Abstract
Melanoma-associated antigen family protein-D1 (MAGE-D1) is a recently identified p75 neurotrophin receptor intracellular binding protein and functions as an adaptor that mediates multiple signaling pathways, including Dlx/Msx-mediated transcription. Here, a new regulatory function for MAGE-D1 in tumor cell motility and adhesion to endothelium is described. MAGE-D1 over-expression suppressed HeLa cell and BEL7402 cell migration, invasion, and adhesion to the monolayer of ECV304 cells. We also report that MAGE-D1 over-expression disrupted actin cytoskeleton rearrangement induced by hypoxia and down-regulated hypoxia inducible factor 1-dependent luciferase gene expression. These findings provide new insight into the ability of MAGE-D1 to suppress the motility and adhesion response of tumor cells by interfering with actin cytoskeleton reorganization and hypoxia inducible factor 1-dependent gene expression.
INTRODUCTION
Melanoma-associated antigen family protein-D1 (MAGE-D1) (also designated NRAGE or Dlxin-1) was identified as a p75 neurotrophin receptor intracellular binding protein and belongs to the MAGE protein family (Salehi et al., Citation2000). MAGE-D1 has several interaction partners, including p75 neurotrophin receptor, the inhibitor of apoptosis proteins ITA and XIAP, the UNC5H1 axon guidance receptor, the homeobox proteins Dlx5, Dlx7, Msx1, and Msx2, the receptor tyrosine kinase Ror2, and the ubiquitin ligase Praja1 (Salehi et al., Citation2000; Jordan et al., Citation2001; Williams et al., Citation2003; Kuwajima et al., Citation2004; Masuda et al., 2001, 2003; Sasaki et al., Citation2002), all of which are involved in apoptosis. It has been reported that MAGE-D1 could arrest cell cycle through a p53-dependent pathway (Wen et al., Citation2004), suggesting that it may interact with a common set of cell cycle regulators. Subsequent studies revealed that MAGE-D1 is a universally expressed gene and highly conserved (Hennuy et al., Citation2000) and may function as an adaptor protein that mediates multiple signaling pathways (Salehi et al., Citation2000; Sasaki et al., Citation2005).
Hypoxia is widely recognized as a common feature of tumor and ischemic tissues and alters fundamental and physiologically important intracellular pathways. Several lines of evidence have highlighted that hypoxia may facilitate the expression of many genes, which are involved in regulation of tumor cell proliferation, migration, and adhesion (Harris, Citation2002). Adhesion of tumor cells to endothelial cells is proposed to figure heavily in cancer metastasis and is involved in the vasculogenesis associated with tumor progression (Hakomori, Citation2002; Tei et al., Citation2002). It has been reported that MAGE-D1 is structurally homologous to necdin, which served as a postmitotic neuron-specific growth suppressor, induced growth arrest (Taniura et al., Citation1998) and could negatively regulate hypoxia inducible factor 1 α (HIF-1α) stability and angiogenesis (Moon et al., Citation2005). However, the relationship between MAGE-D1 and tumor cell migration and adhesion to endothelium in response to hypoxic stress has not been previously explored.
The aim of this study is to evaluate whether MAGE-D1 is involved in the regulation of tumor cell proliferation, migration, invasion, and adhesion on endothelium under hypoxic conditions. Considering that cell migration, invasion, and adhesion are highly organized and integrated processes, the regulation of all the components involved, including actin and its partners, must be closely coordinated (Eccles, Citation2004). Specifically, we evaluated the effects of MAGE-D1 over-expression under hypoxic conditions on actin organization and HIF-1 transcriptional activity in cells.
MATERIALS AND METHODS
Cell Lines and Culture Conditions
BEL7402 cells (human hepatocellular carcinoma cell line), HeLa cells, purchased from the cell bank of Shanghai Institute of Cell Biology, ECV304 cells (a spontaneously transformed human umbilical vein endothelial cell line) and HEK293 cells (Ad5 E1-transformed human embryo kidney cell line), obtained from China Center for Type Culture Collection, were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco/BRL, Gaithersburg, MD) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μ g /ml streptomycin and maintained at 37°C in a humidified atmosphere of 5% CO2. Hypoxic incubation was performed in a tightly sealed hypoxic cultural chamber (Changjin Science Technologies Co., Ltd., Changsha, China). Cells were placed in the humidified chamber flushed with a gas mixture of 1% O2, 5% CO2, and balanced with nitrogen (N2). The hypoxic chamber containing cell-culture dishes was placed in a culture incubator for the indicated period of time.
