ABSTRACT
Chronic obstructive pulmonary disease (COPD) has been regarded as a disease of smokers, but the prevalence of non-smoking COPD patients have been reported to be considerable. We investigated differences in clinical characteristics between smoking and non-smoking COPD patients. We used data from the Korea COPD Subgroup Study (KOCOSS) database, which is a multicenter cohort that recruits patients from 54 medical centres in Korea. Comprehensive comparisons of smoking and non-smoking COPD patients were performed based on general characteristics, exacerbations, symptom scores, radiological findings, and lung-function tests. Of the 2477 patients included in the study, 8.1% were non-smokers and 91.9% were smokers. Non-smoking COPD patients were more likely to be female and to have a higher body mass index and lower level of education. Non-smoking COPD patients had more comorbidities, including hypertension, osteoporosis, and gastroesophageal reflux disease, and experienced more respiratory and allergic diseases. No significant differences in exacerbation rates, symptom scores, or exercise capacity scores were observed between the two groups. Smoking COPD patients had more emphysematous lung according to the radiological findings, and non-smoking patients had more tuberculosis-destroyed lung and bronchiectasis. Lung-function testing revealed no significant difference in the forced expiratory capacity in 1 sec between the two groups, but smokers had more rapid lung-function decline in the 5 years of follow-up data. We found differences in general characteristics and radiological findings between smoking and non-smoking COPD patients. No significant differences in exacerbation or symptom scores were observed, but decline in lung function was less steep in non-smoking patients.
Supplemental data for this article is available online at https://doi.org/10.1080/15412555.2022.2053088 .
Introduction
Chronic obstructive pulmonary disease (COPD) is a major clinical disorder that causes substantial morbidity and is reported to be the third leading cause of mortality worldwide [Citation1, Citation2]. The prevalence of COPD has been increasing and 4–10% of the global population currently suffers from this disease [Citation3, Citation4]. COPD poses a serious economic and social burden contributing $18 billion in direct costs in the United States, and 29.4 million years lost due to disability worldwide [Citation5–8].
COPD is characterised by persistent airflow obstruction caused by various risk factors. Among these risk factors, cigarette smoking is the leading cause of COPD, which hastens the rate of decline of lung function in cigarette users and reverts to the normal rate of decrease in those who have quit smoking [Citation9]. Hence, COPD is often regarded as a disease of smokers, and a large proportion of observational and interventional studies have enrolled patients with a smoking history of more than 10 pack-years or 100 packs in their lifetime [Citation10–13]. However, COPD patients include a considerable proportion of non-smokers, which can range from 15–50% worldwide [Citation14]. The proportion of non-smoking COPD patients is higher in developing than in developed countries, suggesting that non-cigarette risk factors for COPD, such as indoor/outdoor pollution, infection, or occupational exposure, are more common in developing countries [Citation10, Citation14, Citation15]. Korea rapidly developed from an extremely poor to a developed country within 60 years, so its risk factors for exposure may have rapidly changed and may have significant effects for non-smoking COPD patients.
Despite the significant proportion of non-smoking COPD patients, most studies have focussed on smokers, so little is known about the non-smoking phenotype. COPD is a heterogeneous disease, so it is necessary to further investigate whether the clinical characteristics and the efficacy of treatments identified in smoking COPD patients correspond with those in non-smoking COPD patients. Several studies have analysed the clinical characteristics of non-smoking COPD patients, but the results have been inconsistent [Citation10, Citation13, Citation16–19].
In this study, we investigated the clinical characteristics of non-smoking COPD compared with smoking COPD patients in terms of general characteristics, the rate of acute exacerbations, symptom and exercise capacity scores, radiological features, and lung function. We also analysed the difference in the rate of decline of 5-year lung function between the two groups.
