ABSTRACT
This review article provides an overview of supercritical fluid chromatography (SFC) techniques for the separation of amino acids and peptides and modifications of mobile phases in SFC for the separation of highly polar and hydrophilic compounds. Furthermore, it covers SFC applications for the chiral and achiral separations of amino acids and peptides. In this review, detailed explanations of possible and already performed achiral and chiral separations by individual amino acids and peptides are presented. Different stationary phases, separation conditions such as backpressure and temperature, and compositions of the mobile phase with possible additives for each amino acid and peptide are summarized.
INTRODUCTION
Liquid chromatography (LC) is commonly used for the separation and analysis of hydrophilic and highly polar analytes. Supercritical fluid chromatography (SFC) began to develop in this direction too and pushed the limits of chiral and achiral separations. Due to the miscibility of CO2 with organic solvents and changing the mobile phase with modifiers and additives, separations over a wide polarity range of both non-polar long-chain alkyl components and small polar components, such as amino acids, are possible. Because amino acids are the constituents of peptides and proteins, it is important to develop accurate and efficient techniques for their separation and purification. Many methods have been described for such separations – from electrophoresis, LC methods, thin-layer chromatography (TLC), gas chromatography (GC), to ion-exchange chromatography (IEC) and others, with IEC and high-performance liquid chromatography (HPLC) as most commonly used .[Citation1,Citation2] Since the biological activity of amino acids also depends on their stereoisomeric configuration (D- and L-), the chiral analysis and separation of amino acids and peptides are very important. Very common techniques, in addition to SFC, for chiral separations are LC and GC at analytical scale, and LC at preparative scale .[Citation3]
SFC has emerged as a technique for separating components using supercritical fluids as mobile phases .[Citation4] This has many advantages, such as a short analysis time, high chromatographic efficiency, low cost, and, above all, the non-toxicity of the mobile phase with the use of CO2, making SFC an increasingly popular technique for separation of different compounds. SFC uses the high permeability of gases and the property of liquids to dissolve materials into their components. It is considered a “hybrid” of GC and LC .[Citation5] Several reviews of separating amino acids and peptides have already been published, [Citation2,Citation6–8] including Molineau’s et al. [Citation9] review of biomolecule separations with pressurized CO2 mobile phases. The present work focused on the development of SFC, its modification to separate very polar and hydrophilic compounds and a detailed overview of recent analyses and chiral and achiral separations of amino acids and peptides with SFC.
PROPERTIES OF AMINO ACIDS AND PEPTIDES
Amino acids are involved in most metabolic pathways and processes that are crucial for the growth of organisms .[Citation10] Therefore, the synthesis, separation, and purification of amino acids are both important and interesting. Since pharmaceutical, peptide research, and other applications demand the production of enantiomerically pure compounds, chiral separations have become an important analytical task. There are different classes of amino acids () that need to be distinguished for further analysis. Nonpolar amino acids are those that have a hydrophobic R-group, with a small dipole moment and a tendency to be repelled from water. Next, polar and zwitterionic amino acids are those whose R-group has polar functional groups. The third class is acidic amino acids, which have carboxylic acid on their side chains that give them acidic (i.e., can donate a proton) properties. At neutral pH, all functional groups will be ionized, resulting in an overall charge of −1. The last group is basic amino acids, whose side groups are basic (i.e., can accept a proton) and exist in an overall charge of +1 at neutral pH.
Figure 1. Classification of 20 common amino acids considering their volume, hydropathy, and chemical classes as well as their charge and polarity [Citation11].
![Figure 1. Classification of 20 common amino acids considering their volume, hydropathy, and chemical classes as well as their charge and polarity [Citation11].](/cms/asset/71e7ea68-733d-4bfa-9957-a7cd3b539df4/lspr_a_2038625_f0001_oc.jpg)
When trying to separate amino acids, two characteristics can be exploited, namely the differences in charge and the differences in R-group polarity. Charge differences are affected by pH values. Increasing the pH deprotonates the acid functional group and makes the net charge on an amino acid more negative, while decreasing the pH protonates both functional groups and consequently makes the net charge more positive. On the other hand, the R-group polarity can also be exploited, as it determines whether a given amino acid will be soluble or insoluble in the solvent. Furthermore, the zwitterionic properties of amino acids also play an important role in their separation. With these characteristics, LC, as well as SFC, are more powerful and preferable separation techniques, since GC analyses are not possible unless analytes are subjected to some form of derivatization to increase their volatility.
Most studies of amino acid separation refer to chromatographies .[Citation2] Frank et al. [Citation12] were the first group examining the separation of amino acids by GC using a chiral stationary phase. Later a lot of research on amino acid separation was done with GC .[Citation13–15] However, LC is today one of the most widely used methods, most notably HPLC, [Citation16–18] which is the most popular among the various LC techniques .[Citation19] The separation of amino acids in the field of biochemical research is important because it is possible to further investigate the structure and composition of proteins, determine amino acids in biological tissues and fluids, and determine the presence of free amino acids in food products to estimate food values .[Citation20] Peptides usually contain both ionizable acidic and basic side chains, so they have a characteristic isoelectric point. The net charge of the peptides and the polarity in the aqueous solution varies with pH. Further, hydrophobicity/hydrophilicity and the number of charged groups present are essential factors in the separation of these compounds .[Citation21] The difference between some peptides may only be in a single amino acid. This is called a single amino acid polymorphism (SAAP), while a single amino acid chiral polymorphism (SAACP) is when peptides differ only by an inversion of chirality of a single stereogenic center .[Citation22,Citation23] The separation of molecules like peptides or polypeptides often requires selective techniques, such as LC methods. More specifically reversed-phase liquid chromatography (RPLC) or LC combined with mass spectrometry (LC-MS), are the favored methods to achieve this, due to their robustness, applicability, and high speed of biomolecule analysis .[Citation24–26]
SUPERCRITICAL FLUID CHROMATOGRAPHY
In recent years one of the separation processes which has been investigated by many researchers is SFC. This was originally developed as a form of gas chromatography almost 60 years ago by Klesper et al. .[Citation27] A few years later, Sie et al. [Citation28,Citation29] started using CO2 as a mobile phase and studied its behavior from theoretical and experimental points of view. The physicochemical aspects of using supercritical CO2 as a mobile phase were investigated by Bartmann and Schneider .[Citation30] It is the most commonly used fluid in pure form or combined with co-solvent .[Citation31] Moreover, the SFC process can also be performed when CO2 is liquid, i.e. in the sub-critical range .[Citation32] Another advantage of this approach is the abundance of this material since it is a by-product in many industries .[Citation33,Citation34] Because of all the features listed above and because of its mild critical temperature (31.1°C) and pressure (73.8 bar), it is ideal for pharmaceutical applications. On the other hand, it does have some disadvantages as it reacts with primary and secondary aliphatic amines. Nevertheless, due to the frequent reversible reaction in the case of primary amines, the original product can still be retrieved .[Citation35,Citation36] Supercritical CO2 is a non-polar solvent and has similar polarity to hexane. The general rule is that if a compound dissolves in hexane, it is also expected to dissolve in supercritical CO2. This selectively dissolves water-insoluble substances such as fats, fatty acids, vegetable oils, and hydrophobic substances. The supercritical CO2 itself does not dissolve hydrophilic components such as sugars, proteins, and minerals (e.g., salts and metals) .[Citation37,Citation38] It is known that the solubility of an analyte can be significantly modulated by changing the pressure and temperature conditions.