Production of Recombinant Adenovirus
A replication-defective adenoviral vector expressing human MAGE-D1 (Ad-MAGE-D1) was generated as described previously (Wen et al., Citation2004). The adenoviral vector expressing the green fluorescence protein (Ad-GFP) was also generated and performed as control adenovirus. Recombinant adenovirus particles were packaged and amplified in HEK293 cells.
Western Blot Analysis
HeLa cells or BEL7402 cells infected with the indicated adenoviral vectors for 48 h were washed twice with cold phosphate buffered saline (PBS), lysed in RIPA buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and cocktail of protease inhibitors (Roche). Protein concentrations were determined with the Bradford method. Samples were subjected to 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes (Bio-Rad) and subsequently blocked in PBS-Tween 20 containing 3% BSA. The membranes were incubated with rabbit anti-human MAGE-D1 polyclonal antibody (Upstate) at 4°C overnight, after washing three times with PBS-Tween 20, followed by incubation with alkaline phosphatase conjugated secondary antibody (anti-rabbit IgG, Santa Cruz, CA, USA). The alkaline phosphatase buffer (pH 9.5) containing NBT and BCIP (Sigma) was used as detection system.
Cell Proliferation Assay
Cell proliferation was investigated using direct cell counting and the Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Gaithersburg, MD) assays. For cell counting assay, HeLa cells or BEL7402 cells (3 × 104 cells/well) infected with the indicated adenovirus at a multiplicity of infection (MOI) of 50 were seeded in 24-well plates in DMEM supplemented with 10% FBS at 37°C in a humidified atmosphere of 5% CO2. Cell proliferation was analyzed by direct counting of cells in triplicate wells every 24 h by trypan blue exclusion using a hemocytometer. Cell Counting Kit-8 allows sensitive colorimetric assays for the determination of the number of viable cells in cell proliferation assay by utilizing WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] to produce a water-soluble formazan dye upon reduction in the presence of an electron carrier. The amount of the formazan dye generated by the activity of dehydrogenases in cells is directly proportional to the number of living cells. Therefore, CCK-8 assay was also used to evaluate the effect of MAGE-D1 on cell proliferation according to the manufacturer's instructions. Briefly, quadruple samples of HeLa cells or BEL7402 cells were grown on 96 well plates and were infected with 50 MOI of the indicated adenovirus. After 24, 48, 72, and 96 h, 10 μ l of CCK-8 solution were added to each well and incubation again for another 1 h, and the absorbance at 450 nm was measured by using a microplate reader. Absorbances were converted to percentages for comparison with the untreated control. Three separate experiments were performed.
Wound-Healing Assay
BEL7402 or HeLa cells infected with 50 MOI of the indicated adenovirus for 48 h were grown to confluence in 6-well plates. A wound was made by using a pipette tip to scrape off the cells. The phase contrast images of the wounds were recorded after incubation for 16 h under normoxic and hypoxic conditions, and cells that had migrated to the wounded areas were counted for quantification of cell migration from five random microscopic fields (× 200) per well from triplicate wells, and three separate experiments were performed.
Transwell Invasion Assay
Cell invasion assay was performed using 6.5 mm, 8.0 μ m pore size transwell (Costar, Cambridge, MA) as previously described (Leavesley et al., Citation1993) with some modifications. After serum-starved overnight in serum-free DMEM, BEL7402 or HeLa cells infected with 50 MOI of the indicated adenovirus for 48 h were collected by using trypsin-EDTA in PBS and suspended at 3 × 105 cells/ml in serum-free medium supplemented with 0.1% bovine serum albumin (BSA). Cells (3 × 104) were seeded into transwell inserts precoated with Matrigel (1 μ g/ml, diluted with serum-free DMEM, BD Biosciences). Inserts containing the cells were placed into a 24-well plate containing DMEM supplemented with 10% FBS and incubated for 8 h at 37°C under normoxic or hypoxic conditions. Inserts were then carefully removed and the cells attached to the upper side were scraped off with a cotton swab. Cells migrating through the filter were fixed with methanol for 15 min and stained with Giemsa solution for 30 min. Filters were cut out of the inserts and mounted onto glass slide. The migratory cells were counted from five random microscopic fields (× 200) per insert from triplicate wells, and three separate experiments were performed.
