non-asthma related), 2427(3.56%) had emergency asthma hospitalizations, and 3158(4.63%) had emergency department visits. The median length of hospital stay was estimated as 3.00 with an interquartile range of 4.00. The majority of the asthma patients were prescribed ICS (n = 26034, 38.17%) and SABA (n = 24428, 35.81%), respectively. A total of 2636(3.86%) collected long-acting beta-2 agonist (LABA), 90(0.13%) were prescribed with the combined ICS/LABA at baseline, 1046(1.53%) had Leukotriene receptor antagonists (LTRA). Additional medications prescribed to the study cohort included Inhaled mast cell stabilizers, theophylline, short-acting muscarinic antagonist (SAMA), and others.
Bivariate analysis
summarizes the output of the unadjusted failure time ratio (uFTR) in the bivariate analysis and associated 95% confidence interval (95% CI) and p-values. In selecting patient factors associated with the time-to-COPD diagnosis from baseline patient’s characteristics, we set the maximum significance level of “at most 0.20” to include as many covariates as possible into the multivariate model. Based on the results, any covariates that were significantly related to time-to-COPD onset with significant levels ranging from “0.00–0.20” were included in the multivariate model. Significant factors identified in the bivariate analysis were male sex, patients’ age category (<30 years, 30–40 years, 40 + years), length of hospital stay (days), emergency admission, asthma exacerbation, Charlson comorbidity index, history of tobacco use/nicotine dependence, asthma-related comorbidities, asthma severity levels (mild, moderate, and severe), SABA overuse, and medication adherence levels (“high”, “low”). For instance, male sex patients were at an increased risk of developing COPD, with a shortened time to COPD onset with an unadjusted time ratio of 0.52; 95% CI: (0.47–0.58). Also, compared to patients aged “less than 30 years”, individuals who were “40 years and older” were 98% more likely to develop COPD faster (i.e. uFTR: 0.02, 95% CI: 0.01–0.03).
The patient history of tobacco use/nicotine dependence’ at baseline was statistically and clinically relevant at the bivariate level with an increased risk of COPD diagnosis (uFTR: 0.21, 95% CI: 0.08-0.52). That is, asthma patients who frequently smoke tobacco compared to nonsmokers speed up their risk of COPD incidence by 79%. The patient-level effect of asthma exacerbation, Charlson’s comorbidity index, PDC and MPR varies significantly over the follow-up period in the mixed effect AFT analysis.
Multivariate analysis
The results of the multivariate analysis are summarized in and 3a (supplementary material) with adjusted failure time ratios (aFTR) and their corresponding 95% confidence interval (95% CI). Patient demographic factors associated with increased risk of COPD diagnosis with a faster time to COPD incidence from baseline were male sex (aFTR: 0.62, 95% CI: 0.56–0.68) and patients aged “≥40 years” (0.03; 95% CI: 0.02–0.04, p < 0.0001) Thus, male sex patients have 38% shorter time to COPD onset compared to the female sex patients. Also, the time-to-incidence of COPD was 97% shorter among older adults (40 years and older) than individuals below the age of 30 years.
Table 3. Multivariate analysis of risk factors for time-to-COPD incidence-PDC model*.
With regards to healthcare utilization patient factors, patients who had asthma admission and stayed in the hospital for at least a day were significantly associated with increased risk of time-to-COPD diagnosis (aFTR: 0.13, 95% CI: 0.12–0.15 for 1 day; and 0.07, 95% CI: 0.06–0.09 for ≥2 days) compared to no hospitalizations. In other words, asthma patients who were hospitalized for more than a day were 93% more likely to develop COPD faster than patients who were not hospitalized. Similarly, patients with history of asthma emergency admission (aFTR: 0.21, 95% CI: 0.17–0.25), asthma exacerbation (aFTR: 0.81, 95%CI: 0.70–0.94), tobacco use or nicotine dependence (aFTR: 0.29, 95%CI: 0.13, 0.68) have an increased risk of early COPD diagnosis. Thus, individuals who were exposed to tobacco smoking were 71% more likely to develop COPD faster than non-tobacco smokers. Also, compared to asthma patients who did not experience exacerbations, patients who experienced asthma exacerbations over time were 19% more likely to speed-up their risk of future COPD incidence.