The maximum achievable polarity index of pure CO2 solvent is slightly over 2.0 .[Citation39] For comparison, at the bottom of the polarity index scale is pentane, with a value of 0.0, and at the top is water with a polarity index of 10.2 .[Citation40] The number for CO2 is still very non-polar, making CO2 unsuitable for working with polar analytes. The solution is to add a polar organic solvent as co-solvent. One of the first applications of the use of supercritical CO2 and a co-solvent in the mobile phase was presented by Klesper and Hartmann .[Citation41] Methanol and n-pentane were used as co-solvents for the separation of the homologous species of styrene oligomers. The polarity range of solutes has expanded tremendously with the addition of organic solvents and additives, with strong acids, bases or salts often used. Many times, however, water is used as an additive, especially for the separation of highly polar compounds such as polypeptides .[Citation42] However, capillary SFC, where mostly neat CO2 is used as the mobile phase, only allows the separation of less polar compounds (e.g., derivatized amino acids), so it is necessary to emphasize the importance of packed column SFC, as it allows the use of co-solvents, which enable separation of highly polar compounds (e.g., free amino acids) .[Citation43] Therefore, the SFC technique is useful for the separation of non-polar and polar components .[Citation44]
Considering the hydrophobicity and hydrophilicity of the R-groups of amino acids, it should be clarified that amino acids with hydrophobic R-groups are easier to separate with SFCs than those with hydrophilic R-groups. Above all, the most difficult to separate in SFCs are those amino acids that have basic hydrophilic residues (e.g., histidine) (see the Supplementary Material for classifications of amino acids based on side-chain properties, e.g., hydropathy, volume, chemical, charge, and polarity). Recently, however, the technique has evolved tremendously for the separation of chiral components [Citation45] or enantiomers from racemates .[Citation46] Separation and determination of ethers, [Citation47] lipids, [Citation48] fat- and water-soluble vitamins, [Citation49] ketones, [Citation50] saponins, [Citation51] steroids [Citation52] and many other compounds is possible with SFC. Peptides up to 40 mers were also separated, but it is not a suitable technique for separating large biomolecules such as proteins .[Citation53] Furthermore, peptide isomers that differ in the position of one amino acid (pair of peptide isomers, where glycine is in the position of valine, and vice versa) were also well separated in a short time .[Citation54,Citation55] Regarding the separation of peptides, the nature of these is also important, as it is easier to separate hydrophobic or lipophilic polymers with SFC than hydrophilic or lipophobic polar ones .[Citation56]
Initially, method development begins on a laboratory/analytical scale. The scheme of laboratory-scale SFC is shown in . A basic laboratory-scale chromatograph consists of a tank for the mobile phase, unit for pressure control, tank for co-solvent/modifier, pumps, Rheodyne injection valve or automatic injector, separation column, detector, and Tescom valve backpressure regulator .[Citation58] From the analytical scale equipment, it is possible to obtain basic system parameters with a very small amount of material and to perform design and optimization to evaluate process performance and operating conditions .[Citation59]
Figure 2. Flow scheme of laboratory-scale SFC: V1-V3 – valves, AV – automated valve, PG – pressure gauge [Citation57].
![Figure 2. Flow scheme of laboratory-scale SFC: V1-V3 – valves, AV – automated valve, PG – pressure gauge [Citation57].](/cms/asset/0bdf0659-80b9-4e25-8f46-b43f1eb437f8/lspr_a_2038625_f0002_b.gif)
Recently the physicochemical (e.g., high diffusivity, low viscosity, zero surface tension, and tunable solvent strength) properties of supercritical fluids, which are very relevant for the operation of SFC, were elucidated by Miller, Pinkston, and Taylor .[Citation60] The speed, sensitivity, and resolution of chromatographic methods are very important features. But on the other hand, ecological aspects such as lower consumption of toxic organic solvents, greater user safety, lower costs, faster analysis, and less sample preparation are also significant .[Citation61,Citation62] In this context, modern SFC in the field of separation techniques has also evolved, [Citation63] and due to its high efficiency, speed and robustness it has become a desirable meth .[Citation64] Furthermore, the miscibility of supercritical CO2 with liquid organic solvents over a wide range of pressures and temperatures is also an important feature, as it provides wide polarity ranges. All these advantages, in addition to continuous improvements in the analytical SFC instrumentation (development of backpressure regulators, pumps, autosamplers, etc. [Citation65]) and easy hyphenation with mass spectrometry (MS), [Citation66] enable bioanalysis with good separation capacity and high throughput .[Citation67] Today SFC is used mainly on an analytical scale [Citation68–70] for the separation and characterization of bioactive compounds [Citation71] and has also been explored in preparative separations .[Citation72–75] The preparative SFC is shown in .
Figure 3. Flow scheme of pilot preparative to production scale SFC: 1 - CO2 tank, 2 - CO2 cooler, 3 - CO2 pump, 4 - CO2 heater, 5 - modifier tank, 6 - modifier pump, 7 - column with piston, 8 - sample tank, 9 - detector, 10 - flow meter, 11 – evaporator [Citation76].
![Figure 3. Flow scheme of pilot preparative to production scale SFC: 1 - CO2 tank, 2 - CO2 cooler, 3 - CO2 pump, 4 - CO2 heater, 5 - modifier tank, 6 - modifier pump, 7 - column with piston, 8 - sample tank, 9 - detector, 10 - flow meter, 11 – evaporator [Citation76].](/cms/asset/9a85b4d6-a25c-4884-a23a-bb6f01018893/lspr_a_2038625_f0003_b.gif)
SFC is one of the fastest-growing analytical techniques for achiral and chiral small-molecule pharmaceutical separations .[Citation77–79] Because nowadays it is important to be environmentally friendly and provide the greenest technology possible, SFC has been developed in the context of green analytical chemistry .[Citation44] Due to its many good features, SFC is becoming an increasingly attractive and widely used method. Some of the advantages over other techniques are the short analysis time, the possibility of using a high flow rate, the low viscosity of the mobile phase and consequently high chromatographic efficiency, low costs, ease of recovery of the purified sample in preparative SFC, and, above all, lower toxicity of the mobile phase .[Citation80] Moreover, another advantage is the compatibility of almost all stationary phases with CO2/co-solvent mixtures, resulting in a wider range of accessible analytes and incomparable separation options, about normal-phase LC (NPLC), reversed-phase LC (RPLC), hydrophilic interaction liquid chromatography (HILIC) and GC methods .[Citation81]
ADAPTING MOBILE AND STATIONARY PHASES FOR SEPARATIONS OF VERY POLAR AND HYDROPHILIC COMPOUNDS
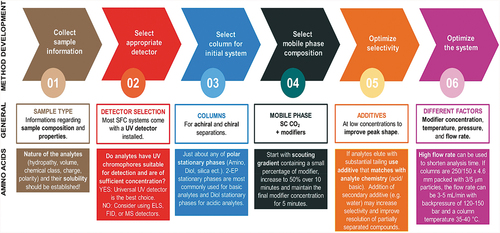
The coupling of SFC with MS has greatly expanded the range of possible applications in recent years due to improvements in the sensitivity of MS, which in turn enables the analysis of compounds at very low concentrations on nano and even picomole scale .[Citation57,Citation82–85] Analysis of compounds with a wide range of polarity is possible, [Citation86] however, the analysis of polar and ionizable compounds (e.g., amino acids, peptides, polar drugs, metabolites) is much more complex. Typically, for the separation of such compounds, the mobile phase consists of 2 to 40% of co-solvents and polar additives .[Citation87,Citation88] The most used co-solvent is methanol (MeOH), which represents about 75% of all used co-solvents, [Citation89,Citation90] followed by ethanol (EtOH), isopropanol (IPA), and acetonitrile (ACN) .[Citation91] Co-solvents are used to influence the polarity of neat CO2, the polarity of the stationary phase (by adsorbing to its surface), the critical pressure and temperature, and the density of the mobile phase. These factors change analyte solubility, and more importantly, the elution strength of the mobile phase and separation selectivity .[Citation92] The choice of a good stationary phase with variable selectivity for resolving difficult peak pairs in the SFC is extensive, and it is quite simple to adjust the stationary phase to the solvent, as seen in . Many times, the choice of different stationary phases is possible for specific separation. As the polarity of the analyte increases, a more polar phase must also be selected for separation.