Methods
Study population and data collection
The Korea COPD subgroup study (KOCOSS) is a prospective observational cohort study that explored the epidemiological and clinical characteristics of COPD patients registered at 54 referral hospitals in South Korea [Citation20, Citation21]. Fifty-four participating centres were mostly tertiary hospital that covers every regions of the South Korea. The main goal of this cohort is to identify phenotypes of COPD and evaluate prognostic value of each phenotypes. Patients were consecutively recruited as they visited for clinical assistance. Inclusion criteria were patients > 40 years old who were confirmed to have fixed airway obstruction by pulmonary function tests (PFTs), which is defined as post-bronchodilator forced expiratory volume in 1 sec (FEV1)/forced vital capacity (FVC) < 70% of the normal predicted value. Enrolled patients were evaluated at their first visit and periodically at 6-month intervals. Patient information was collected using a complete case report form (CRF), which was completed by a doctor or trained nurse. We extracted the data from the KOCOSS cohort registered between April 2012 and October 2018 to evaluate the clinical characteristics of non-smoking COPD patients.
Clinical parameters
We collected patient data including age, sex, body mass index (BMI), educational level (>9 years of national education), residence (urban or rural), comorbidities, moderate to severe depression (Beck Depression Inventory score > 18), and inhaler use. Exposure to COPD risk factors was analysed, including tobacco smoking (current, ex-, or never-smoker) and biomass exposure. Current or ex-smokers were categorised as the smoking COPD group and never-smokers were categorised as the non-smoking COPD group. Differences in the clinical characteristics were analysed between the two groups.
Exacerbation was defined by acute worsening of respiratory symptoms that needs additional therapy [Citation6]. The number of annual COPD exacerbations during the first year of follow-up was evaluated between the two groups. Exacerbations that required administration of antibiotics or systemic corticosteroids on an outpatient basis were defined as moderate exacerbations. Severe exacerbations were defined as exacerbations that led to hospitalisation or an emergency room visit [Citation22].
Differences in symptoms and exercise capacity were also evaluated between the smoking and non-smoking COPD groups based on various scores, including chronic bronchitis symptoms, the modified Medical Research Council (mMRC) score, the St. George’s Respiratory Questionnaire (SGRQ) score, the COPD Assessment Test (CAT) score, and the 6-min walking distance test (6MWT). Chronic bronchitis symptoms were defined as the presence of cough and phlegm for 3 months per year over 2 consecutive years [Citation23]. Differences in the results of the CAT and SGRQ scores were also evaluated.
Study patients received PFTs at the baseline and repeated annually, including the flow-volume curve, lung volume, and diffusion capacity of carbon monoxide (DLCO). PFTs were performed by the protocol suggested by ERS/ATS [Citation24]. Source of predicted values was based on equation suggested by Choi et al. [Citation25]. We extracted 5-year PFT follow-up data to analyse differences in lung function between the two groups.
Radiological findings
The chest X-ray and computed tomography (CT) results reported by radiologists were collected. These results were primarily categorised as normal and abnormal findings. Abnormal findings were subdivided into emphysematous changes, TB-destroyed lung, airway wall thickening, or bronchiectasis. Findings that did not fit in these categories were excluded from analyses.
Statistical analysis
Statistical analyses were performed using R software (ver. 3.6.3; The R Foundation for Computing, Vienna, Austria). All data are presented as mean and standard deviation or as numbers (percentages). We analysed clinical differences between the smoking and non-smoking COPD groups. Intergroup comparisons were performed with Student’s t-test for continuous variables and Pearson’s χ2 test for categorical variables. Differences in risk of exacerbations between non-smoking COPD and smoking COPD were analysed with binominal regression model. Variables including age, sex, BMI, post-bronchodilator FEV1 and previous exacerbation history were adjusted in the regression model. We used a linear mixed model to analyse differences in the annual decline of FEV1 between the two groups. Missing data was managed with pairwise deletion. A P-value < 0.05 was considered significant.
Ethics
Written informed consent was received from all participants in this study. Confidentiality of all personal information was secured. The study protocol was approved by the ethics committees at each participating medical centre. The names of the approving ethics committees are provided in the Supplementary Material.