Likewise, patients with greater comorbid conditions measured by CCI showed a greater risk of COPD diagnosis. Thus, individuals with increased comorbidity burden were 72% more likely to have a faster diagnosis of COPD compared to patients with fewer or no preexisting conditions. Patients with a history of severe asthma were associated with a greater risk of COPD diagnosis with 90% shorter time to COPD incidence (aFTR: 0.10, 95% CI: 0.08–0.12) compared to those with mild asthma after controlling for relevant covariates, confounders and the random components. Patients with optimal medications adherence assessed by PDC ≥ 0.80 over time were significantly less likely to develop COPD with a prolonged time to the disease onset (aFTR: 1.83, 95% CI: 1.54, 2.17) compared to the non-adherent patients. Thus, patients who optimally adhered to their prescribed medications over time were 83% more likely to slow down or delay future incidence of COPD. Furthermore, the use of more than 2 SABA canisters within a year (12 months) compared to the appropriate use (≤ 2 canisters) was a significant predictor for COPD incidence. In addition, the identified risk factors accelerating time-to-COPD incidence have been summarized in Figure S1 in the supplementary material.
Figure 1. Plot of association between the effect of patient factors and time to COPD diagnosis.
The model was aadjusted for Sex, age, emergency admissions, tobacco use/nicotine dependence, obesity; Charlson Comorbidity Index, asthma-related comorbidities, sinusitis, and upper respiratory infection
Where ICS = Inhaled corticosteroids; MA = Medication adherence; PDC = Proportion of Days Covered; MPR = Medication Possession Ratio; SABA = Short acting beta-2 agonist
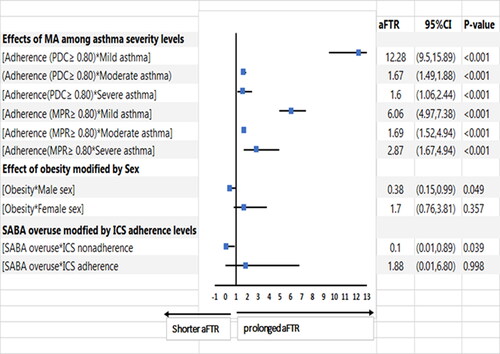
presents the effect modification of medication adherence by asthma severity, sex by obesity, and SABA overuse by ICS adherence and risk of time to COPD diagnosis. Medication adherence ≥0.80 assessed by both PDC and MPR in mild asthma was associated with the greatest protection for delayed COPD diagnosis in asthma patients. History of obesity in male sex asthma patients was a significant risk factor for early diagnosis of COPD.
Discussion
In this study, we examined the role of asthma patients’ demographic factors, lifestyle factors, healthcare utilization factors, medication adherence, SABA usage, and other risk factors on the progression to diagnosis of COPD. Significant risk factors found to accelerate time to COPD incidence in patients with asthma included male sex, older patients (40 years and older), history of tobacco use, patients with increased length of hospital stay, asthma exacerbation including asthma-related hospitalization, patients with increased comorbidity burden (CCI score ≥1), moderate and severe asthma, SABA overuse, obese male sex, and medication non-adherence among asthma patients over time.
Of note, the risk factors linking asthma and its progression to COPD have not been thoroughly investigated. Whereas most asthma patients have mild to moderate disease that can easily be controlled with medications, a small cluster of asthma patients are not well controlled [Citation35]. It has been well established that a significant proportion (representing 5–10%) of asthma patients develop persistent airflow obstruction despite optimal therapy [Citation36, Citation37]. Such patients are more likely to have a poorer prognosis [Citation38]. The persistent obstruction in severe asthma patients over time becomes indistinguishable from the chronic airflow limitation seen in patients with physician-diagnosed COPD.
This is one of the few epidemiological cohort studies to investigate and identify individual risk factors associated with increased risk of COPD in patients with asthma using a large population-based cohort study. The longitudinal nature of the database (clustered survival data) necessitated the use of the mixed-effects log-logistic AFT model to account for time-dependent covariates, clustered components, and unobserved covariates in the model. The findings of this study are partly consistent with the limited existing studies. For instance, a previous cohort study by To and colleagues (2016) [Citation10], found cigarette smoking, “BMI ≥ 30”, and higher exposure to particulate matter (PM2.5) as significant risk factors for developing COPD in asthma patients. Also, in a study to quantify the risk of developing COPD in Ontario women with asthma, low level of education, high BMI, rurality, and increased levels of cigarette smoking were associated with increased risk of COPD incidence. However, exposure to fine particulate matter was not significantly associated with the risk of COPD in asthma patients [Citation11]. Indeed, the results of the latter study, which was restricted to women, suffered from the limitation where the estimates may not accurately represent lifelong exposure to air pollutants. To date, limited epidemiological studies have examined the risk factors for developing COPD in asthma patients using a large population-based study.
