Abstract
This study aimed to conduct a meta-analysis to investigate whether short-term exposure to fine (PM2.5) and coarse (PM10) particulate matter was associated with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) hospitalization, emergency room visit, and outpatient visit at different lag values. PubMed, Embase, and the Cochrane Library were searched for relevant papers published up to March 2021. For studies reporting results per 1-µg/m3 increase in PM2.5, the results were recalculated as per 10-µg/m3 increase. We manually calculated the RRs for these two studies and transferred the RRs to estimate 10 µg/m3 increases in PM2.5. Automation tools were initially used to remove ineligible studies. Two reviewers independently screened the remaining records and retrieved reports. Twenty-six studies (28 datasets; 7,018,419 patients) were included. There was a significant association between PM2.5 and AECOPD events on lag0 (ES = 1.01, 95%CI: 1.01-1.02, p < 0.001; I2=88.6%, Pheterogeneity<0.001), lag1 (ES = 1.00, 95%CI: 1.00-1.01, p < 0.001; I2=82.5%, Pheterogeneity<0.001), lag2 (ES = 1.01, 95%CI: 1.01-1.01, p < 0.001; I2=90.6%, Pheterogeneity<0.001), lag3 (ES = 1.01, 95%CI: 1.00-1.01, p < 0.001; I2=88.9%, Pheterogeneity<0.001), lag4 (ES = 1.00, 95%CI: 1.00-1.01, p < 0.001; I2=83.7%, Pheterogeneity<0.001), and lag7 (ES = 1.00, 95%CI: 1.00-1.00, p < 0.001; I2=0.0%, Pheterogeneity=0.743). The subgroup analyses showed that PM2.5 influenced the rates of hospitalization, emergency room visits, and outpatient visits. Similar trends were observed with PM10. The risk of AECOPD events (hospitalization, emergency room visit, and outpatient visit) was significantly increased with a 10-µg/m3 increment in PM2.5 and PM10 from lag0 to lag7.
List Of Abbreviations: particulate matter (PM2.5 and PM10); acute exacerbation of chronic obstructive pulmonary disease (AECOPD); Chronic obstructive pulmonary disease (COPD); Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA); Effect sizes [Citation48]; confidence intervals (CIs)
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by significant airflow limitation associated with chronic inflammatory responses in airways and lungs, resulting in the destruction of lung tissue [Citation1]. COPD has several complications, including acute exacerbation, respiratory failure, and pulmonary hypertension. The 4-year mortality rate of COPD patients ranges from 28% (for mild-to-moderate COPD cases) to 62% (for moderate-to-severe COPD cases) [Citation1].
The deterioration of symptoms beyond the daily variation range leading to the use of additional therapy is known as an acute exacerbation of COPD (AECOPD), a medical emergency associated with significant morbidity and mortality [Citation1, Citation2]. COPD exacerbations are commonly caused by viral or bacterial infections, including pneumonia, and air pollution (e.g. tobacco smoke, ozone, and occupational exposure) [Citation1, Citation2]. Other risk factors include a history of acute exacerbations, ambient temperature changes, emphysema, chronic bronchitis, severe COPD at baseline, deteriorating airflow limitation, and an increase in the ratio of pulmonary to the aorta [Citation1–7]. The prognosis of AECOPD is poor. Indeed, the in-hospital mortality rates of patients with COPD exacerbations are generally around 2.5% and 10% in those with hypercarbia [Citation1, Citation2, Citation8], and the all-cause mortality rate within 3 years of the index hospitalization can be as high as 49% [Citation1, Citation9].
The effects of air pollution on respiratory health have been extensively studied [Citation10–16]. Substantial epidemiological evidence has demonstrated that ground-level ambient pollutants such as nitrogen dioxide (NO2), sulfur dioxide (SO2), and ozone (O3) are strongly associated with AECOPD [Citation13, Citation17]. Among various air pollutants, fine particulate matter (PM2.5) and coarse particulate matter (PM10) are considered to be the most hazardous because they can easily reach the lower airways and carry many toxic components that trigger a variety of adverse responses [Citation18–24]. According to a previous meta-analysis [Citation13] published in 2016, short-term exposure to PM2.5 significantly increased the burden of AECOPD, but this study only assessed the outcomes of AECOPD patients in terms of emergency hospitalization and mortality. Similarly, the meta-analysis published in 2013 showed that ambient PM10 was associated with COPD hospitalization and mortality [Citation25].
The effects of air pollution on COPD are not necessarily immediate, and a lag can be observed between exposure and the detrimental outcomes. Indeed, since the publication of the meta-analyses presented above, relevant time-series studies [Citation26–38] and case-crossover studies [Citation39–44] were published regarding the association between PM2.5 exposure and AECOP hospitalization, emergency room visit, and outpatient visit.
Aims
Therefore, the present study aimed to conduct a meta-analysis to explore whether PM2.5 and PM10 were associated with AECOPD hospitalization, emergency room visit, and outpatient visit at different lag values.