Results
General characteristics
Among the 2477 patients enrolled in this study, 200 (8.1%) were non-smokers and 2277 (91.9%) were smokers. lists the clinical characteristics of non-smokers and smokers. The mean age was 69.2 ± 8.0 years and there was no significant difference in smoking history. Non-smoking COPD patients had a higher proportion of females (57.5% vs. 3.3%, respectively), a higher BMI (23.8 ± 3.6 vs. 22.9 ± 3.3, respectively), and were less educated (38.1% vs. 45.8%, respectively). However, no significant differences were observed in residence, biomass exposure, or inhaler use. Non-smokers had more hypertension (46.7% vs. 38.9%, respectively), osteoporosis (10.6% vs 3.5%, respectively), and gastroesophageal reflux disease (GERD) (14.1% vs. 8.9%, respectively) than smokers. Non-smoking COPD patients had more bronchiectasis (15.5% vs. 6.7%, respectively) and asthma (47.4% vs. 31.0%, respectively), and experienced more pulmonary tuberculosis (36.7% vs. 24.0%, respectively) and respiratory infection during childhood (28.9% vs. 18.7%, respectively).
Table 1. General characteristics of non-smoking COPD and smoking COPD.
Exacerbations, symptoms, and functional exercise capacity
lists the differences in COPD exacerbations between the non-smoking and smoking COPD groups. No significant differences in moderate-to-severe exacerbations or severe exacerbations were observed at the 1-year follow-up. Additionally, there were no significant difference in risk of exacerbation frequency regarding smoking history (). Furthermore, no significant differences were observed in symptoms, exercise capacity using chronic bronchitis symptoms, mMRC score, the SGRQ score, the CAT score, or the 6MWT between the non-smoking and smoking COPD groups ().
Table 2. Difference of COPD exacerbation in non-smoking and smoking COPD.
Table 3. Differences in the risk of exacerbations between non-smoking COPD and smoking COPD.
Table 4. Differences of symptoms between non-smoking and smoking COPD patients.
Radiological findings
lists the radiological findings for non-smoking and smoking COPD patients. The chest X-ray results of the non-smoking patients tended to be more abnormal than smoking patients, but there was no significant difference. Smoking patients had more emphysematous changes, and non-smoking patients had more TB-destroyed lungs and bronchiectasis. Airway wall thickening tended to be found more frequently in non-smoking patients, but there was no significant difference between the groups. Similarly, the chest CT results tended to reveal more abnormal findings in non-smoking patients than smoking patients, but there was no significant difference. Emphysematous changes were more common in smoking COPD patients and bronchiectatic changes were more common in non-smoking patients. TB-destroyed lung and airway wall thickening tended to be more common on chest CT in non-smoking patients, but the differences were not significant.
Table 5. Comparisons of radiologic features between non-smoking and smoking COPD patients.
Pulmonary function tests
lists differences in the PFT results between the non-smoking and smoking COPD groups. Absolute values of FEV1 were lower for non-smoking COPD patients than smoking patients (1.4 ± 0.5 vs. 1.7 ± 1.3, respectively) and the % predicted value was not significantly different. Absolute and % predicted FVC values were lower in non-smoking patients than smoking patients (2.6 ± 0.8 vs. 3.3 ± 0.8 L, 80.7 ± 18.9 vs. 85.3 ± 18.1%, respectively). The FEV1/FVC ratio was higher in non-smoking patients than smoking patients (55.9 ± 11.0 vs. 50.7 ± 12.5, respectively). The DLCO and ratio of residual volume and total lung capacity (RV/TLC) did not differ. presents the decrease in the longitudinal trajectory of FEV1. By analysis with linear mixed model, the loss of FEV1 in smokers was 41.5 ml per year, which was significantly higher than in non-smokers, whose FEV1 loss was 2 ml per year (p = 0.03).
Figure 1. Difference of FEV1 decline between smoking and non-smoking COPD.
FEV1 Forced expiratory volume in 1 second, COPD Chronic obstructive pulmonary disease.