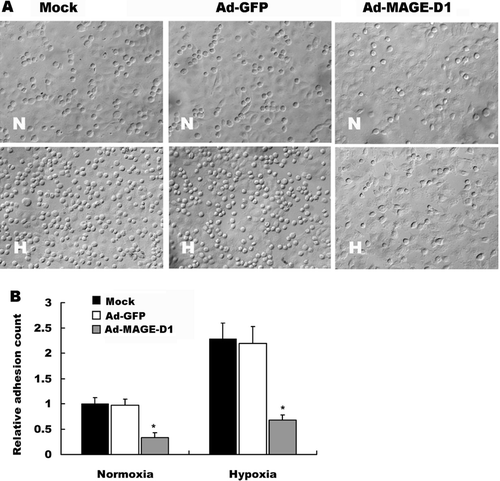
Tumor Cell Adhesion to Endothelial Cell Assay
ECV304 cells were grown to confluence on glass cover slips in 6-well plates maintained in DMEM supplemented with 10% FBS. HeLa cells infected with 50 MOI of the indicated adenovirus for 48 h were prelabeled with DAPI (Sigma) at 20 μ g/ml at 37°C for 20 min, and washed twice with PBS, and then were harvested, and 300 μ l of cell suspension (1 × 106 cells/well) were added to the monolayer of ECV304 cells and incubated at 37°C for 45 min under normoxic or hypoxic conditions. Cells were then gently washed three times with adhesion buffer containing DMEM supplemented with 0.1% BSA and fixed in 1% glutaraldehyde in PBS. DAPI-labeled tumor cells were counted under a fluorescent microscope from five random fields (× 200) per well from duplicate wells. The adherent tumor cells were identified and differentiated from the underlying layer of ECV304 cells by detection of DAPI-labeled tumor cells. Degree of adhesion was calculated by the ratio of the number of the adhesive cells to normoxic mock control, and four separate experiments were performed. In order to show both the adherent tumor cells and the underlying layer of ECV304 cells in the identical field of vision, the images were captured under an optic microscope.
Immunofluorescence and Image Analysis
After infection with the indicated adenovirus at a MOI of 50 for 36 h, HeLa cells were washed with PBS, fixed with 4% paraformaldehyde in PBS for 30 min, and permeabilized with 0.2% Triton X-100 in PBS for 5 min. For filamentous actin (f-actin) staining, cells were incubated with rhodamine-conjugated phalloidin (Sigma) at 37°C for 1 h. The fluorescent images were captured with a fluorescent microscope and a SPOT CCD camera.
Luciferase Reporter Gene Assay
HeLa cells and BEL7402 cells infected with the indicated adenovirus were seeded in 6-well plates and co-transfected with hypoxia-inducible factor-1(HIF-1)-dependent VEGF promoter reporter gene construct (5×HRE/pGL3/VEGF/E1b (Shibata et al., Citation1998), kindly provided by Prof. M. Hiraoka, Kyoto University, Kyoto, Japan) and β -galactosidase expression vector according to a conventional calcium phosphate precipitation technique. After overnight incubation, cells were washed twice with PBS and incubated again at 37°C for 16 h in DMEM supplemented with 10% FBS. After incubation for another 24 h under normoxic or hypoxic conditions, cells were then lysed using reporter lysis buffer (Promega), and luciferase activity was determined using an analytical luminescence luminometer according to the manufacturer's instructions. Luciferase activity was normalized for transfection efficiency using the corresponding β -galactosidase activity.
Statistical Analysis
The data were expressed as the mean ± standard deviations (mean ± SD) from 3–5 independent experiments. Statistical comparisons using analysis of variance (ANOVA) were performed with SPSS 8.0 software for Windows and p values less than 0.05 were considered statistically significant.
RESULTS
Western Blot for MAGE-D1
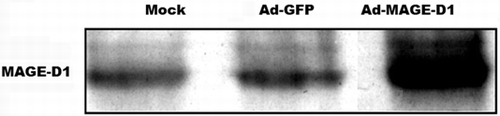
With Western blot analysis for MAGE-D1, we searched for the gene product of the transgene MAGE-D1. Even though low levels of MAGE-D1 could be detected in both mock- and Ad-GFP-treated HeLa cells, over-expression of MAGE-D1 was observed in Ad-MAGE-D1 infected cells ().