Our findings contribute to the existing knowledge that male sex, exposure to tobacco smoking, older patients (≥40), asthma exacerbations, obese male patients, asthma severity, SABA overuse, medication non-adherence and increased burden of comorbidities elucidate the diagnosis of COPD in asthma patients. Sex related differences exist in patients with COPD and other related obstructive airway diseases (OADs) such as ACO [Citation39–42]. Emerging evidence indicates that female sex is more susceptible to COPD and this could be attributed to increased tobacco use in women [Citation39–42]. However, the mechanism explaining this phenomenon is largely unknown.
Whereas previous studies found frequent use of SABA overuse as a major risk factor for severe asthma exacerbations [Citation28, Citation43], our study further found increased risk of COPD among asthma patients who overused their SABA. Additionally, the effect of SABA overuse among asthma patients who did not adhere to prescribed ICS was a significant risk factor for COPD development with a 90% shorter time to COPD onset (aFTR: 0.1, 95% CI:0.01–0.89; see ). Current clinical practice guidelines such as Global Initiative for Asthma (GINA) [Citation44], British thoracic society (BTS) [Citation45], and Canadian Thoracic Society (CTS) [Citation46] recommend against the use of SABA as monotherapy and increasingly endorse the use of ICS as the first-line controller therapy for all ages. Recent documented evidence show that substituting SABA with fast-acting LABA/ICS therapy reduces patients’ risk of severe exacerbations by one-third [Citation47]. Thus, healthcare providers (HCPs) particularly clinicians who treat patients with asthma are encouraged to adhere to the recommendations in the treatment guidelines to improve best practice of the management of the disease. Consequently, interventions such as decision support tools, feedback and audit, and clinical pharmacy support [Citation48] are encouraged to improve HCPs compliance to the treatment guidelines recommendations.
Also, patients who had a greater percent of medication adherence over time were significantly less likely to experience early diagnosis of COPD compared to non-adherent patients. The association between medication adherence and risk of COPD was modified by the various levels of asthma severity as mild asthma patients had a prolonged time to developing COPD than the moderate and severe patients.
Limitations
Despite the strength of this study, some limitations that could have influenced the study to some extent are worth noting. First, is the completeness of the list of patients’ factors identified from the PopData BC administrative databases. There is a potential for unmeasured confounding variables, that is, the database used was limited to some important clinical data such as laboratory findings, pulmonary function tests, environmental factors and some lifestyle variables. Unmeasured variables could have introduced residual confounding into the model. However, we adopted a robust statistical approach and proxy variables to account for these unmeasured confounders.
Second, we have used prescription data as a proxy for measuring medication adherence in asthma patients. There is the risk that we may have under-or-overestimated the exposure due to the occurrence of primary non-adherence (patients never file a prescription written by their provider) or secondary non-adherence (fill a prescription but partially or never consumed medication). The third relates to the potential ascertainment bias or misclassification associated with asthma and COPD diagnosis from the DAD, MSP, and the PharmaNet databases. However, previous studies have demonstrated that these databases are highly valid for confirmed asthma in obstructive airway diseases, especially for COPD diagnosis [Citation12, Citation20].
Conclusion
In conclusion, the present large population-based study with an 18-year follow-up period highlights some important risk factors that are likely to accelerate asthma patients’ progression to COPD diagnosis. Of note, some individual factors such as adherence to asthma medications over time (PDC ≥ 0.80) delay and slows down their risks of developing COPD over time. Healthcare providers and policymakers should emphasize greater medication adherence as preventive and interventions measures capable of reducing the risk of COPD in asthma patients. Patient-specific education and counseling should be intensified to increase their awareness of the importance of adhering to prescribed medications over time, minimizing unhealthy lifestyles such as cigarette smoking and sedentary lifestyles leading to obesity, particularly in male patients.
Disclaimer
All inferences, opinions, and conclusions drawn in this study are those of the authors, and do not reflect the opinions or policies of the Data Steward(s) of population data BC.
Acknowledgments
We acknowledge the Population Data BC (PopData) for their effort in providing a partial waiver for the acquisition of the data.
Conflict of interest
The authors declare no competing interests and that this study has not been published previously in a similar form.
Funding
This study was supported by the Dean of Faculty of Medicine, Memorial University of Newfoundland Research Support Fund; Research, and Graduate Studies (RGS), Faculty of Medicine, Memorial University of Newfoundland; and the TPMI/NL SUPPORT Educational scholarship.
References
- World Health Organization (WHO). Asthma: key facts 2021. [Internet]. [cited 2021 Aug 9]. Available from: https://www.who.int/news-room/fact-sheets/detail/asthma.