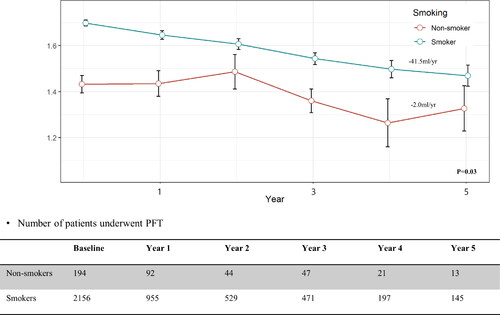
Table 6. Differences of lung function between non-smoking and smoking COPD patients.
Discussion
This study revealed differences in clinical characteristics between non-smoking and smoking COPD patients. We found that non-smoking COPD patients were more likely to be female, have a higher BMI, be less educated, and have more comorbidities, such as hypertension, osteoporosis, or GERD, compared to smoking COPD patients. Also, non-smoking patients had a more frequent history of respiratory and allergic diseases, including bronchiectasis, tuberculosis, childhood respiratory infection, and asthma. However, no significant differences in the acute exacerbation rate or symptom scores were observed between the groups.
These differences in the clinical characteristics were comparable to previous studies. Most studies have found that non-smoking COPD patients are more likely to be female and have a higher BMI, but some studies have also reported that non-smoking COPD patients are younger than smoking COPD patients [Citation16–19]. A previous study reported that osteoporosis is consistently more common in non-smoking COPD patients, but rheumatoid arthritis and depression are also common in those patients [Citation13]. This may be a consequence of a sexual difference in the prevalence of these diseases [Citation26–28]. Our study found that depression tended to be more common in non-smoking patients. Previous studies have also yielded differing results with regard to the prevalence of cardiovascular disease and hypertension. A Tunisian population study reported that cardiovascular disease and hypertension are more common in non-smoking patients [Citation16]. Our study found that hypertension was more common in non-smoking patients. However, a single-centre Chinese study and a Korean nationwide population study reported no significant differences between smoking and non-smoking COPD groups [Citation13, Citation17]. Thus, further studies are needed to clarify the differences in cardiovascular risk between smoking and non-smoking COPD patients.
Recent studies have shown various lung function trajectories in those who eventually becomes COPD [Citation11, Citation29]. Most important factors are known to be early life disadvantages and chronic risk factor exposures, such as tobacco smoking. Early life disadvantages that halt lung growth is reported to be various, including low birthweight, preterm delivery, childhood smoking exposure and history of respiratory diseases (i.e. asthma, respiratory infection, chronic bronchitis) in young age [Citation30]. In our study, as non-smoking COPD patients had more history of asthma and childhood respiratory infection, these patients may be more associated with early life disadvantages compared to smokers. Non-smoking COPD patients had more frequent TB-destroyed lungs and bronchiectasis, but less emphysematous changes compared to smoking COPD patients. Airway wall thickening tended to be more frequent in non-smoking patients. Previous studies have consistently reported that emphysema is more likely to be present in smokers, whereas small airways are more likely diseased in non-smokers [Citation10, Citation17–19, Citation31]. These findings were also shown in the pathological findings of COPD patients. Necropsy findings of biomass-exposed lung reveal more lung fibrosis and thickened pulmonary artery intima, but less emphysema and goblet cell metaplasia compared to tobacco-exposed lung [Citation32]. Also, bronchoscopic biopsies of COPD patients reveal that biomass COPD patients develop more small-airway diseases than smoking COPD patients [Citation31]. These findings suggest that different pathophysiological processes are caused by different risk factors. Also, as our study included more structurally damaged lung, such as TB-destroyed lung and bronchiectasis in non-smoking COPD patients, subtyping these phenotypes and comparing them with the airway-dominant type of non-smoking COPD may be interesting in a future study.
The PFTs showed that the absolute value of FEV1 was lower in non-smoking COPD patients, but the % predicted value was comparable between the groups. The difference in the absolute value may have been the result of a sexual difference between the two groups. Previous studies have reported either better [Citation10, Citation17] or comparable [Citation16, Citation18, Citation19] outcomes after analysing differences in lung function between the two groups. Salvi et al. found that smoking COPD patients develop a steeper FEV1 decline trajectory compared to non-smoking COPD patients (−130 ml vs. −80 ml/2 years, respectively) [Citation19]. This is consistent with the results of our 5-year analysis (−41.5 ml vs. −2 ml/year, respectively).