Ad-MAGE-D1 Infection Suppressed Tumor Cell Proliferation
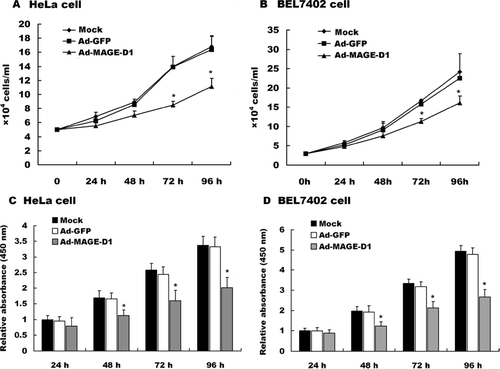
To examine the role of MAGE-D1 on tumor cell proliferation, BEL7402 cells or HeLa cells were transduced with the indicated adenovirus and cell proliferation was measured by CCK-8 assay and cell counting every 24 h. As shown in , Ad-MAGE-D1 infection significantly suppressed both BEL7402 cell and HeLa cell proliferation relative to mock- and Ad-GFP-treated cells. No significant differences in cell proliferation were detected between mock- and Ad-GFP-infected cells. The results indicated that MAGE-D1 over-expression suppresses tumor cell proliferation.
Figure 2 Ad-MAGE-D1 infection suppressed tumor cell proliferation. (A) and (B), Quantitative analysis of the proliferation of HeLa cells (A) and BEL7402 cells (B) infected with 50 MOI of the indicated adenovirus was performed by counting cells every 24 hours. Data are shown as cells/ml per well for triplicate wells from three independent experiments. *p < 0.05 vs. mock. (C) and (D), Cell proliferation for HeLa cells (C) and BEL7402 cells (D) was measured in a CCK-8 assay. Each dataset represents the ratio of the mock control after incubation for 24 h. Data are shown as the mean ± SD of three independent experiments.*p < 0.05 compared with the indicated mock control.
Ad-MAGE-D1 Infection Inhibited Tumor Cell Migration and Invasion
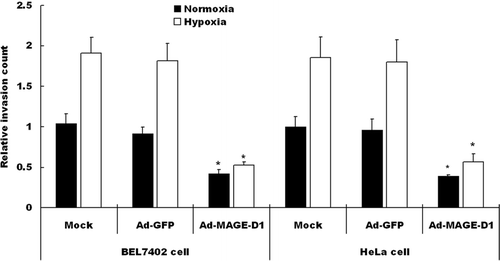
To investigate whether over-expression of MAGE-D1 interferes with cell migration, wound-healing and transwell invasion assays were assessed. Under hypoxic conditions, the migration of either HeLa cells or BEL7402 cells across a wound area was significantly increased relative to cells under normoxic conditions (). After transduction with Ad-MAGE-D1, however, the number of migratory cells was reduced significantly relative to mock treated cells in wound-healing assays (, ). No significant differences in cell migration were detected between Ad-GFP-treated and mock-cells. Furthermore, the reduction in cell migration did not appear to be due to decreased cell viability. Under these conditions Ad-MAGE-D1-infected cells did not display detectable or significant increases in the frequencies of TUNEL-or pyknotic nuclei-positive cells (data not shown). Similar results were also shown in transwell invasion assays in BEL7402 and HeLa cells (). Taken together, these results clearly demonstrated that over-expression of MAGE-D1 impairs the migration and invasion of tumor cells, especially under hypoxic conditions.
Figure 3 Ad-MAGE-D1 infection inhibited tumor cell migration in wound healing assay. (A) and (B), photomicrographs (× 200 magnification) showing the migration of HeLa cells infected with 50 MOI of the indicated adenovirus into the scraped area after 16 h under normoxic (A) or hypoxic (B) conditions, respectively. (C) and (D), Quantitative analysis of the migration for HeLa cells (C) and BEL7402 cells (D) after incubation for 16 h by counting the number of cells that had migrated to the wounded areas. Data are shown as the mean ± SD from three independent experiments. Results are expressed as a percentage of the migration in normoxic mock control. *p < 0.01 vs. mock.
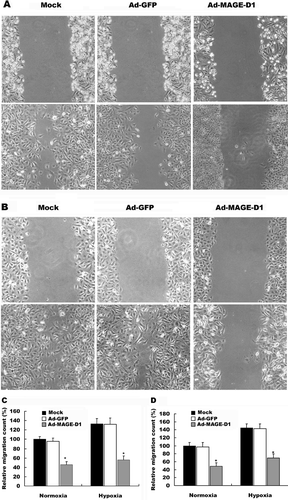
Figure 4 Ad-MAGE-D1 infection decreased the invasion of tumor cells. Cells transduced with 50 MOI of the indicated adenovirus for 48 h were plated on the transwell inserts, and transwell invasion assays were performed. Quantitative analysis of invasion after incubation for 8 h under normoxic or hypoxic conditions was performed by counting cells on membranes that were stained with Giemsa solution. Each experiment was performed in triplicate, and three separate experiments were performed. Data are expressed relative to normoxic mock condition. Results are shown as the mean ± SD *p < 0.01 vs. mock condition.