Materials and methods
Literature search
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation45]. Based on the PICO principle [Citation46], PubMed, Embase, and the Cochrane library were searched for available papers published up to March 2021 using the MeSH term ‘Pulmonary Disease’, and ‘Chronic Obstructive’, as well as relevant key words. The eligibility criteria were 1) patients with COPD; 2) patients exposed to PM2.5 or PM10; 3) the occurrence of AECOPD as shown by hospitalization, emergency room visit, or outpatient visit; 4) language was limited to English. The study selection process was performed by two investigators (** and **). Discrepancies in the final list of included studies were resolved by discussion with a third investigator (**) until a consensus was reached. The reference lists of the included reports were screened for additional potentially eligible studies.
Data extraction
The data on study characteristics (authors, year of publication, study design, country and city/district where the study was performed, sex percentage, and sample size), exposure parameters (average concentration of PM2.5 and PM10, analytical model, and lag time of the event), and hospitalization for AECOPD were independently extracted by two authors (Liniuniu and Wangliyun). Any disagreements were solved by discussion. Some articles could be separated into independent datasets because each article reported AECOPD hospitalization and emergency room visit separately.
Outcome
The primary outcome was all events or evidence of AECOPD, including hospitalization, outpatient visit, and emergency treatment.
Quality of the evidence
To our knowledge, there were no validated quality assessment scales recommended for time-series and case-crossover studies [Citation47]. Thus, we evaluated the qualities of the evidence were based on the possible biases in the research team, first author, and institution where the study was performed, and the relevant data were analyzed. The authority of the journals was also a critical component of quality assessment.
Statistical analysis
All analyses were performed using STATA SE 14.0 (StataCorp, College Station, TX, USA). Not all studies reported their outcomes as risk ratio (RR). Some used odds ratio (OR) to evaluate the association between PM2.5 or PM10 and AECOPD. In our meta-analysis, RR and OR were treated as the same parameter. For studies that reported the RR for 1-µg/m3 increase in PM2.5, we manually calculated the RRs for these studies and converted the RRs to estimate 10 µg/m3 increases in PM2.5. Effect sizes [Citation48] and the corresponding 95% confidence intervals (CIs) were used to compare the outcomes. Statistical heterogeneity among these studies was calculated using Cochran’s Q-test and I2 index. An I2 >50% and p < 0.10 for the Q-test indicated high heterogeneity, and the random-effects model was used; otherwise, the fixed-effects model was applied. A P-value ≤0.05 was considered statistically significant. We only assessed the potential publication bias on lag0, lag1, lag2, lag3, lag4, and lag5 by funnel plots and Egger’s test because more than 10 studies reported the RRs on these lag points. As for outcomes with less than 10 studies, the funnel plots and Egger’s test could yield misleading results and were not recommended [Citation47]. Subgroup analyses were performed according to the individual event types (hospitalization, emergency room visit, and outpatient visit. Sensitivity analyses were also performed by sequentially excluding each study and observing whether the summary results changed significantly.
Results
Retrieval of the studies
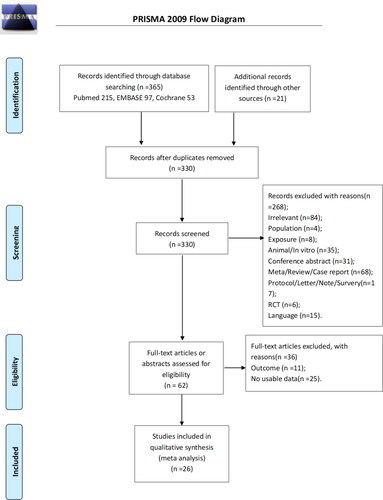
presents the study selection process. The initial search identified 386 records, and two were found from other sources. A total of 330 records were left after removing the duplicates and screened, and 268 were excluded. The remaining 62 papers were assessed for eligibility, and 36 were excluded because of inappropriate outcomes (n = 11) and no usable data (n = 25). Finally, 26 studies were included, and 28 datasets were analyzable [Citation26–30, Citation39–42, Citation49–53] because the studies by Pothirat et al. [Citation28] and Jo et al. [Citation27], respectively, contained two datasets that could be assessed separately.
presents the characteristics of selected studies. There were six case-crossover studies [Citation39–44], 14 time-series studies (16 datasets) [Citation26–38], three cross-sectional studies [Citation49, Citation50, Citation53], two ecological studies [Citation51, Citation52], and one cohort study [Citation54], involving a total of 7,018,419 patients. The assessed lag varied from 0-2 to 0-14 days, except for one study evaluating 0-5 years [Citation54]. A summarized table () shows the overall outcomes on each lag day and subgroup analyses stratified by the type of admission.
Table 1. Literature search and study characteristic.