In our study, only 8.1% of the COPD patients were non-smokers. However, the prevalence of non-smoking COPD was much higher than in previous studies. An international multicenter population-based Burden of Obstructive Lung Disease (BOLD) study reported that 23.3% of moderate-to-severe COPD patients are non-smokers. Nonetheless, there are some discrepancies in the figures between study populations. Analysis of another Korean population study using data from the Korean National Health and Nutrition Examination Survey (KNHANES) indicated that 30.7% of COPD patients are non-smokers [Citation13]. The proportion of non-smokers reported among East Asian COPD patients is similar to that in the present study (China: 38.6%, Japan: 34%) [Citation10, Citation15]. The proportion of non-smokers has been reported to be lower in most Western countries (United States: 24.9%, United Kingdom 22.9%, Spain 23.4%) [Citation33–35] but much higher in an Austrian study (47.3%) [Citation36]. We assumed that a large proportion of non-smoking COPD patients in Korea are underdiagnosed, as tobacco smoking is usually considered a necessary condition for diagnosing COPD. As the KNHANES is a nationally representative database using a multistage clustered probability design, the prevalence of non-smoking COPD patients was revealed to be as high as in other countries [Citation13, Citation37]. The relatively higher prevalence of non-smoking COPD patients in Asian countries may be the result of more frequent exposure to non-tobacco risk factors, including biomass, occupational exposure, or household air pollution compared to Western countries [Citation38]. Respiratory infections associated with poor socioeconomic status, such as tuberculosis, may also be a reason for the higher prevalence of non-smoking COPD [Citation39]. Finally, COPD it may also result from risk factors during early life, including maternal/childhood malnutrition, childhood infection, preterm birth, and low birth weight, which are more prevalent in developing countries [Citation30, Citation39].
Some limitations of the current study should be considered. First, as this study primarily enrolled patients from tertiary hospitals, our data may not correspond to the general population. Considering regional differences in exposure to some non-smoking risk factors such as biomass, non-smoking COPD patients may have less access to a tertiary hospital. This may have lowered the proportion of non-smoking COPD patients and affected the study outcome. Second, as there were major differences in the sexual proportion between non-smoking COPD and smoking COPD patients, the outcomes may have been affected by a sexual effect. Further studies that adjust for other confounding factors may be needed. Third, the proportion of male in this database was 92.3%, which was relatively high compared to the proportion reported in other nationwide studies [Citation40, Citation41]. This may be associated with poor self-recognition of the disease in the female patients that result in infrequent visitation to tertiary hospital [Citation42]. Finally, radiologic findings in this study were reported by a single radiologist in each participant centres. As presence of each radiologic patterns were depended on the radiologists’ decision, there may be some bias. Also, the number of sample for the radiologic findings were relatively small.
Despite these limitations, our study is valuable as we analysed comprehensive aspects of non-smoking COPD patients, including general characteristics, symptom scores, exacerbation rates, radiological findings, and PFTs. Our data consist of a large population enrolled from 54 different medical centres. Finally, we investigated the decline in lung function over 5 years to reveal different lung-function trajectories between the two groups. No previous study has shown long-term differences in lung-function trajectories between smoking and non-smoking COPD patients.
Conclusions
We studied differences in clinical characteristics between smoking and non-smoking COPD patients. Non-smoking COPD patients were more likely to be female with more comorbidities, and to have a more frequent history of tuberculosis, asthma, or bronchiectasis. These histories were associated with the progression of non-smoking COPD. Although the rate of lung-function decline was lower in the non-smoking COPD group, most of the important clinical parameters, including respiratory symptoms and the exacerbation rate, did not differ significantly compared to the smoking COPD group. These results indicate that non-smoking COPD should be treated cautiously and equally with smoking COPD. Further studies are needed to investigate the best treatment options for non-smoking COPD patients.