Ad-MAGE-D1 Infection Decreased the Adhesion of Tumor Cells to ECV304 Cells
Adhesion of tumor cells to endothelial cells is proposed to figure heavily in cancer metastasis and during vasculogenesis associated with tumor progression (Hakomori, Citation2002; Tei et al., Citation2002). To investigate the role of MAGE-D1 in the adhesion of tumor cells to endothelial cells, HeLa cells were transduced with Ad-MAGE-D1 and tested for their ability to adhere to a monolayer of ECV304 cells. Under hypoxic conditions, the number of adherent HeLa cells bound to the ECV304 monolayer increased significantly relative to cells under normoxic conditions (). After infection with Ad-MAGE-D1, however, the number of attached HeLa cells dramatically decreased, especially under hypoxic conditions, when compared with mock- or Ad-GFP-treated cells (, ). Similar results were also shown in adhesion of BEL7402 cells to endothelial cells (data not shown). These results suggested that MAGE-D1 over-expression in tumor cells suppresses hypoxia-induced tumor cell-adhesion to endothelial cells.
Figure 5 Ad-MAGE-D1 infection decreased adhesion of tumor cells to endothelial cells. (A) ECV304 cells were grown to confluence on glass cover slips in 6-well plates. A suspension of HeLa cells infected with the indicated adenovirus was added onto the monolayer of ECV304 cells. After 45 min of incubation at 37°C in normoxia (N) or hypoxia (H), cells were washed, counted, and photographed. (B) Quantitative evaluation of the data from adhesion of HeLa cells on the monolayer of ECV304 cells. Each assay was performed twice in triplicate. Results are shown as the mean ± SD, expressed as fold increase relative to normoxic mock control. *p < 0.01 vs. the indicated normoxic mock control.
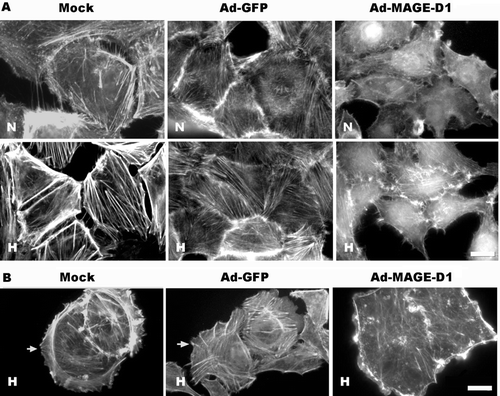
Ad-MAGE-D1 Infection Disrupted Actin Cytoskeletal Arrangement and Lamellipodia Formation in Tumor Cells
The actin cytoskeleton with its polymerization dynamics is central to many aspects of cellular activities, such as cytokinesis, phagocytosis, cell migration, adhesion, polarity, and morphology (Lee and Kay, Citation2003). All the components involved in cell migration and adhesion, including actin, must be closely coordinated, and membrane ruffling and lamellipodia formation are associated with cell protrusion during cell movement (Hall, Citation1998; Ridley, Citation2001). Thus we investigated whether Ad-MAGE-D1 infection could interfere with the actin cytoskeleton arrangement and the formation of lamellipodia in HeLa cells, processes involved in cell movement and migration. As shown in , hypoxia promoted normal appearance of the actin cytoskeleton to parallel, well-developed stress fibers across the cell bodies in both mock-and Ad-GFP-infected cells (). Meanwhile, HeLa cells were able to form lamellipodia at the leading edges of migrating cells (). In contrast, Ad-MAGE-D1 infection significantly altered stress fiber morphology and led to disrupted actin stress fibers () and lamellipodia actin bundling in hypoxia (). Similar results were also shown in BEL7402 cells (data not shown). Thus these findings clearly demonstrated that MAGE-D1 plays negative roles in the regulation of actin reorganization and in the formation of lamellipodia.