Figure 4. Specific mobile and stationary phases in SFC selected according to the polarity of soluble substances [Citation54].
![Figure 4. Specific mobile and stationary phases in SFC selected according to the polarity of soluble substances [Citation54].](/cms/asset/0859a185-e00a-4206-9ec4-7509b90b68e9/lspr_a_2038625_f0004_oc.jpg)
In most cases, the addition of a co-solvent is not sufficient to obtain a chromatographic separation of components that are very polar. By using polar additives (in a concentration range of 0.1–2% [Citation93]), improvements in component separation, peak shape, and selectivity are possible .[Citation94–96] The additives commonly used in SFC separations are acids (e.g., formic (FA), acetic (HAc), and trifluoroacetic (TFA) acid [Citation96,Citation97]), strong bases (e.g., amines and ammonium hydroxide [Citation97,Citation98]), and organic salts (e.g., ammonium formate and acetate [Citation94,Citation97,Citation99–101]). Water also has a promising effect on the separation of highly polar components as an additive. A small amount of water (1–5%) alone or with other additives makes it possible to separate polar components and improve peak shapes .[Citation54] Moreover, as water is inorganic and moderately volatile, it can improve compatibility with MS .[Citation88] Water has very low solubility in supercritical CO2, [Citation102] but the addition of a co-solvent allows water to be mixed with supercritical CO2. Water is more polar and becomes acidic in contact with CO2 due to the formation and dissociation of carbonic acid .[Citation42,Citation94,Citation103] Based on such modified mobile phases, elution and separations of highly polar and hydrophilic compounds are possible .[Citation83] shows and explains the flow chart of method development for SFC in general and for amino acid separation. It can also be helpful in establishing the final method for the separation of very polar and hydrophilic compounds.
SEPARATION OF AMINO ACIDS AND PEPTIDES WITH SFC
Chiral Separations
Enantiomer analysis is especially important for the characterization of chiral drugs in the pharmaceutical industry and in food science, as chiral analysis can provide valuable information on the safety, quality, traceability, and bioactivity of the analyzed samples .[Citation104,Citation105] The biological activity of molecules depends on chirality since enantiomers can trigger different reactions in a living organism because different enantiomers bind to different receptors .[Citation106] High diffusivity and low viscosity using a mobile phase with supercritical CO2 make SFC a widely used technique for separating chiral compounds .[Citation107] Several general SFC strategies for fast chiral separations, including screening and fine-tuning of the method, have already been described .[Citation108] In the chiral separation of enantiomers, a particular component must interact enantioselectively with the enantiomers to be separated .[Citation109] For that, chiral selectors are required. Among the various chiral selectors, the most commonly used are cation and anion exchangers (e.g., aminosulfonic acid-based chiral strong cation exchangers, Chinchona alkaloid-based chiral weak anion exchangers), [Citation110] zwitterionic chiral selectors (e.g., quinine and quinidine-based zwitterionic CSPs), [Citation111] and macrocyclic antibiotic-based materials (e.g., teicoplanin aglycone) .[Citation112] While the most used and universal chiral stationary phases are made with derivatized macrocrystalline cellulose or amylose coated on silica .[Citation54] Given the fact that the polarity of free and derivatized amino acids is very different, the choice of the composition of the stationary and mobile phases is also distinctive. Ordinarily, less polar derivatized amino acids are analyzed on polysaccharide chiral selectors, while very polar free amino acids are best analyzed on ionic chiral selectors .[Citation113]
SFC applications concerning amino acid derivatives analysis have mostly been focused on chiral separations. Extremely important are the facts that, for example, some D-amino acids have long been considered non-functional, and with the introduction of enantiomeric separations have gained an important role in many physiological processes in living organisms, including the human body, and have also been associated with many diseases (e.g., schizophrenia, age-related disorders, and amyotrophic lateral sclerosis) .[Citation114] Early research related to chiral/enantiomeric separation of derivatized amino acids appeared in the late 1980s .[Citation115] Chiral separations of amino acids and amino acid derivatives with SFC using different stationary and mobile phases are depicted in . Dobashi et al. [Citation116] studied the enantiomer resolution of D and L-alpha-amino acid derivatives on chiral valine-diamide phases with supercritical CO2 and methanol as a co-solvent. The column temperature was 40°C and the pressure used was 200 bar. Enantiomer separation was carried out in less than five minutes, except for the tryptophan derivative, which showed the highest retention. D-enantiomers were always eluted before L-enantiomers. Lajkó et al. [Citation117] carried out separations of the enantiomers of Nα-proteinogenic amino acids on alkaloid-based zwitterionic CSPs. Amino acids were protected with Nα-Fmoc and with additional protecting groups (t-butyloxycarbonyl, t-butyl, O-t-butyl, triphenylmethyl, and Nω-2,2,4,6,7-pentamethyl-dihydro benzofuran-5-sulfonyl) to make them more appropriate for peptide separation protocols. The study was performed at 40°C and 150 bar. On Chiralpak™ ZWIX (+), all D-enantiomers eluted before L-enantiomers, while on Chiralpak™ ZWIX (-) the elution sequence was opposite.
Table 1. Chiral separations of amino acids and amino acid derivatives with SFC using different stationary and mobile phases
Additionally, a variable-temperature study was performed to investigate its effect on chromatographic parameters. Retention time slightly increased, while the selectivity decreased with increasing temperature. Lajkó et al.’s findings are in line with those of Lou et al. .[Citation118] Changing the temperature can improve the enantioselectivity and capacity factor. Enantioselectivity decreases with increasing temperature, while the column pressure has no connection with enantioselectivity. However, it should be considered that the mobile and stationary phases and the nature of the analyte (e.g., free amino acids or derivatized amino acids) strongly influence the effect of the temperature on enantioresolution, and such studies are difficult to compare. Furthermore, in the conditions applied by Lou et al., [Citation118] the enantioselectivity was not affected by the mobile phase density. However, it should be noted that the researchers used an uncommon column, with OV-225-L-valine-tert.-butylamide chiral stationary phase, as otherwise in most cases mobile phase density does affect enantioselectivity in SFC .[Citation131]
Liu et al. [Citation119] investigated chiral separations of 111 chiral compounds, including N-protected and native amino acids, with macrocyclic glycopeptide CSPs at 31°C and 100 bar. Because of the decrease in enantioselectivity with increasing temperature, a low and constant temperature was selected. Furthermore, constant outlet pressure was used due to the effect of decreasing enantioresolution factors with rising pressure. Seventy percent of the compounds were separated in less than 4 min, and a maximum of 15 min was required to separate the remaining compounds. Regarding the separations of N-protected amino acids, most baseline separations could be achieved with 40–60% MeOH in the mobile phase, but the addition of 0.1% TEA was also required, meaning that the stationary phase and the solutes were in negatively charged forms. On the other hand, due to the nature of the polarity of native, underivatized amino acids, 2–2.8% of water and/or 0.3% of glycerol with 0.1–0.15% TEA and TFA needed to be added to the mobile phase to increase the solubility of polar analytes and to improve peak shapes.