Author contributions
Conceptualisation: Choi JY, Yoon HK
Methoodology: Yoon HK, Choi JY, Kim JW, Kim YH
Formal analysis: Choi JY, Yoon HK
Data curation: Choi JY, Kim JW, Kim YH, Yoo KH, Jung K, Lee JH, Um S, Lee W, Park D, Yoon HK
Investigation: Choi JY, Kim JW, Kim YH, Yoo KH, Jung K, Lee JH, Um S, Lee W, Park D, Yoon HK
Writing-original draft preparation: Choi JY
Writing-review and editing Choi JY, Kim JW, Kim YH, Yoo KH, Jung K, Lee JH, Um S, Lee W, Park D, Yoon HK
Approval of final manuscript: All authors
Declaration of interest statement
The authors have no conflicts of interest.
Data availability statement
The datasets supporting the conclusions of this article are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27(2):397–412. DOI:10.1183/09031936.06.00025805
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. DOI:10.1164/rccm.201204-0596PP
- Halbert RJ, Isonaka S, George D, et al. Interpreting COPD prevalence estimates: what is the true burden of disease? Chest. 2003;123(5):1684–1692. DOI:10.1378/chest.123.5.1684
- Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med. 2017;5(9):691–706. DOI:10.1016/s2213-2600(17)30293-x
- Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Respir Med. 2020;8(6):585–596. DOI:10.1016/s2213-2600(20)30105-3
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of COPD, global initiative for chronic obstructive lung disease 2021. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf. Accessed December 23, 2020.
- López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23. DOI:10.1111/resp.12660
- Kim C, Kim Y, Yang D-W, et al. Direct and indirect costs of chronic obstructive pulmonary disease in Korea. Tuberc Respir Dis (Seoul). 2019;82(1):27–34. DOI:10.4046/trd.2018.0035
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–1648. DOI:10.1136/bmj.1.6077.1645
- Takiguchi H, Takeuchi T, Niimi K, et al. Proportion and clinical characteristics of non-asthmatic non-smokers among adults with airflow obstruction. PLoS One. 2018;13(5):e0196132. DOI:10.1371/journal.pone.0196132
- Martinez FJ, Han MK, Allinson JP, et al. At the root: Defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(12):1540–1551. DOI:10.1164/rccm.201710-2028PP
- Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. DOI:10.3109/15412550903499522
- Lee SH, Hwang ED, Lim JE, et al. The risk factors and characteristics of COPD among nonsmokers in Korea: an analysis of KNHANES IV and V. Lung. 2016;194(3):353–361. DOI:10.1007/s00408-016-9871-6
- Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–743. DOI:10.1016/S0140-6736(09)61303-9
- Zhou Y, Wang C, Yao W, et al. COPD in Chinese nonsmokers. Eur Respir J. 2009;33(3):509–518. DOI:10.1183/09031936.00084408
- Denguezli M, Daldoul H, Harrabi I, et al. COPD in nonsmokers: reports from the Tunisian population-based burden of obstructive lung disease study. PLoS One. 2016;11(3):e0151981. DOI:10.1371/journal.pone.0151981
- Zhang J, Lin XF, Bai CX. Comparison of clinical features between non-smokers with COPD and smokers with COPD: a retrospective observational study. Int J Chron Obstruct Pulmon Dis. 2014;9:57–63. DOI:10.2147/copd.S52416
- Ji W, Lim MN, Bak SH, et al. Differences in chronic obstructive pulmonary disease phenotypes between non-smokers and smokers. Clin Respir J. 2018;12(2):666–673. DOI:10.1111/crj.12577
- Salvi SS, Brashier BB, Londhe J, et al. Phenotypic comparison between smoking and non-smoking chronic obstructive pulmonary disease. Respir Res. 2020;21(1):50. DOI:10.1186/s12931-020-1310-9
- The Korea COPD Subgroup Study. Available from: http://www.kocoss.kr/.