Association between PM2.5 and AECOPD events on lag0
Seventeen datasets could be analyzed for the relationship between particulate matters and AECOPD events on lag0. There was a significant association between PM2.5 and AECOPD events on lag0 (ES = 1.01, 95%CI: 1.01-1.02, p < 0.001; I2=88.6%, Pheterogeneity<0.001) ( and ). Subgroup analyses showed significant associations between PM2.5 and hospitalization (ES = 1.01, 95%CI: 1.00-1.02, p < 0.001; I2=91.7%, Pheterogeneity<0.001), emergency room visit (ES = 1.01, 95%CI: 1.01-1.01, p < 0.001; I2=0.0%, Pheterogeneity=0.533), and outpatient visit (ES = 1.01, 95%CI: 1.00-1.03, p < 0.001) ( and ). Significant associations of PM2.5 (ES = 1.01, 95%CI: 1.01-1.02, p < 0.001; I2=91.7%, p < 0.001) and PM10 (ES = 1.00, 95%CI: 0.99-1.02, p < 0.001) with hospitalization were also found ().
Figure 2. Forest plots for the association between PM2.5 and AECOPD hospitalization, outpatient, and emergency room visit on lag0 (A), lag1 (B), lag2 (C), lag3 (D), lag4 (E), lag5 (F), lag7 (G), lag0-1 (H), lag0-2 (I), and lag0-3 (J).

Association between PM2.5 and AECOPD events on lag1
Fourteen datasets analyzed the correlation of PM2.5 with AECOPD events on lag1. There was a significant association between PM2.5 and AECOPD events on lag1 (ES = 1.00, 95%CI: 1.00-1.01, p < 0.001; I2=82.5%, Pheterogeneity<0.001) ( and ). Subgroup analyses showed a significant association between PM2.5 and hospitalization (ES = 1.00, 95%CI: 1.00-1.00, p < 0.001; I2=0.0%, Pheterogeneity=0.482), outpatient visit (ES = 1.00, 95%CI: 1.00-1.00, p < 0.001; I2=0.0%, Pheterogeneity=0.934), and emergency room visit (ES = 1.02, 95%CI: 1.01-1.03, p < 0.001; I2=71.7%, Pheterogeneity=0.007) ( and ).
Association between PM2.5 and AECOPD events on lag2
Pooled data of fourteen datasets showed a significant association between PM2.5 and AECOPD events on lag2 (ES = 1.01, 95%CI: 1.01-1.01, p < 0.001; I2=90.6%, Pheterogeneity<0.001) ( and ). Subgroup analyses showed a significant association between PM2.5 and hospitalization (ES = 1.01, 95%CI: 1.00-1.01, p < 0.001; I2=84.4%, Pheterogeneity<0.001), outpatient visit (ES = 1.00, 95%CI: 1.00-1.00, p < 0.001; I2=1.7%, Pheterogeneity=0.313), and emergency room visit (ES = 1.03, 95%CI: 1.01-1.04, p < 0.001; I2=89.0%, Pheterogeneity<0.001) ( and ).
Association between PM2.5 and AECOPD events on lag3
Pooled analyses of eleven datasets suggested a significant association between PM2.5 and AECOPD events on lag3 (ES = 1.01, 95%CI: 1.00-1.01, p < 0.001; I2=88.9%, Pheterogeneity<0.001) ( and ). Subgroup analyses showed significant associations between PM2.5 and hospitalization (ES = 1.01, 95%CI: 1.00-1.02, p < 0.001; I2=94.0%, Pheterogeneity<0.001), outpatient visit (ES = 1.00, 95%CI: 1.00-1.00, p < 0.001; I2=74.4%, Pheterogeneity=0.048), and emergency room visit (ES = 1.01, 95%CI: 1.00-1.02, p < 0.001; I2=0.0%, Pheterogeneity=0.889) ( and ). Significant correlations of hospitalization with PM2.5 (ES = 1.01, 95%CI: 1.01-1.02, p < 0.001; I2=94.0%, p < 0.001) and PM10 (ES = 1.03, 95%CI: 1.00-1.06, p < 0.001) were also demonstrated ().
Association between PM2.5 and AECOPD events on lag4
There was a significant association between PM2.5 and AECOPD events on lag4 (ES = 1.00, 95%CI: 1.00-1.01, p < 0.001; I2=83.7%, Pheterogeneity<0.001) based on the pooled analysis of ten datasets ( and ). Subgroup analyses showed significant associations between PM2.5 and hospitalization (ES = 1.01, 95%CI: 1.00-1.01, p < 0.001; I2=90.1%, Pheterogeneity<0.001), outpatient visit (ES = 1.00, 95%CI: 1.00-1.00, p < 0.001; I2=86.7%, Pheterogeneity=0.006), and emergency room visit (ES = 1.00, 95%CI: 0.98-1.03, p < 0.001; I2=66.2%, Pheterogeneity=0.052) ( and ). Subgroup analyses showed significant associations of hospitalization with PM2.5 (ES = 1.01, 95%CI: 1.00-1.01, p < 0.001; I2=90.1%, p < 0.001) and PM10 (ES = 1.03, 95%CI: 1.00-1.06, p < 0.001) ().