- Dharmage S, Perret J, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7(246):246.
- Silva G, Sherrill D, Guerra S, et al. Asthma as a risk factor for COPD in a longitudinal study. Chest. 2004;126(1):59–65. DOI:10.1378/chest.126.1.59
- Tai A, Tran H, Roberts M, et al. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax. 2014;69(9):805–810. DOI:10.1136/thoraxjnl-2013-204815
- Tagiyeva N, Devereux G, Fielding S, et al. Outcomes of childhood asthma and wheezy bronchitis: a 50-year cohort study. Am J Respir Crit Care Med. 2016;193(1):23–30. DOI:10.1164/rccm.201505-0870OC
- Omori K, Iwamoto H, Yamane T, et al. Clinically remitted childhood asthma is associated with airflow obstruction in Middle-aged adults. Respirology. 2017;22(1):86–92. DOI:10.1111/resp.12860
- Aanerud M, Carsin AE, Sunyer J, et al. Interaction between asthma and smoking increases the risk of adult airway obstruction. Eur Respir J. 2015;45(3):635–643. Available from: DOI:10.1183/09031936.00055514
- Svanes C, Sunyer J, Plana E, et al. Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65(1):14–20. DOI:10.1136/thx.2008.112136
- Asamoah-Boaheng M, Acheampong L, Tenkorang EY, et al. Association between early history of asthma and COPD diagnosis in later life: a systematic review and Meta-analysis. Int J Epidemiol. 2018;47(6):1865–1876. DOI:10.1093/ije/dyy207
- To T, Zhu J, Larsen K, Canadian Respiratory Research Network, et al. Progression from asthma to chronic obstructive pulmonary disease is air pollution a risk factor? Am J Respir Crit Care Med. 2016;194(4):429–438. DOI:10.1164/rccm.201510-1932OC
- To T, Zhu J, Gray N, et al. Asthma and chronic obstructive pulmonary disease overlap in women incidence and risk factors. Ann Am Thorac Soc. 2018;15(11):1304–1310. DOI:10.1513/AnnalsATS.201802-078OC
- Bedouch P, Marra C, FitzGerald J, et al. Trends in asthma related direct medical costs from 2002 to 2007 in British Columbia, Canada: a population based-cohort study. PLoS One. 2012;7(12):e50949– 8. DOI:10.1371/journal.pone.0050949
- Population Data BC (PopData BC). Population data British Columbia [Internet]. 2021. Available from: https://www.popdata.bc.ca/.
- Canadian Institute for Health Information (CIHI) [creator]. Discharge Abstract Database (Hospital Separations). V2. Population Data BC [publisher]. Data Extract. MOH 2020. [Internet]. British Columbia; 2019. Available from: http://www.popdata.bc.ca/data.
- British Columbia Ministry of Health (BC MOH) [creator]. Medical Services Plan (MSP) Payment Information File. V2. Population Data BC [publisher]. Data Extract. MOH 2020. [Internet]. 2019. Available from: http://www.popdata.bc.ca/data.
- British Columbia Ministry of Health (BC MOH) [creator]. PharmaNet. V2. Population Data BC [publisher]. Data Extract. Data Stewardship Committee 2020. [Internet]. 2020. Available from: http://www.popdata.bc.ca/data.
- British Columbia Ministry of Health (BC MOH) [creator]. Consolidation File (MSP Registration & Premium Billing). V2. Population Data BC [publisher]. Data Extract. MOH(2020) [Internet]. 2020. Available from: http://www.popdata.bc.ca/data.
- Population Data BC(PopData BC). Data available [Internet]. 2021. Available from: https://www.popdata.bc.ca/data.
- Prosser R, Carleton B, Smith M. Identifying persons with treated asthma using administrative data via latent class modelling. Health Serv Res. 2008;43(2):733–754. DOI:10.1111/j.1475-6773.2007.00775.x
- Chen W, FitzGerald JM, Sin DD, et al. Excess economic burden of comorbidities in COPD: a 15-year population-based study. Eur Respir J. 2017;50(1):1700393. [Internet]. Available from: DOI:10.1183/13993003.00393-2017
- Friedman HS, Navaratnam P, McLaughlin J. Treatment and outcomes—adherence and asthma control with mometasone furoate versus fluticasone propionate in adolescents and young adults with mild asthma. J Asthma. 2010;47(9):994–1000. DOI:10.1080/02770903.2010.513076
- Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:217047. DOI:10.1155/2015/217047
- Korgaonkar S, BanahanIIIB, Pittman E, et al. Effect of depression on adherence to controller medications and healthcare resource utilization in asthma patients. Value Heal. 2018;21:S231. DOI:10.1016/j.jval.2018.04.1567
- Engelkes M, Janssens HM, De Jongste JC, et al. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2015;45(2):396–407. Available from: DOI:10.1183/09031936.00075614
- Núñez JE, Núñez E, Fácila L, et al. Prognostic value of charlson comorbidity index at 30 days and 1 year after acute myocardial infarction. Rev Esp Cardiol. 2004;57(9):842–849.