Later, Stringham [Citation120] separated 36 of 45 basic compounds, including four underivatized amino acids, with amylose tris-(3,dimethyl phenyl carbamate) chiral stationary column 20% of EtOH containing 0.1ethane sulfonic acid (ESA) in the mobile phase. It was found that the addition of acid was already required during the preparation of the analytes for successful separation, and the acid-free mobile phase did not result in elution of the peaks. The cause is probably in the formation of salts from the basic compound and ESA, which are then separated as intact salts throughout SFCs. Further West et al. [Citation121] studied packed-column SFC with two polysaccharide-based chiral stationary phases to separate six amino acid derivatives (PP, PFP, PBzt, oMPP, oMPFP, PPY). Lux™ Cellulose-1 enabled enantiorecognition for five out of six racemates, and Lux™ Cellulose-2 complemented Lux™ Cellulose-1, as it enabled enantiorecognition of two out of six racemates, but one of the two was the one (PPY) that Lux™ Cellulose-1 did not recognize. Therefore, the separation of five racemates was studied on Lux™ Cellulose-1, while Lux™ Cellulose-2 was studied with only two racemates. SFC was performed at 30°C, 150 bar, and with a modifier proportion of 10%. For better resolution, the percentage of modifier was lowered from 10 to 3%.
Payagala et al. [Citation122] investigated the applicability of three polymeric CSPs in SFC for the application of amino acids and other chiral compounds. With simple free-radical-initiated polymerization in solution, CSPs were synthesized based on three (1S,2S)-(−)-1,2-diphenylethylenediamine derivatives and were evaluated and compared with commercial polymeric CSP in HPLC and SFC. In SFC, 65 of 100 racemates were separated with 24 baseline separations. Among all of these, a derivatized amino acid N-(3,5-dinitrobenzoyl)-DL-leucine was separated only with commercial P-CAP-DP CSP, with 10% EtOH and 0.1% TFA in the supercritical CO2 mobile phase. Wang et al. [Citation123] studied enantioseparation on 14 racemates, including amino acid derivatives. They used chemically bonded cationic β-cyclodextrin perphenylcarbamoylated derivatives as CSPs. Dansyl amino acids were separated using 2-propanol as a modifier, as it has a lower polarity compared to MeOH and does not react as much with the substituents on the cyclodextrin rim, allowing better enantioseparation. On the other hand, the alkyl chain length of the substituent at a position to the carboxylic acid group affects retention and selectivity. Shorter alkyl chains form looser inclusion complexes with a cyclodextrin cavity, so retention and selectivity are lower for dansyl amino acids with a shorter alkyl chain.
Another study by Sánchez-Hernández et al. [Citation124] reported the enantioseparation of aromatic amino acids with SFC, carried out on glycopeptide-based CSP. As analytes had no acidic or basic group in the residue, just MeOH and H2O were necessary as additives to the mobile phase. The effects of pressure and temperature were also evaluated. The optimized parameters were 100 bar and 35°C, as lower pressure (90 bar) can cause slightly higher retention and broader peaks, while higher pressure (150 bar) causes lower retention and higher pressure drops. As the temperature increased (from 20 to 40°C), the resolution and retention increased, as well as the analysis time. It took less than 7 min for individual separation and identification of amino acids. Wohlrab et al. [Citation125] studied the enantioseparation of N-protected amino acids and other basic analytes with HPLC and SFC. SFC was carried out at 40°C and 150 bar. Because the HPLC separations were performed at 20°C, the researchers also performed a separation with subcritical CO2 at 20°C to compare the measurements. Enantioselectivity increased at 20°C, but resolution decreased. In addition, chromatographic conditions were investigated for increasing the ratio of a polar co-solvent (alcohol) with subcritical CO2, and with that retention times decreased. Interestingly, under certain conditions, no enantiomers were separated by HPLC, while the same analytes were separated by SFC. The reason could be in the physical-chemical properties of CO2, which is a non-polar solvent but has the property of accepting protons which makes it completely miscible with protic organic solvents.
Vera et al. [Citation126] compared the chiral separation of D- and L-fluorenylmethyl oxycarbonyl (FMOC) chloride amino acids with HPLC and SFC conditions. RPLC gave the best resolution between enantiomers in the range of amino acids, but with the addition of higher concentrations of formic acid (2%) in the mobile phase at SFC allowed similar resolution and a shorter run time. SFC exhibited the best resolution per minute overall separations. A comparison of chromatograms is shown in . Vera et al. [Citation126] also noted the advantage of SFC over HPLC due to the lower consumption of organic solvents, which reduces environmental as well as economic costs.
Figure 6. Comparison of chromatograms of FMOC-DL-Alanine-OH in RP-HPLC and SFC. Experimental conditions: column: Lux™ (250 mm × 4.6 mm, 3 μm); mobile phase: 60:40 composition of supercritical CO2 and MeOH with 0.1 or 2% formic acid; flow rate: 3 mL/min; detection mode: Agilent DAD UV-detector [Citation126].
![Figure 6. Comparison of chromatograms of FMOC-DL-Alanine-OH in RP-HPLC and SFC. Experimental conditions: column: Lux™ (250 mm × 4.6 mm, 3 μm); mobile phase: 60:40 composition of supercritical CO2 and MeOH with 0.1 or 2% formic acid; flow rate: 3 mL/min; detection mode: Agilent DAD UV-detector [Citation126].](/cms/asset/259f0f39-055f-4b5e-b2e7-aec454c042d0/lspr_a_2038625_f0006_oc.jpg)
Recently, Khater et al. [Citation127] separated 13 pairs of enantiomers belonging to the group of phenylthiohydantoin (PTH)-amino acids. To examine the thermodynamic behaviors of enantioseparations, temperatures from 5 to 40°C were applied on cellulose and amylose-based CSPs. The thermodynamic relationship between temperature (T) and chromatographic retention factor (k) was expressed using the Van’t Hoff equation and the separation factor (α) was defined from it. An equilibrium between enthalpy and entropy, where enantiomers coelute (α = 1), exists at the isoelution temperature (Tiso), while below this temperature the separation is enthalpically-driven. Here, enantioselectivity decreases with increasing temperature. In contrast, over the Tiso, the separation is entropically-driven, and with temperature the enantioselectivity increases. It was observed, that when the temperature rises above 20–30°C, both CSPs underwent structural changes. The trend of comparing retention and enantioselectivity values at different CSPs is important. The values of ln(α) can be related to the energy of interactions in the chromatographic system, so the diversity of these values is reflected in the diversity of the chiral cavities in the CSP. In the cellulose-based phase, where all cavities are similar, all chiral cavities were affected by temperature to the same extent. On the contrary, in the amylose-based phase, cavities may be more diverse, the chiral cavities were affected differently, which enables more diverse separation factors and exhibit a diverse level of sensitivity to temperature changes. This means that the stationary phase with amylose may allow more options and variety of chiral binding, and thus the analytes can access different cavities.