Figure 6 Ad-MAGE-D1 infection impaired lamellipodia formation and actin cytoskeletal reorganization induced by hypoxia in HeLa cells. Cells infected with 50 MOI of the indicated adenovirus were grown on glass cover slips in 6-well plates. After 4 h incubation in either normoxia (N) or hypoxia (H), cells were fixed and permeabilized, and f-actin structures were stained with rhodamine-conjugated phalloidin to visualize actin cytoskeleton organization (A), and the lamelipodia formation in migrating ECV304 cells (B). Scale bar, 10 μ m.
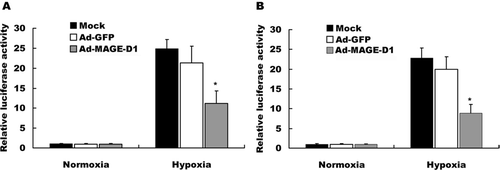
Ad-MAGE-D1 Infection Decreased HIF-1-Dependent Reporter Gene Expression
To evaluate further whether MAGE-D1 over-expression mediates its effects via HIF-1-dependent transcription, we performed luciferase reporter gene assays. As shown in , BEL7402 cells (A) and HeLa cells (B) transiently transfected with 5xHRE/pGL3/VEGF/E1b showed remarkable transcriptional induction in hypoxia, but induction was blocked by transduction with Ad-MAGE-D1 relative to mock and Ad-GFP infected cells. Together, these data indicated that MAGE-D1 might be an inhibitor of the induction of VEGF promoter activity in response to hypoxic stress.
Figure 7 Ad-MAGE-D1 infection down-regulated hypoxia-induced HIF-1-dependent reporter gene expression. BEL7402 cells (A) and HeLa cells (B) infected with the indicated adenovirus were seeded in 6-well plates and transfected with 5xHRE/pGL3/VEGF/E1b, respectively. At 28 h post-transfection, cells were exposed to hypoxia for another 20 h. Luciferase and β -gal activities were measured. Relative luciferase activity was determined by the ratio of luciferase to β -gal activity and normalized to the value obtained in normoxic mock control cells. Data represent the mean ± SD of triplicate samples from a typical experiment, expressed as fold increase with respect to normoxic mock control. *p < 0.01 vs. the mean of hypoxic mock control cells.
DISCUSSION
The current study provides the first evidence for a role of MAGE-D1 in the negative regulation of cell migration and adhesion under hypoxic conditions. This conclusion is supported by the observation that Ad-MAGE-D1 infection significantly inhibits tumor cell proliferation, migration, invasion, and tumor cell adhesion onto endothelium, disrupts actin cytoskeleton organization and lamellipodia formation, and down-regulates HIF-1-dependent gene expression. These findings indicated that MAGE-D1 might be a novel negative regulator in the modulation of tumor metastasis.
Previous studies have indicated that MAGE-D1 serves as a regulator of apoptosis and transcriptional modulators, and is on adapter protein that links the MAGE homology domain with Msx/Dlx homeodomain proteins, which are thought to play important roles in organogenesis and cellular differentiation (Salehi et al., Citation2000; Kuwajima et al., Citation2004; Harris, Citation2002). Recent evidence indicates that MAGE-D1 expression is widely distributed in mature rat brains (Barrett et al., Citation2005) and many tumors and normal tissues (Barker and Salehi, Citation2002; Chomez et al., Citation2001), suggesting that MAGE-D1 participates in multiple cellular signaling processes.
Cell proliferation and migration play an important role in the maintenance of vascular function (Ettenson and Gotlieb, Citation1994). Our initial experiments investigated the effect of over-expression of MAGE-D1 on the proliferation of tumor cells in vitro. We demonstrated that over-expression of MAGE-D1 suppressed the proliferation of both BEL7402 cells and HeLa cell. There is substantial evidence that tumor cells arrest and the formation of adhesive interactions between tumor cells and endothelial cells are crucial steps in the metastatic process (Zetter, Citation1990; Engers and Gabbert, Citation2000). Since hypoxia is regarded as a strong stimulus for tumor invasion and metastasis, we next evaluated whether MAGE-D1 regulates cell migration and invasion in both normoxia and hypoxia. Furthermore, considering MAGE-D1 expression is usually restricted to subsets of cells involved in neural development, branching organ development, and tumor progression (Frade, Citation2000; Roux and Barker, Citation2002), we expected MAGE-D1 to enhance cell migration. Interestingly, our present study demonstrated that MAGE-D1 over-expression inhibits migration and invasion of tumor cells in both the wound-healing assay and transwell invasion assay, especially in hypoxia. These findings provided the first evidence that MAGE-D1 has a role in anti-metastasis of tumors, particularly in response to hypoxic stress.