Association between PM2.5 and AECOPD events on lag5
According to pooled analysis of ten datasets, there was a significant association between PM2.5 and AECOPD events on lag5 (ES = 1.00, 95%CI: 1.00-1.00, p < 0.001; I2=75.7%, Pheterogeneity<0.001) ( and ). Subgroup analyses showed significant associations between PM2.5 and hospitalization (ES = 1.00, 95%CI: 1.00-1.01, p < 0.001; I2=84.3%, Pheterogeneity<0.001), emergency room visit (ES = 0.99, 95%CI: 0.96-1.03, p < 0.001; I2=77.8%, Pheterogeneity=0.011), and outpatient visit (ES = 1.00, 95%CI: 1.00-1.00, p < 0.001) ( and ).
Association between PM2.5 and AECOPD event on lag7
Pooled estimate from three datasets showed a significant association between PM2.5 and AECOPD events on lag7 (ES = 1.00, 95%CI: 1.00-1.00, p < 0.001; I2=0.0%, Pheterogeneity=0.743) (). Subgroup analyses showed significant associations between PM2.5 and hospitalization (ES = 1.00, 95%CI: 1.00-1.00, p < 0.001; I2=0.0%, Pheterogeneity=0.510), and emergency room visit (ES = 0.98, 95%CI: 0.88-1.08, p < 0.001) (). Subgroup analyses showed significant correlations of hospitalization with PM2.5 (ES = 0.98, 95%CI: 1.00-1.08, p < 0.001) and PM10 (ES = 1.03, 95%CI: 1.01-1.05, p < 0.001) ().
Association between PM2.5 and AECOPD events on lag0-1
There was a significant association between PM2.5 and AECOPD events on lag0-1 (ES = 1.01, 95%CI: 1.00-1.03, p < 0.001; I2=82.0%, Pheterogeneity<0.001) based on five datasets (). Subgroup analyses showed significant associations between PM2.5 and hospitalization (ES = 1.00, 95%CI: 0.99-1.03, p < 0.001; I2=85.0%, Pheterogeneity<0.001), and emergency room visit (ES = 1.03, 95%CI: 1.01-1.04, p < 0.001) ().
Association between PM2.5 and AECOPD events on lag0-2
There was a significant association between PM2.5 and AECOPD events on lag0-2 (ES = 1.02, 95%CI: 1.02-1.03, p < 0.001; I2=75.0%, Pheterogeneity<0.001) based on eight datasets (). Subgroup analyses showed significant associations between PM2.5 and hospitalization (ES = 1.02, 95%CI: 1.01-1.03, p < 0.001; I2=54.8%, Pheterogeneity=0.065), and emergency room visit (ES = 1.04, 95%CI: 1.01-1.06, p < 0.001) ().
Association between PM2.5 and AECOPD events on lag0-3
Pooled analysis of four datasets showed a significant association between PM2.5 and AECOPD events on lag0-3 (ES = 1.03, 95%CI: 1.02-1.04, p < 0.001; I2=0.0%, Pheterogeneity=0.600) (). Subgroup analyses showed significant associations between PM2.5 and hospitalization (ES = 1.03, 95%CI: 1.02-1.04, p < 0.001; I2=0.0%, Pheterogeneity=0.916), emergency room visit (ES = 1.03, 95%CI: 1.01-1.05, p < 0.001), and outpatient visit (ES = 1.08, 95%CI: 1.01-1.16, p < 0.001) (). Significant associations of hospitalization with PM2.5 (ES = 1.03, 95%CI: 1.01-1.05, p < 0.001) and PM10 (ES = 1.01, 95%CI: 1.01-1.01, p < 0.001) were also revealed ().
Sensitivity analysis
Sensitivity analysis results showed that no individual study could influence the overall association relationship between PM2.5 and AECOPD events on lag0, lag1, lag2, lag3, lag4, and lag5 ().
Publication bias
The funnel plot indicated that one study [Citation32] had a significant publication bias ().
Discussion
The results showed that AECOPD events were significantly increased with an increment of 10 µg/m3 in PM2.5 from lag0 to lag7. Similar results were observed with PM10, as well as with the individual event types.
These results were supported by a previous meta-analysis by Li et al. published in 2016 [13]. Their review [Citation13] included 59 studies that examined the association between various air pollutants (O3, CO, NO2, SO2, PM10, and PM2.5) and AECOPD and reported that short-term exposure to those pollutants increased the burden of AECOPD. They also reported that those associations were stronger on lag0 and lag3, and the present study observed associations at all time points from lag0 to lag7. Wang et al. [Citation55] performed a meta-analysis of studies from mainland China, Hong Kong, Macao, and Taiwan and showed an association of PM10 and PM2.5 with hospitalization burden of COPD, but they did not examine the outcomes based on days since exposure. Nevertheless, why the associations were stronger on lag0 and lag3 but not on lag1 or lag5 in previous studies was difficult to explain. Based on the sub-analysis of PM2.5, Li et al. [Citation13] observed that the main source of heterogeneity was the differences in lag periods and confounders among the studies. These two sources of bias were identified as early as 2001 [56]. In addition, the weaker or lack of association on lag5 could be due to a decreased inflammatory response with elapsing time from exposure. Still, despite the early recognition of the lag effect, these previous meta-analyses did not consider it, mainly because of a lack of data. In the present meta-analysis, the lag periods varied from 0-2 to 0-7 among the included studies. Moreover, our meta-analysis included over 7 million patients and showed that the associations were significant at all lag points, suggesting that the risk of AECOPD remains significant for 7 consecutive days following PM2.5 and PM10 exposure.