The separation of the 13 native amino acid enantiomers was studied with different polysaccharide-based chiral selectors by Lipka et al. .[Citation128] The separation with eight different columns with coated or covalently immobilized chiral selectors and with the addition of 20–40% ethanol or methanol as a co-solvent at 40°C and 150 bar was compared. Five out of 8 CSPs (Chiracel™ OD, Chiralpak™ AS-H, Chiralpak™ AD, Chiralpak™ IB, and Chiralpak™ IA) did not provide separation of any pair of enantiomers. Lux™ Amylose-2 resolved only enantiomers of Phe, while Lux™ Cellulose-2 separated enantiomers of Phe, Val, Ile, and Leu. The cellulose-based column (3,5-dichlorophenylcarbamate), Lux™-I-Cellulose-5, showed the best ability to separate the enantiomers, as it exhibited recognition ability for the enantiomers of 10 amino acids. The composition of the mobile phase and the nature of the chiral selector play a key role in the differentiation of the enantiomers. Raimbault et al. [Citation129] described the enantioresolution of 19 amino acids from two food supplements, tablets containing a mixture of proteinogenic amino acids and capsules containing taurine and theanine. To achieve elution of all amino acids, a wide elution gradient ranging from 90% CO2 to 100% co-solvent was employed at 25°C and 150 bar. For elution of the most basic amino acids, it was necessary to add water and ammonium formate.
Recently, Miller and Yue [Citation130] performed chiral separations of 18 underivatized amino acids in SFC with a chiral crown ether derived column CROWNPAK® CR-I (+). Detection was performed with MS, and the type of modifier and acidic additives were optimized, while the role of water in the mobile phase was determined. The use of IPA as a modifier resulted in the separation of nine amino acids, while the use of EtOH or MeOH alone resulted in the separation of 17 of the 18 amino acids, with only histidine, the most basic amino acid, not resolved. This is in line with previous observations, as amino acids with a basic side group are more difficult for SFC. Furthermore, all amino acids were separated by gradient elution, with all three modifiers: MeOH, EtOH, or IPA with the addition of 5% H2O and 0.5% TFA. Because EtOH provided exceptional resolution, the influence of water and the type and concentration of acid additives on the separations were checked only with EtOH as the modifier. The addition of 5% water achieved sharper peaks and better resolution for all amino acids except histidine, while the addition of 10% resulted in decreased resolution (compared to 5% H2O). Among HAc, FA, and TFA, TFA, the strongest acid, resulted in the separation of 17 of the 18 amino acids, and with its addition, the resolution was also improved. Stronger acids provide full protonation, thus enabling the interaction of crown ether and ammonium ion (-NH3+) and the consequent enantioselectivity and chiral recognition. Moreover, a higher concentration of TFA increased the resolution. It is important to emphasize the effect of the acid additive on histidine in particular. Near baseline resolution was achieved with 0.8% TFA, while 1.5 and 2.0% TFA enabled baseline separation of DL-histidine. For all amino acids, the D-enantiomer eluted first and was separated using gradient methods, and then transferred to optimized isocratic conditions. Detailed methodologies of possible and already performed chiral separations by individual amino acids, which include CSPs, separation conditions such as temperature and backpressure, and the composition of the (modified) mobile phase with possible other additives are listed in the Supplementary Material.
Achiral Amino Acid Separations
So far, only a few studies have been devoted to the achiral analysis of amino acids by SFC. Different such achiral separations using different stationary and mobile phases are depicted in . Most amino acids lack suitable chromophores, so MS is usually used to detect them .[Citation83,Citation144] Previously, UV and FID detection was used .[Citation132,Citation145] In 1989 Berger et al. [Citation132] separated 24 PTH-amino acids using a packed cyanopropyl Zorbax™ column. Separation was achieved in less than 20 min at 40°C and 240 bar. Veuthey et al. [Citation133] separated amino acids with SFC after a pre-derivatization step with FMOC and (+)-1-(9-fluorenyl)ethyl chloroformate (FLEC) reagents in 40 min at 42°C and 270 bar. Further, they separated three FLEC-amino acid diastereomers on bare silica with low polar modifier concentration on supercritical CO2. L-enantiomer eluted before D-enantiomer, and a further advantage of SFC over HPLC is that the excess of FLEC reagents is eluted with the acetone solvent and does not interfere with the amino acid peaks.
Table 2. Achiral separations of amino acids and peptides with SFC using different stationary and mobile phases
Wolrab et al. [Citation134] directly coupled SFC to MS for the analysis of amino acids and related compounds. They described a method for separating metabolites from the tryptophan metabolic pathway and other amino acids present. Supercritical CO2 and co-solvent (MeOH, EtOH, or 1:1 mixture of MeOH: ACN) were used for the mobile phase. For better ionization and separation, they included NH4FA or NH4OAc as additive. They were added to the modifiers to support the ionization of the analytes and improve peak shapes. They successfully separated all the components of the standard mix at 40°C and 150 bar. The efficiency of ionization using electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) and positive and negative ion modes was also compared. APCI was found to be more suitable for amino acids with polar side chains, while ESI provided better ionization efficiency of amino acids having hydrophobic residues.
Huang et al. [Citation135] studied the presence of amino acids in various teas (green tea, Oolong tea, black tea, and Pu-erh tea). Ten different columns were used for separation with SFC. Due to the polarity of the analytes, the analytes have no retention on the Zorbax™ C18 column. More polar stationary phases (Venusil™ PFP, Venusil™ NP, and Venusil™ Imidazolyl) resulted in increased analyte retention on the column and co-elution of some peaks. The other six hydrophilic stationary phases (Zorbax™ RX-SIL, Cosmosil™ 5X-SIL, Unitary™ Diol, Venusil™ HILIC, Unitary™ XAmide, and Unitary™ NH2) resulted in successful separation, shorter retention, and better resolution. All eleven amino acids could be baseline separated with a Unitary XAmide column. The addition of TFA and water in the supercritical CO2 mobile phase significantly improved the separation of amino acids, as shown in . The study was carried out at 25°C and 100 bar. In all tea samples, L-theanine was the most abundant amino acid. Green tea had the highest total content of amino acids, followed by Oolong tea, while black and Pu-erh teas contained a much lower total amino acid content.
Figure 7. Effects of ammonium acetate, trifluoroacetic acid, and water in the mobile phase on the separation of amino acids by SFC: 1. Leucine, 2. Valine, 3. Gamma Aminobutyric Acid, 4. Phenylalanine, 5. Glycine, 6. Theanine, 7. Threonine, 8. Serine, 9. Glutamine, 10. Asparagine, 11. Histidine. Column: Unitary™ XAmide (250 mm × 4.6 mm, 5 μm); mobile phase: supercritical CO2 with indicated additives: 3 mL/min; 25°C; 100 bar; detection mode: selected ion monitoring mode using the precursor ions. Adapted from [Citation135].