Adhesion of cancer cells to endothelial cells is known to be involved in the hematogenous metastasis of cancer (Hakomori, Citation2002). Here, we found that hypoxia significantly facilitates tumor cell-adhesion to the endothelium. In contrast, the capacity of HeLa cells transduced with Ad-MAGE-D1 to adhere to the monolayer of ECV304 cells was effectively inhibited under conditions of hypoxia, indicating that MAGE-D1 might be a negative regulator in hypoxia adhesion of induced tumor cells to endothelial cells.
It is generally accepted that actin stress fiber formation is often associated with cell adhesion, while membrane ruffling and lamellipodia formation are associated with cell migration (Hall, Citation1998; Ridley, Citation2001). We found that Ad-MAGE-D1 infection resulted in disruption of the hypoxia-induced actin stress fibers assembly in contacted HeLa cells and decreased the lamellipodia formation in migrating cells, which is consistent with the suppression of tumor cell motility and adhesion to endothelium. Taken together, these data suggested that inhibition of cell motility and adhesion by MAGE-D1, might be through its regulation of actin reorganization and the formation of lamellipodia.
Since cancer cells undergo distinct metabolic changes to cope with their hypoxic environment, hypoxia-induced cell adhesion and migration are achieved at least partly by the action of HIF-1 (Pennacchietti et al., Citation2003; Ceradini et al., Citation2004). HIF-1 is known to be intimately involved in the promotion of tumor angiogenesis by triggering the transcription of VEGF in cancer cells to overcome their hypoxic environments (Semenza, Citation2001; Harris, Citation2002). In the present study, we found that MAGE-D1 over-expression down-regulated HIF-1-dependent transcription in tumor cells. Taken together, inhibition of the migration and invasion of tumor cells by MAGE-D1 presumably mainly acts through its down-regulation of hypoxia-induced gene expression, including VEGF in tumor cells.
In conclusion, the data presented in this study demonstrated the influence of MAGE-D1 on tumor cell function, and confirmed that MAGE-D1 may be a novel inhibitor of tumor cell proliferation, migration, invasion, and adhesion on endothelium. Furthermore, the inhibition of cell migration, invasion, and adhesion on endothelium by MAGE-D1 might be modulated through down-regulating HIF-1-dependent gene expression and induction of actin cytoskeleton reorganization.
ACKNOWLEDGMENTS
We thank Prof. M. Hiraoka for 5xHRE/pGL3/ VEGF/E1b. This work was supported in part by grants from Natural Science Foundation of the Department of Education of Jiangsu Province and the project of the Subjects Group for Life Science of Yangzhou University (China).
REFERENCES
- Barker P A, Salehi A. The MAGE proteins: emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J Neurosci Res 2002; 67: 705–712
- Barrett G L, Greferath U, Barker P A, Trieu J, Bennie A. Co-expression of the p75 neurotrophin receptor and neurotrophin receptor-interacting melanoma antigen homolog in the mature rat brain. Neuroscience 2005; 133: 381–392
- Ceradini D J, Kulkarni A R, Callaghan M J, Tepper O M, Bastidas N, Kleinman M E, Capla J M, Galiano R D, Levine J P, Gurtner G C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004; 10: 858–864
- Chomez P, De Backer O, Bertrand M, De Plaen E, Boon T, Lucas S. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res 2001; 61: 5544–5551
- Eccles S A. Parallels in invasion and angiogenesis provide pivotal points for therapeutic intervention. Int J Dev Biol. 2004; 48: 583–598
- Engers R, Gabbert H E. Mechanisms of tumor metastasis: cell biological aspects and clinical implications. J Cancer Res Clin Oncol 2000; 126: 682–692
- Ettenson D S, Gotlieb A I. Endothelial wounds with disruption in cell migration repair primarily by cell proliferation. Microvasc Res 1994; 48: 328–337
- Frade J M. NRAGE and the cycling side of the neurotrophin receptor p75. Trends Neurosci 2000; 23: 591–592
- Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA 2002; 99: 10231–10233
- Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279: 509–514