There is a wealth of literature about the relationship between air pollutants and health [Citation12], COPD [Citation11, Citation14–16], and AECOPD [Citation10, Citation56, Citation57]. PM2.5 can absorb chemicals from the environment, inducing oxidative stress in the airways [Citation58–60], impairing the protective function of the immune system [Citation60, Citation61], and triggering inflammatory responses that further damage the respiratory system in COPD patients [Citation60, Citation62]. Nevertheless, the associations of AECOPD with smoking and pneumonia have been well documented [Citation48, Citation63, Citation64], but data are still lacking about the link with air pollutants. It is, of course, complicated by the presence of a wide variety of pollutants both indoors and outdoors. The present meta-analysis and previous ones [Citation13, Citation55] supported the effect of short-term exposure to air pollutants on AECOPD, but the impact of long-term exposure was not studied. Indeed, long-term exposure to air pollutants is a well-known risk factor for cardiovascular events and mortality [Citation65–67]. Nevertheless, one meta-analysis revealed the association between PM2.5 and COPD risk [Citation68]. The associations were observed at all lag values, suggesting that the effects of PM exposure activate systemic immunity and that this activation remains. Still, the exact mechanisms involved will have to be examined. The subgroup analyses showed that the individual AECOPD events (i.e. hospitalization, emergency room visits, and outpatient visits) were associated with PM2.5 exposure. Although these events indicate different severity levels of the patients’ condition, they nevertheless indicate that the patients feel ill, and they seek consultation according to their perceived degree of illness severity. Future studies should refine the relationship between PM2.5 and AECOPD.
The results of the present meta-analysis must be explained with its limitations. First, the included studies were controlled for crucial covariates such as weather, temperature, humidity, and day of the week, but the adjusted covariates were different among the included studies. Second, 16 of 26 studies were from China, which might cause bias in the results. Indeed, many households in China still used coal for heating and cooking [Citation69], which could predispose COPD patients to disease exacerbation when they were exposed to outdoor PM2.5. Third, only the parameters in single-pollutant models were extracted for analysis purposes. The underlying interactive effects might exist among pollutants. It needs further analysis. Finally, despite being statistically significant, the ESs were small and must be interpreted with caution.
Conclusions
In conclusion, this meta-analysis showed that the risk of AECOPD events (hospitalization, emergency room, and outpatient visits) was significantly increased with an increment of 10 µg/m3 in PM2.5 and PM10 from lag0 to lag7. The present study enabled the investigation of the association between short-term exposure to PM2.5 and PM10 and AECOPD risk. Nevertheless, result interpretation must be cautious due to the study limitations. Future studies should focus on the impact of relatively longer lag-time points on AECOPD events.
Relevance to clinical practice
Short-term exposure to PM2.5 and PM10 were reported to increase the burden of AECOPD significantly. Therefore, this study aimed to conduct a meta-analysis to explore whether PM2.5 and PM10 were associated with AECOPD hospitalization, emergency room visit, and outpatient visit. The results showed that the risk of AECOPD events (hospitalization, emergency room visit, and outpatient visit) was significantly increased with an increment of 10 µg/m3 in PM2.5 and PM10 from lag0 to lag7.
Impact statement
What does this paper contribute to the wider global clinical community?
The results showed that an increment of 10 µg/m3 in particulate matter PM2.5 and PM10 from lag0 to lag7 might significantly increase the AECOPD events, including hospitalization, emergency room, and outpatient visits.
Author contributions
Niuniu Li and Liyun Wang carried out the studies, participated in collecting data, and drafted the manuscript. Niuniu Li and Liyun Wang performed the statistical analysis and participated in its design. Kun Ji and Jianling Ma participated in the acquisition, analysis, or interpretation of data and drafted the manuscript. All authors read and approved the final manuscript.
Statement
Manuscripts have been read and approved by all authors to meet the authorship requirements of this journal, and each author believes that the manuscript represents honest work if that information is not provided in another form.
Health and safety
This study does not involve any experimental operations, so there are no risks that might be involved in experiments or procedures, or that might involve instructions, materials, or formulas. This article complies with the relevant rules.
Supplemental Material
Download PDF (325.9 KB)Declaration of interest
The authors report no conflict of interest.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Additional information
Funding
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of COPD; 2019. Available from https://goldcopd.org/gold-reports/.
- Evensen AE. Management of COPD exacerbations. Am Fam Physician. 2010;81(5):607–613.
- Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. DOI:10.1056/NEJMoa1203830
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. DOI:10.1056/NEJMoa0909883
- Mullerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. DOI:10.1378/chest.14-0655
- Li MH, Fan LC, Mao B, et al. Short-term exposure to ambient fine particulate matter increases hospitalizations and mortality in COPD: a systematic review and Meta-analysis. Chest. 2016;149(2):447–458. DOI:10.1378/chest.15-0513
- Du Q, Jin J, Liu X, et al. Bronchiectasis as a comorbidity of chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0150532. DOI:10.1371/journal.pone.0150532
- Patil SP, Krishnan JA, Lechtzin N, et al. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163(10):1180–1186. DOI:10.1001/archinte.163.10.1180
- Gudmundsson G, Gislason T, Lindberg E, et al. Mortality in COPD patients discharged from hospital: the role of treatment and co-morbidity. Respir Res. 2006;7:109. DOI:10.1186/1465-9921-7-109
- Zhu RX, Nie XH, Chen YH, et al. Relationship between particulate matter (PM2.5) and hospitalizations and mortality of chronic obstructive pulmonary disease patients: a Meta-Analysis. Am J Med Sci. 2020;359(6):354–364. DOI:10.1016/j.amjms.2020.03.016
- Park J, Kim HJ, Lee CH, et al. Impact of long-term exposure to ambient air pollution on the incidence of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Environ Res. 2021;194:110703. DOI:10.1016/j.envres.2020.110703
- Lee KK, Bing R, Kiang J, et al. Adverse health effects associated with household air pollution: a systematic review, meta-analysis, and burden estimation study. Lancet Glob Health. 2020;8(11):e1427–e1434. DOI:10.1016/S2214-109X(20)30343-0
- Li J, Sun S, Tang R, et al. Major air pollutants and risk of COPD exacerbations: a systematic review and Meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:3079–3091. DOI:10.2147/COPD.S122282
- Zhang S, Li G, Tian L, et al. Short-term exposure to air pollution and morbidity of COPD and asthma in east asian area: a systematic review and meta-analysis. Environ Res. 2016;148:15–23. DOI:10.1016/j.envres.2016.03.008
- Song Q, Christiani DC, Xiaorong W, et al. The global contribution of outdoor air pollution to the incidence, prevalence, mortality and hospital admission for chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Environ Res Public Health. 2014;11:11822–11832. DOI:10.3390/ijerph111111822
- Schikowski T, Adam M, Marcon A, et al. Association of ambient air pollution with the prevalence and incidence of COPD. Eur Respir J. 2014;44(3):614–626. DOI:10.1183/09031936.00132213
- Ghanbari Ghozikali M, Heibati B, Naddafi K, et al. Evaluation of chronic obstructive pulmonary disease (COPD) attributed to atmospheric O3, NO2, and SO2 using air Q model (2011-2012 year). Environ Res. 2016;144(Pt A):99–105. DOI:10.1016/j.envres.2015.10.030
- Li T, Hu R, Chen Z, et al. Fine particulate matter (PM2.5): the culprit for chronic lung diseases in China. Chronic Dis Transl Med. 2018;4(3):176–186.
- Xing YF, Xu YH, Shi MH, et al. The impact of PM2.5 on the human respiratory system. J Thorac Dis. 2016;8(1):E69–74.
- Chen CH, Wu CD, Chiang HC, et al. The effects of fine and coarse particulate matter on lung function among the elderly. Sci Rep. 2019;9(1):14790. DOI:10.1038/s41598-019-51307-5
- Wu W, Jin Y, Carlsten C. Inflammatory health effects of indoor and outdoor particulate matter. J Allergy Clin Immunol. 2018;141(3):833–844. DOI:10.1016/j.jaci.2017.12.981
- Hamanaka RB, Mutlu GM. Particulate matter air pollution: effects on the cardiovascular system. Front Endocrinol (Lausanne). 2018;9:680.
- Zielinski M, Gasior M, Jastrzebski D, et al. Influence of particulate matter air pollution on exacerbation of chronic obstructive pulmonary disease depending on aerodynamic diameter and the time of exposure in the selected population with coexistent cardiovascular diseases. Adv Respir Med. 2018;86:227–233.