![Figure 7. Effects of ammonium acetate, trifluoroacetic acid, and water in the mobile phase on the separation of amino acids by SFC: 1. Leucine, 2. Valine, 3. Gamma Aminobutyric Acid, 4. Phenylalanine, 5. Glycine, 6. Theanine, 7. Threonine, 8. Serine, 9. Glutamine, 10. Asparagine, 11. Histidine. Column: Unitary™ XAmide (250 mm × 4.6 mm, 5 μm); mobile phase: supercritical CO2 with indicated additives: 3 mL/min; 25°C; 100 bar; detection mode: selected ion monitoring mode using the precursor ions. Adapted from [Citation135].](/cms/asset/4b94e51d-6674-41bd-90bf-75baedc22523/lspr_a_2038625_f0007_oc.jpg)
Konya and colleagues [Citation83] developed a new method using SFC and tandem MS for the simultaneous analysis of polar metabolites. Using 11 columns, the separation of 20 proteogenic amino acids was optimized. The retention time, peak shape, and chromatographic separation of two pairs of amino acids with the same multiple reaction monitoring (MRM) transition (e.g., Gln/Lys and Ile/Leu) were important factors in selecting the optimal column and mobile phase. They found that replacing FA with TFA shortens the retention time of almost all amino acids and dramatically improves peak shapes. However, only five (Viridis® BEH, Viridis® HSS C18SB, Torus™ Diol, DCpack® P4VP, and CROWNPAK® CR-I (+)) out of 11 columns were able to separate the Gln/Lys pair, with the CROWNPAK® CR-I (+) column being the only one separating the Ile/Leu pair. Furthermore, it was found that amino acid separations with the same MRM transitions are improved by increasing the ratio of CO2 in the mobile phase to 70%. Therefore, a CROWNPAK® CR-I (+) column with a mobile phase composition of 70% CO2 and 30% modifier under isocratic elution was selected as the most optimal for the separation of polar metabolites. It would also be important to point out that there is a trend of using smaller particles and even superficially porous particles in chiral separations using SFC, and in the case of chiral amino acid separations we observe that particles of size 3 and 5 μm are still used.
Achiral Separations of Peptides
Blackwell and Stringham [Citation136] were already studying the separation of a group of small polypeptides (leucine enkephalin, des-Tyr-leucine enkephalin, methionine enkephalin, oxytocin, and bradykinin) in 1999. Bradykinin, which contained the largest number of amino acid residues, was the last of these to be eluted. A study of the effect of temperature (10–60°C) on the gradient elution of polypeptides was also carried out. As the temperature increased the retention also rise, but a greater selectivity between the peaks of the polypeptides and impurities was detected at the expense of lower efficiency. Achiral separations of peptides with SFC using different stationary and mobile phases are depicted in . Zheng et al. [Citation53] showed that 40-mer peptides, including a variety of basic and acidic residues, can be separated with SFC. This is of great importance to rapidly perform determinations and separations of bioactive peptides in complex biological matrixes, which is useful for pharmaceutical and other branches of bioindustries. TFA was used as additive in the supercritical CO2/MeOH mobile phase to suppress ionization of the carboxylic acid groups and to protonate amino groups of peptides. A higher concentration of additive allowed less retention and fronting peak shapes, indicating electrostatic reflectance between peptides and some part of the functional groups in the stationary phase. The ethylpyridine bonded silica was the only stationary phase to allow successful elution of polypeptides, which is attributed to the deactivation of silanol groups due to the hydrogen bond between silanols and pyridine functional groups on the stationary phase. Also, the pyridine aromatic ring causes a steric barrier and consequently limited access to the silica surface.
Tognarelli et al. [Citation137] explored the SFC separation of five peptides ranging in molecular weight from 238.2 to 1046.2 Da. The separation lasted less than 12 min, compared to the HPLC method which lasted nearly 1 hour. Patel et al. [Citation138] demonstrated that a packed-column SFC is suitable for separating water-soluble peptides of the same mass, composition, and charge that differ only in the sequence of amino acids. Furthermore, it was also shown [Citation55] that polar, high molecular mass polypeptides could be separated using ion-pairing SFC. Liu et al. [Citation139] studied the separation of peptides among other hydrophilic compounds by SFC using water-containing modifiers at 35 and 40°C and 100 bar. Shao et al. [Citation140] used SFC to develop a method for the separation of five cyclosporin analogs. These are cyclic peptides and contain eleven amino acids but differ at the side chain of their amino acid residues. With an increase in temperature (0–80°C) the selectivity remained almost the same, but the efficiency of the column was increased as the widths of the peaks were significantly reduced. Baseline resolution for all five peaks was achieved at 50°C and resolution continued to improve up to 80°C. With increasing temperature, the retention times were shifted for approx. 3 min longer at 80°C compared to 0°C. Increasing back pressure (100–300 bar), the retention times decreased, and the selectivity was significantly improved. In summary, the best conditions for separating cyclosporin analogs with SFC are at 80°C and 300 bar back pressure using ethanol modified supercritical CO2 mobile phase as a mobile phase on a bare silica column due to the directly exposed hydroxyl group on the silica surface.
Enmark et al. [Citation141] compared the separation of the peptide gramicidin, using either isocratic or gradient elution, and found that gradient elution is about three times more robust than isocratic elution. Desfontaine et al. [Citation142] studied the suitability of SFC-MS for the analysis of lipophilic and highly hydrophilic substances. To cover the widest possible range of analytes, a gradient from 2 to 100% co-solvent in CO2 was systematically used. The best conditions for the separation of a wide range of chemical compounds have proved to be bare silica or silica bonded with a zwitterionic ligand for the stationary phase at 40°C and 120 bar. A considerable amount of salt in the mobile phase makes it possible to reduce the retention, to increase the solubility, and to obtain the corresponding peak shapes for ionizable species. Schiavone et al. [Citation143] reported the potential of SFC for the purification of peptides and proteins. An organic-aqueous modifier-based preparative SFC method was developed for the purification of peptides. Purification of bradykinin and insulin were demonstrated using preparative SFC for peptide purification. Peptides were dissolved in water or TFA solution. The mobile phase modifier was 3:1 MeOH:ACN with 5%/0.2% H2O/TFA additive. Purification was carried out at 25°C and 100 bar. However, the analyzed proteins (ubiquitin, cytochrome C, and apomyoglobin) changed during the SFC process and were unable to return to their original higher-order structure.
Detailed methodologies of possible and already performed achiral separations by individual amino acids and peptides are listed in the Supplementary Material. Different stationary phases, separation conditions such as temperature and backpressure, and the composition of the (modified) mobile phase with possible other additives are also included.
CONCLUSIONS
While SFC is not a new concept, it has experienced incredible commercial success in the last few years, driven by the introduction of new instruments with improved performance and robustness, and hyphenation to MS. SFC also has major advantages in the separation of amino acids and peptides () over the well-known HPLC and GC methods, which are still considered to be standard approaches for the analysis of complex samples. Analysis of a wider molecular weight range of compounds is possible with SFC compared to GC, along with good detection of chromophore-free compounds, separation of thermally labile compounds at low temperatures, and low viscosity and high diffusivity, allowing the SFC method to be three to five times faster than HPLC, among other advantages .[Citation148,Citation149] Mixing polar organic solvents with non-polar CO2 allows the elution of polar compounds, while more polar additives such as TFA, TEA, and water further extend the polarity range of solutes suitable for SFC .[Citation150] Direct coupling of SFC with MS makes the technique even easier to operate. Although the number of published studies on separating amino acids and peptides in recent years is relatively low, the interest in the achiral and chiral separation of these compounds continues to increase. The applicability of SFC in chiral separations of amino acids has been demonstrated to be successful in numerous reports. Most applications have been developed on polysaccharide-based CSPs while applying a chiral selector was also described as an effective method for separating enantiomers of amino acids. Regarding the achiral separations of amino acids and peptides, SFC provides an extremely wide choice of stationary phases and a wide range of operating parameters. Compared to HPLC, these properties show the strength of the SFC technique, which allows a wider range of selectivity.