- Kaplan A, Szefler SJ, Halpin DMG. Impact of comorbid conditions on asthmatic adults and children. NPJ Prim Care Respir Med. 2020;30(1):1–10.
- Boulet L. Influence of comorbid conditions on asthma. Eur Respir J. 2009;33(4):897–906. DOI:10.1183/09031936.00121308
- Nwaru BI, Ekström M, Hasvold P, et al. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872. Available from: DOI:10.1183/13993003.01872-2019
- Ismaila A, Corriveau D, Vaillancourt J, et al. Impact of adherence to treatment with fluticasone propionate/salmeterol in asthma patients. Curr Med Res Opin. 2014;30(7):1417–1425. DOI:10.1185/03007995.2014.908827
- Firoozi F, Lemière C, Beauchesne MF, et al. Development and validation of database indexes of asthma severity and control. Thorax. 2007;62(7):581–587. DOI:10.1136/thx.2006.061572
- Wang Y. Estimation of accelerated failure time models with random effects. 2006.
- Faruk A. The comparison of proportional hazards and accelerated failure time models in analyzing the first birth interval survival data. J Phys Conf Ser. 2018;974(1):1–10.
- Swindell WR. Accelerated failure time models provide a useful statistical framework for aging research. Exp Gerontol. 2009;44(3):190–200. DOI:10.1016/j.exger.2008.10.005
- Abdul-Fatawu M. Accelerated failure time models: an application in insurance attrition. J Risk Manag Insur. 2020;24(2):1–18.
- Ten Brinke A. Risk factors associated with irreversible airflow limitation in asthma. Curr Opin Allergy Clin Immunol. 2008;8(1):63–69. DOI:10.1097/ACI.0b013e3282f3b5b5
- Lange P, Parner J, Vestbo J, et al. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339(17):1194–1200. DOI:10.1056/NEJM199810223391703
- Ulrik C, Lange P. Decline of lung function in adults with bronchial asthma. Am J Respir Crit Care Med. 1994;150(3):629–634. DOI:10.1164/ajrccm.150.3.8087330
- Panizza J, James A, Ryan G, et al. Mortality and airflow obstruction in asthma: a 17-year follow-up study. Intern Med J. 2006;36(12):773–780. DOI:10.1111/j.1445-5994.2006.01214.x
- Barnes PJ. Sex differences in chronic obstructive pulmonary Disease Mechanisms. Am J Respir Crit Care Med. 2016;193(8):813–824. DOI:10.1164/rccm.201512-2379ED
- Wheaton A, Pleasants R, Croft J, et al. Gender and asthma-chronic obstructive pulmonary disease overlap syndrome. J Asthma. 2016;53(7):720–731. DOI:10.3109/02770903.2016.1154072
- Han MLK, Postma D, Mannino DM, et al. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med. 2007;176(12):1179–1184. DOI:10.1164/rccm.200704-553CC
- Jain N, Thakkar M, Jain N, et al. Chronic obstructive pulmonary disease: does gender really matter? Lung India. 2011;28(4):258–262. DOI:10.4103/0970-2113.85686
- Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343(5):332–336. DOI:10.1056/NEJM200008033430504
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention-update [Internet]. 2019 [cited 2021 Mar 3]. pp. 1–163. Available from: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf.
- British Thoracic Society (BTS). British guideline on the management of asthma-A national clinical guideline. Revised edition. 2019.
- Lougheed M, Lemière C, Dell SD, Canadian Thoracic Society Asthma Committee, et al. Canadian thoracic society asthma management continuum-2010 consensus summary for children six years of age and over, and adults. Can Respir J. 2010;17(1):15–24. DOI:10.1155/2010/827281
- Sobieraj DM, Weeda ER, Nguyen E, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent Asthma: A Systematic Review and Meta-analysis. JAMA—J Am Med Assoc. 2018;319(14):1485–1496. DOI:10.1001/jama.2018.2769
- Okelo SO, Butz AM, Sharma R, et al. Interventions to modify health care provider adherence to asthma guidelines: a systematic review. Pediatrics. 2013;132(3):517–534. DOI:10.1542/peds.2013-0779