- Kwon SO, Hong SH, Han YJ, et al. Long-term exposure to PM10 and NO2 in relation to lung function and imaging phenotypes in a COPD cohort. Respir Res. 2020;21(1):247. DOI:10.1186/s12931-020-01514-w
- Zhu R, Chen Y, Wu S, et al. The relationship between particulate matter (PM10) and hospitalizations and mortality of chronic obstructive pulmonary disease: a meta-analysis. COPD. 2013;10(3):307–315. DOI:10.3109/15412555.2012.744962
- Hwang SL, Lin YC, Guo SE, et al. Fine particulate matter on hospital admissions for acute exacerbation of chronic obstructive pulmonary disease in southwestern Taiwan during 2006-2012. Int J Environ Health Res. 2017;27(2):95–105. DOI:10.1080/09603123.2017.1278748
- Jo YS, Lim MN, Han YJ, et al. Epidemiological study of PM2.5 and risk of COPD-related hospital visits in association with particle constituents in Chuncheon, Korea. Int J Chron Obstruct Pulmon Dis. 2018;13:299–307. DOI:10.2147/COPD.S149469
- Pothirat C, Chaiwong W, Liwsrisakun C, et al. Acute effects of air pollutants on daily mortality and hospitalizations due to cardiovascular and respiratory diseases. J Thorac Dis. 2019;11(7):3070–3083. DOI:10.21037/jtd.2019.07.37
- Qiu H, Yu IT, Wang X, et al. Cool and dry weather enhances the effects of air pollution on emergency IHD hospital admissions. Int J Cardiol. 2013;168(1):500–505. DOI:10.1016/j.ijcard.2012.09.199
- Raji H, Riahi A, Borsi SH, et al. Acute effects of air pollution on hospital admissions for asthma, COPD, and bronchiectasis in Ahvaz, Iran. Int J Chron Obstruct Pulmon Dis. 2020;15:501–514. DOI:10.2147/COPD.S231317
- Sun Q, Liu C, Chen R, et al. Association of fine particulate matter on acute exacerbation of chronic obstructive pulmonary disease in Yancheng, China. Sci Total Environ. 2019;650(Pt 2):1665–1670. DOI:10.1016/j.scitotenv.2018.09.278
- Sun XW, Chen PL, Ren L, et al. The cumulative effect of air pollutants on the acute exacerbation of COPD in Shanghai, China. Sci Total Environ. 2018;622-623:875–881. DOI:10.1016/j.scitotenv.2017.12.042
- Tao Y, Mi S, Zhou S, et al. Air pollution and hospital admissions for respiratory diseases in Lanzhou, China. Environ Pollut. 2014;185:196–201. DOI:10.1016/j.envpol.2013.10.035
- Tian L, Ho KF, Wang T, et al. Ambient carbon monoxide and the risk of hospitalization due to chronic obstructive pulmonary disease. Am J Epidemiol. 2014;180(12):1159–1167. DOI:10.1093/aje/kwu248
- Tian Y, Liu H, Zhao Z, et al. Association between ambient air pollution and daily hospital admissions for ischemic stroke: a nationwide time-series analysis. PLoS Med. 2018;15(10):e1002668. DOI:10.1371/journal.pmed.1002668
- Wang Z, Zhou Y, Zhang Y, et al. Association of change in air quality with hospital admission for acute exacerbation of chronic obstructive pulmonary disease in Guangdong, China: a province-wide ecological study. Ecotoxicol Environ Saf. 2021;208:111590. DOI:10.1016/j.ecoenv.2020.111590
- Wong TW, Lau TS, Yu TS, et al. Air pollution and hospital admissions for respiratory and cardiovascular diseases in Hong Kong. Occup Environ Med. 1999;56(10):679–683. DOI:10.1136/oem.56.10.679
- Xu Q, Li X, Wang S, et al. Fine particulate air pollution and hospital emergency room visits for respiratory disease in urban areas in Beijing, China, in 2013. PloS One. 2016;11(4):e0153099. DOI:10.1371/journal.pone.0153099
- Alman BL, Pfister G, Hao H, et al. The association of wildfire smoke with respiratory and cardiovascular emergency department visits in Colorado in 2012: a case crossover study. Environ Health. 2016;15(1):64. DOI:10.1186/s12940-016-0146-8
- Belleudi V, Faustini A, Stafoggia M, et al. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology. 2010;21(3):414–423. DOI:10.1097/EDE.0b013e3181d5c021
- Kloog I, Chudnovsky AA, Just AC, et al. A new hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmos Environ (1994). 2014;95:581–590. DOI:10.1016/j.atmosenv.2014.07.014
- Li R, Jiang N, Liu Q, et al. Impact of air pollutants on outpatient visits for acute respiratory outcomes. Int J Environ Res Public Health. 2017;14(1):47. DOI:10.3390/ijerph14010047
- Santus P, Russo A, Madonini E, et al. How air pollution influences clinical management of respiratory diseases. a case-crossover study in Milan. Respir Res. 2012;13:95. DOI:10.1186/1465-9921-13-95
- Weichenthal SA, Lavigne E, Evans GJ, et al. Fine particulate matter and emergency room visits for respiratory illness. Effect modification by oxidative potential. Am J Respir Crit Care Med. 2016;194(5):577–586. DOI:10.1164/rccm.201512-2434OC
- Selcuk AA. A guide for systematic reviews: PRISMA. Turk Arch Otorhinolaryngol. 2019;57(1):57–58. DOI:10.5152/tao.2019.4058
- Aslam S, Emmanuel P. Formulating a researchable question: a critical step for facilitating good clinical research. Indian J Sex Transm Dis AIDS. 2010;31(1):47–50. DOI:10.4103/0253-7184.69003
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). London: Cochrane Collaboration; 2019.