Table 3. Advantages and disadvantages of SFC over other separation techniques in the separation of amino acids and peptides .[Citation80,Citation81,Citation146,Citation147].
Finally, the increasing number of published articles focused on the development of the SFC method for the analysis of highly polar and hydrophilic compounds confirms the important role of SFC in the family of separation techniques. Due to its excellent properties and obvious benefits, it will probably become a method of choice for many other applications in the near future.
Author Contributions
M.L. and M.P. conceived and designed the study. K.K. studied the literature and wrote the manuscript. M.L., M.P., and Z.K. reviewed the manuscript. K.K. edited the manuscript. Z.K. and M.L. were responsible for the financial part of the project. All authors accepted the final version of the review.
Supplemental Material
Download MS Word (17.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Wang, X.; Yu, H.; Xing, R.; Characterization, L. P. Preparation, and Purification of Marine Bioactive Peptides. Biomed Res. Int. 2017, 2017, 9746720. DOI: 10.1155/2017/9746720.
- Alia, K. B.; Nadeem, H.; Rasul, I.; Azeem, F.; Hussain, S.; Siddique, M. H.; Muzammil, S.; Riaz, M.; Nasir, S. Separation and Purification of Amino Acids. Applications of Ion Exchange Materials in Biomedical Industries; Inamuddin. Ed. Springer International Publishing: Cham, 2019. 1–11. 10.1007/978-3-030-06082-4_1.
- Dołowy, M.; Pyka, A. Application of TLC, HPLC and GC Methods to the Study of Amino Acid and Peptide Enantiomers: A Review. Biomed. Chromatogr. 2014, 28(1), 84–101. DOI: 10.1002/bmc.3016.
- Yang, L.; Nie, H.; Zhao, F.; Song, S.; Meng, Y.; Bai, Y.; Liu, H.; Novel Online, A. Two-Dimensional Supercritical Fluid Chromatography/Reversed Phase Liquid Chromatography–Mass Spectrometry Method for Lipid Profiling. Anal. Bioanal. Chem. 2020, 412(10), 2225–2235. DOI: 10.1007/s00216-019-02242-x.
- Sethi, N.; Anand, A.; Jain, G.; Srinivas, K.; Chandrul, K. Supercritical Fluid Chromatography-A Hybrid of GC and LC. Chronicles Young Sci. 2010, 1(2), 12.
- Larive, C. K.; Lunte, S. M.; Zhong, M.; Perkins, M. D.; Wilson, G. S.; Gokulrangan, G.; Williams, T.; Afroz, F.; Schoneich, C.; Derrick, T. S., et al. Separation and Analysis of Peptides and Proteins. Anal. Chem. 1999, 71(12), 389R–423R. DOI: 10.1021/a1990013o.
- da Fonseca Ferreira, L. A. R.;. Separation and Purification of Amino Acids. In Dissertation for the Degree of Doctor in Biological and Chemical Engineering. Faculty of Engineering, University of Porto: Portugal, 2008.
- Neda, O.; Vlazan, P.; Pop, R. O.; Sfarloaga, P.; Grozescu, I.; Segneanu, A.-E. Peptide and Amino Acids Separation and Identification from Natural Products. Anal. Chem. 2012, 135–146. DOI: 10.5772/51619.
- Molineau, J.; Hideux, M.; West, C. Chromatographic Analysis of Biomolecules with Pressurized Carbon Dioxide Mobile Phases – A Review. J. Pharm. Biomed. Anal. 2021, 193, 113736. DOI: 10.1016/j.jpba.2020.113736.
- Cesari, M.; Rossi, G. P.; Sticchi, D.; Pessina, A. C. Is Homocysteine Important as Risk Factor for Coronary Heart Disease? Nutr. Metab. Cardiovasc. Dis. 2005, 15(2), 140–147. DOI: 10.1016/j.numecd.2004.04.002.
- Pommié, C.; Levadoux, S.; Sabatier, R.; Lefranc, G.; Lefranc, M.-P. IMGT Standardized Criteria for Statistical Analysis of Immunoglobulin V-REGION Amino Acid Properties. J. Mol. Recognit. 2004, 17(1), 17–32. DOI: 10.1002/jmr.647.
- Frank, H.; Nicholson, G. J.; Bayer, E. Rapid Gas Chromatographic Separation of Amino Acid Enantiomers with a Novel Chiral Stationary Phase. J. Chromatogr. Sci. 1977, 15(5), 174–176. DOI: 10.1093/chromsci/15.5.174.
- Fox, S.; Strasdeit, H.; Haasmann, S.; Brückner, H. Gas Chromatographic Separation of Stereoisomers of Non-Protein Amino Acids on Modified γ-Cyclodextrin Stationary Phase. J. Chromatogr. A. 2015, 1411, 101–109. DOI: 10.1016/j.chroma.2015.07.082.
- Bertrand, M.; Chabin, A.; Brack, A.; Westall, F. Separation of Amino Acid Enantiomers VIA Chiral Derivatization and Non-Chiral Gas Chromatography. J. Chromatogr. A. 2008, 1180(1–2), 131–137. DOI: 10.1016/j.chroma.2007.12.004.
- Pätzold, R.; Schieber, A.; Brückner, H. Gas Chromatographic Quantification of Free D-Amino Acids in Higher Vertebrates. Biomed. Chromatogr. 2005, 19(6), 466–473. DOI: 10.1002/bmc.515.
- Lee, K.-A.; Yeo, S.; Kim, K. H.; Lee, W.; Kang, J. S. Enantioseparation of N-Fluorenylmethoxycarbonyl Alpha-Amino Acids on Polysaccharide-Derived Chiral Stationary Phases by Reverse Mode Liquid Chromatography. J. Pharm. Biomed. Anal. 2008, 46(5), 914–919. DOI: 10.1016/j.jpba.2008.01.012.
- Berkecz, R.; Ilisz, I.; Misicka, A.; Tymecka, D.; Fülöp, F.; Choi, H. J.; Hyun, M. H.; Péter, A. HPLC Enantioseparation of Beta2-Homoamino Acids Using Crown Ether-Based Chiral Stationary Phase. J. Sep. Sci. 2009, 32(7), 981–987. DOI: 10.1002/jssc.200800561.
- Lee, T.; Lee, W.; Hyun, M. H.; Park, J. H. Enantioseparation of Alpha-Amino Acids on an 18-Crown-6-Tetracarboxylic Acid-Bonded Silica by Capillary Electrochromatography. J. Chromatogr. A. 2010, 1217(8), 1425–1428. DOI: 10.1016/j.chroma.2009.12.064.