- Harris A L. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47
- Hennuy B, Reiter E, Cornet A, Bruyninx M, Daukandt M, Houssa P, N'Guyen V H, Closset J, Hennen G. A novel messenger ribonucleic acid homologous to human MAGE-D is strongly expressed in rat Sertoli cells and weakly in Leydig cells and is regulated by follitropin, lutropin, and prolactin. Endocrinology 2000; 141: 3821–3831
- Jordan B W, Dinev D, Le Mellay V, Troppmair J, Gotz R, Wixler L, Sendtner M, Ludwig S, Rapp U R. Neurotrophin receptorinteracting mage homologue is an inducible inhibitor of apoptosis protein-interacting protein that augments cell death. J Biol Chem 2001; 276: 39985–39989
- Kuwajima T, Taniura H, Nishimura I, Yoshikawa K N. Necdin interacts with the Msx2 homeodomain protein via MAGE-D1 to promote myogenic differentiation of C2C12 cells. J Biol Chem 2004; 279: 40484–40493
- Leavesley D I, Schwartz M A, Rosenfeld M, Cheresh D A. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol 1993; 121: 163–170
- Lee H T, Kay E P. FGF-2 induced reorganization and disruption of actin cytoskeleton through PI 3-kinase, Rho, and Cdc42 in corneal endothelial cells. Mol Vis 2003; 9: 624–634
- Matsuda Y, Sasaki A, Shibuya H, Ueno N, Ikeda K, Watanabe K. Dlxin-1, a novel protein that binds Dlx5 and regulates its transcriptional function. J Biol Chem 2001; 276: 5331–5338
- Matsuda T, Suzuki H, Oishi I, Kani S, Kuroda Y, Komori T, Sasaki A, Watanabe K, Minami Y. The receptor tyrosine kinase Ror2 associates with the melanoma-associated antigen (MAGE) family protein Dlxin-1 and regulates its intracellular distribution. J Biol Chem 2003; 278: 29057–29064
- Moon H E, Ahn M Y, Park J A, Min K J, Kwon Y W, Kim K W. Negative regulation of hypoxia inducible factor-1alpha by necdin. FEBS Lett 2005; 579: 3797–3801
- Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio P M. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 2003; 3: 347–361
- Ridley A J. Rho GTPases and cell migration. J Cell Sci 2001; 114: 2713–2722
- Roux P P, Barker P A. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 2002; 67: 203–233
- Salehi A H, Roux P P, Kubu C J, Zeindler C, Bhakar A, Tannis L L, Verdi J M, Barker P A. NRAGE, a novel MAGE protein, interacts with the p75 neurotrophin receptor and facilitates nerve growth factor-dependent apoptosis. Neuron 2000; 27: 279–288
- Sasaki A, Matsuda Y, Iwai K, Ikeda K, Watanabe K. A RING finger protein Praja1 regulates Dlx5-dependent transcription through its ubiquitin ligase activity for the Dlx/Msx-interacting MAGE/Necdin family protein, Dlxin-1. J Biol Chem 2002; 277: 22541–2546
- Sasaki A, Hinck L, Watanabe K. RumMAGE-D the members: structure and function of a new adaptor family of MAGE-D proteins. J Recept Signal Transduct Res 2005; 25: 181–198
- Semenza G L. Regulation of hypoxia-induced angiogenesis: a chaperone escorts VEGF to the dance. J Clin Invest 2001; 108: 39–40
- Shibata T, Akiyama N, Noda M, Sasai K, Hiraoka M. Enhancement of gene expression under hypoxic conditions using fragments of the human vascular endothelial growth factor and the erythropoietin genes. Int J Radiat Oncol Biol Phys 1998; 42: 913–916
- Taniura H, Taniguchi N, Hara M, Yoshikawa K. Necdin, a postmitotic neuron-specific growth suppressor, interacts with viral transforming proteins and cellular transcription factor E2F1. J Biol Chem 1998; 273: 720–728
- Tei K, Kawakami-Kimura N, Taguchi O, Kumamoto K, Higashiyama S, Taniguchi N, Toda K, Kawata R, Hisa Y, Kannagi R. Roles of cell adhesion molecules in tumor angiogenesis induced by cotransplantation of cancer and endothelial cells to nude rats. Cancer Res 2002; 62: 6289–6296
- Wen C J, Xue B, Qin W X, Yu M, Zhang M Y, Zhao D H, Gao X, Gu J R, Li C J. hNRAGE, a human neurotrophin receptor interacting MAGE homologue, regulates p53 transcriptional activity and inhibits cell proliferation. FEBS Lett 2004; 564: 171–176
- Williams M E, Strickland P, Watanabe K, Hinck L. UNC5H1 induces apoptosis via its juxtamembrane region through an interaction with NRAGE. J Biol Chem 2003; 278: 17483–17490
- Zetter B R. The cellular basis of site-specific tumor metastasis. N Engl J Med 1990; 322: 605–612