- Kessler R, Faller M, Fourgaut G, et al. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(1):158–164. DOI:10.1164/ajrccm.159.1.9803117
- Chen C, Liu X, Wang X, et al. Effect of air pollution on hospitalization for acute exacerbation of chronic obstructive pulmonary disease, stroke, and myocardial infarction. Environ Sci Pollut Res Int. 2020;27(3):3384–3400. DOI:10.1007/s11356-019-07236-x
- Chen C, Wang X, Lv C, et al. The effect of air pollution on hospitalization of individuals with respiratory and cardiovascular diseases in Jinan, China. Medicine (Baltimore). 2019;98(22):e15634. DOI:10.1097/MD.0000000000015634
- Ko FW, Tam W, Wong TW, et al. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax. 2007;62(9):780–785. DOI:10.1136/thx.2006.076166
- Liang L, Cai Y, Barratt B, et al. Associations between daily air quality and hospitalisations for acute exacerbation of chronic obstructive pulmonary disease in beijing, 2013–17: an ecological analysis. Lancet Planet Health. 2019;3(6):e270–e279. DOI:10.1016/S2542-5196(19)30085-3
- Pothirat C, Tosukhowong A, Chaiwong W, et al. Effects of seasonal smog on asthma and COPD exacerbations requiring emergency visits in Chiang Mai, Thailand. Asian Pac J Allergy Immunol. 2016;34:284–289.
- Weichenthal S, Pinault LL, Burnett RT. Impact of oxidant gases on the relationship between outdoor fine particulate air pollution and nonaccidental, cardiovascular, and respiratory mortality. Sci Rep. 2017;7(1):16401. DOI:10.1038/s41598-017-16770-y
- Wang K, Hao Y, Au W, et al. A systematic review and meta-analysis on short-term particulate matter exposure and chronic obstructive pulmonary disease hospitalizations in China. J Occup Environ Med. 2019;61(4):e112–e124. DOI:10.1097/JOM.0000000000001539
- Sunyer J. Urban air pollution and chronic obstructive pulmonary disease: a review. Eur Respir J. 2001;17(5):1024–1033. DOI:10.1183/09031936.01.17510240
- Garshick E. Effects of short- and long-term exposures to ambient air pollution on COPD. Eur Respir J. 2014;44(3):558–561. DOI:10.1183/09031936.00108814
- MacNee W, Donaldson K. Exacerbations of COPD: environmental mechanisms. Chest. 2000;117(5):390S–397S. DOI:10.1378/chest.117.5_suppl_2.390S
- Kasemo B, Lausmaa J. Material-tissue interfaces: the role of surface properties and processes. Environ Health Perspect. 1994;102(Suppl 5):41–45. DOI:10.1289/ehp.94102s541
- Ni L, Chuang CC, Zuo L. Fine particulate matter in acute exacerbation of COPD. Front Physiol. 2015;6:294.
- Li XY, Gilmour PS, Donaldson K, et al. Free radical activity and pro-inflammatory effects of particulate air pollution (PM10) in vivo and in vitro. Thorax. 1996;51(12):1216–1222. DOI:10.1136/thx.51.12.1216
- Abbey DE, Burchette RJ, Knutsen SF, et al. Long-term particulate and other air pollutants and lung function in nonsmokers. Am J Respir Crit Care Med. 1998;158(1):289–298. DOI:10.1164/ajrccm.158.1.9710101
- Garcia-Aymerich J, Monso E, Marrades RM, et al. Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation. EFRAM study. Am J Respir Crit Care Med. 2001;164(6):1002–1007. DOI:10.1164/ajrccm.164.6.2006012
- Anthonisen NR. Smoking, lung function, and mortality. Thorax. 2000;55(9):729–730. DOI:10.1136/thorax.55.9.729
- Sanyal S, Rochereau T, Maesano CN, et al. Long-term effect of outdoor air pollution on mortality and morbidity: a 12-year follow-up study for metropolitan France. Int J Environ Res Public Health. 2018;15(11):2487. DOI:10.3390/ijerph15112487.
- Yang Y, Tang R, Qiu H, et al. Long term exposure to air pollution and mortality in an elderly cohort in Hong Kong. Environ Int. 2018;117:99–106. DOI:10.1016/j.envint.2018.04.034
- Burnett R, Chen H, Szyszkowicz M, et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci U S A. 2018;115(38):9592–9597. DOI:10.1073/pnas.1803222115
- Han F, Yang X, Xu D, et al. Association between outdoor PM2.5 and prevalence of COPD: a systematic review and meta-analysis. Postgrad Med J. 2019;95(1129):612–618. DOI:10.1136/postgradmedj-2019-136675
- Duan X, Jiang Y, Wang B, et al. Household fuel use for cooking and heating in China: results from the first Chinese environmental exposure-related human activity patterns survey (CEERHAPS). Appl Energy. 2014;136:692–703. DOI:10.1016/j.apenergy.2014.09.066