- Lee JY, Chon GR, Rhee CK, et al. Characteristics of patients with chronic obstructive pulmonary disease at the first visit to a pulmonary medical center in Korea: the Korea COPD subgroup study team cohort. J Korean Med Sci. 2016;31(4):553–560. DOI:10.3346/jkms.2016.31.4.553
- Mullerova H, Shukla A, Hawkins A, et al. Risk factors for acute exacerbations of COPD in a primary care population: a retrospective observational cohort study. BMJ Open. 2014;4(12):e006171. DOI:10.1136/bmjopen-2014-006171
- Choi JY, Yoon HK, Park SJ, et al. Chronic bronchitis is an independently associated factor for more symptom and high-risk groups. Int J Chron Obstruct Pulmon Dis. 2016;11:1335–1341. DOI:10.2147/copd.S105516
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. DOI:10.1183/09031936.05.00034805
- Choi JK, Paek D, Lee JO. Normal predictive values of spirometry in Korean population. Tuberc Respir Dis. 2005;58(3):230–242. DOI:10.4046/trd.2005.58.3.230
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–911. DOI:10.1016/s0140-6736(01)06075-5
- Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396(10250):565–582. DOI:10.1016/s0140-6736(20)31561-0
- Oh JY, Lee YS, Min KH, et al. Osteoporosis in patients with asthma–chronic obstructive pulmonary disease overlap syndrome. Tuberc Respir Dis. 2018;81(1):73–79. DOI:10.4046/trd.2017.0066
- Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6(7):535–544. DOI:10.1016/S2213-2600(18)30100-0
- Choi JY, Rhee CK. Diagnosis and treatment of early chronic obstructive lung disease (COPD). JCM. 2020;9(11):3426. DOI:10.3390/jcm9113426
- Zhao D, Zhou Y, Jiang C, et al. Small airway disease: a different phenotype of early stage COPD associated with biomass smoke exposure. Respirology. 2018;23(2):198–205. DOI:10.1111/resp.13176
- Rivera RM, Cosio MG, Ghezzo H, et al. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int J Tuberc Lung Dis. 2008;12(8):972–977.
- Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest. 2005;128(3):1239–1244. DOI:10.1378/chest.128.3.1239
- Birring SS, Brightling CE, Bradding P, et al. Clinical, radiologic, and induced sputum features of chronic obstructive pulmonary disease in nonsmokers: a descriptive study. Am J Respir Crit Care Med. 2002;166(8):1078–1083. DOI:10.1164/rccm.200203-245oc
- Penña VS, Miravitlles M, Gabriel R, et al. Geographic variations in prevalence and underdiagnosis of COPD: results of the IBERPOC multicentre epidemiological study. Chest. 2000;118(4):981–989. DOI:10.1378/chest.118.4.981
- Lamprecht B, Schirnhofer L, Kaiser B, et al. Non-reversible airway obstruction in never smokers: results from the Austrian BOLD study. Respir Med. 2008;102(12):1833–1838. DOI:10.1016/j.rmed.2008.07.007
- Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea national health and nutrition examination survey (KNHANES). Int J Epidemiol. 2014;43(1):69–77. DOI:10.1093/ije/dyt228
- Brakema EA, van Gemert FA, van der Kleij R, et al. COPD’s early origins in low-and-middle income countries: what are the implications of a false start? NPJ Prim Care Respir Med. 2019;29(1):6. DOI:10.1038/s41533-019-0117-y
- Myong JP, Yoon HK, Rhee CK, et al. Risk factors for lung function impairment among the general non-smoking Korean population. Int j Tuberc Lung Dis. 2015;19(9):1019–1026. DOI:10.5588/ijtld.14.0929
- Korea National Health & Nutrition Examination Survey. Available from: https://knhanes.cdc.go.kr/knhanes/eng/index.do.
- Jia G, Lu M, Wu R, et al. Gender difference on the knowledge, attitude, and practice of COPD diagnosis and treatment: a national, multicenter, cross-sectional survey in China. COPD. 2018;13:3269–3280. DOI:10.2147/COPD.S176173
- Choi JY, Kim SY, Lee JH, et al. Clinical characteristics of chronic obstructive pulmonary disease in female patients: findings from a KOCOSS cohort. Int J Chron Obstruct Pulmon Dis. 2020;15:2217–2224. DOI:10.2147/copd.S269579