- Majhi, K. C.; Karfa, P.; Madhuri, R. Chromatographic Separation of Amino Acids. Applications of Ion Exchange Materials in Biomedical Industries; Inamuddin. Ed. Springer International Publishing: Cham, 2019. 71–118. 10.1007/978-3-030-06082-4_4.
- Singh, C.; Sharma, C. S.; Kamble, P. R. Amino Acid Analysis Using Ion-Exchange Chromatography: A Review. Int. J. Pharmacognosy. 2014, 1(12), 756–762. DOI: 10.13040/IJPSR.0975-8232.IJP.1(12).756-62.
- Sewald, N.; Jakubke, H.-D. Peptides: Chemistry and Biology; John Wiley & Sons: Bielefeld: Germany, 2015. DOI: 10.1002/9783527626038.
- Wimalasinghe, R. M.; Breitbach, Z. S.; Lee, J. T.; Armstrong, D. W. Separation of Peptides on Superficially Porous Particle Based Macrocyclic Glycopeptide Liquid Chromatography Stationary Phases: Consideration of Fast Separations. Anal. Bioanal. Chem. 2017, 409(9), 2437–2447. DOI: 10.1007/s00216-017-0190-4.
- Zhang, B.; Soukup, R.; Armstrong, D. W. Selective Separations of Peptides with Sequence Deletions, Single Amino Acid Polymorphisms, And/or Epimeric Centers Using Macrocyclic Glycopeptide Liquid Chromatography Stationary Phases. J. Chromatogr. A. 2004, 1053(1–2), 89–99. DOI: 10.1016/J.CHROMA.2004.06.117.
- Underberg, W. J.; Hoitink, M. A.; Reubsaet, J. L.; Waterval, J. C. Separation and Detection Techniques for Peptides and Proteins in Stability Research and Bioanalysis. J. Chromatogr. B Biomed. Sci. Appl. 2000, 742(2), 401–409. DOI: 10.1016/S0378-4347(00)00198-5.
- Mant, C. T.; Hodges, R. S., and Hodges, R. S. High-Performance Liquid Chromatography of Peptides and Proteins : Separation, Analysis, and Conformation; Boca Raton, Florida, USA: CRC Press, 2017. DOi: 10.1201/9780203751947.
- Gilar, M.; Olivova, P.; Daly, A. E.; Gebler, J. C. Two-Dimensional Separation of Peptides Using RP-RP-HPLC System with Different PH in First and Second Separation Dimensions. J Separation Sci. 2005, 28(14), 1694–1703. DOI: 10.1002/jssc.200500116.
- Klesper, E.; Corwin, A. H.; Turner, D. A. High Pressure Gas Chromatography above Critical Temperatures. J. Organic Chem. 1962, No.27, 700–701.
- Sie, S. T.; Rijnders, G. W. A. High-Pressure Gas Chromatography and Chromatography with Supercritical Fluids. III. Fluid-Liquid Chromatography. Sep. Sci. 1967, 2(6), 729–753. DOI: 10.1080/01496396708049735.
- Sie, S. T.; Rijnders, G. W. A. Chromatography with Supercritical Fluids. Anal. Chim. Acta. 1967, 38, 31–44. DOI: 10.1016/S0003-2670(01)80559-6.
- Bartmann, D.; Schneider, G. Experimental Results and Physico-Chemical Aspects of Supercritical Fluid Chromatography with Carbon Dioxide as the Mobile Phase. J. Chromatogr. A. 1973, 83, 135–145. DOI: 10.1016/S0021-9673(00)97034-1.
- Kiran, E.; Debenedetti, P. G.; Peters, C. J. Supercritical Fluids: Fundamentals and Applications; Series E. Applied Sciences; Springer Science & Business Media. Kemer:Antalya, Turkey.2012, Vol. 366.10.1007/978-94-011-3929-8.
- Tarafder, A.; Guiochon, G. Use of Isopycnic Plots in Designing Operations of Supercritical Fluid Chromatography: II. The Isopycnic Plots and the Selection of the Operating Pressure–Temperature Zone in Supercritical Fluid Chromatography. J. Chromatogr. A. 2011, 1218(28), 4576–4585. DOI: 10.1016/j.chroma.2011.05.041.
- Lesellier, E.; West, C. The Many Faces of Packed Column Supercritical Fluid Chromatography – A Critical Review. J. Chromatogr. A. 2015, 1382, 2–46. DOI: 10.1016/j.chroma.2014.12.083.
- Knez, Ž.; Markočič, E.; Leitgeb, M.; Primožič, M.; Knez Hrnčič, M.; Škerget, M. Industrial Applications of Supercritical Fluids: A Review. Energy. 2014, 77, 235–243. DOI: 10.1016/j.energy.2014.07.044.
- Subramaniam, B.; Rajewski, R. A.; Snavely, K. Pharmaceutical Processing with Supercritical Carbon Dioxide. J. Pharm. Sci. 1997, 86(8), 885–890. DOI: 10.1021/js9700661.
- Knez, Ž.; Cör, D.; Knez Hrnčič, M. Solubility of Solids in Sub- and Supercritical Fluids: A Review 2010–2017. J. Chem. Eng. Data. 2018, 63(4), 860–884. DOI: 10.1021/acs.jced.7b00778.
- Peach, J.; Eastoe, J. Supercritical Carbon Dioxide: A Solvent like No Other. Beilstein J. Org. Chem. 2014, 10, 1878–1895. DOI: 10.3762/bjoc.10.196.
- Gupta, R. B., and Shim, -J.-J. Solubility in Supercritical Carbon Dioxide; Boca Raton, Florida, USA: CRC Press, 2006. DOi: 10.1201/9781420005998.
- Lemmon, E. W.; Thermophysical Properties of Fluid Systems. NIST Chemistry Webbook, 1998.
- Solvent Physical Properties https://people.chem.umass.edu/xray/solvent.html ( accessed Jun 24, 2021).
- Klesper, E.; Hartmann, W. Parameters in Supercritical Fluid Chromatography of Styrene Oligomers. J. Polym. Sci. Polym. Lett. Ed. 1977, 15(12), 707–712. DOI: 10.1002/pol.1977.130151201.
- Taylor, L. T.;. Packed Column Supercritical Fluid Chromatography of Hydrophilic Analytes via Water-Rich Modifiers. J. Chromatogr. A. 2012, 1250, 196–204. DOI: 10.1016/j.chroma.2012.02.037.
- Ng, M. H.; Din, A. K. Applications of Packed and Capillary Supercritical Fluid Chromatography in the Separation of Tocochromanols. J Separation Sci. 2020, 43(1), 285–291. DOI: 10.1002/jssc.201900342.
- Dispas, A.; Lebrun, P.; Sassiat, P.; Ziemons, E.; Thiébaut, D.; Vial, J.; Hubert, P. Innovative Green Supercritical Fluid Chromatography Development for the Determination of Polar Compounds. J. Chromatogr. A. 2012, 1256, 253–260. DOI: 10.1016/j.chroma.2012.07.043.
- De Klerck, K.; Mangelings, D.; Clicq, D.; De Boever, F.; Vander Heyden, Y. Combined Use of Isopropylamine and Trifluoroacetic Acid in Methanol-Containing Mobile Phases for Chiral Supercritical Fluid Chromatography. J. Chromatogr. A. 2012, 1234, 72–79. DOI: 10.1016/j.chroma.2011.11.023.
- De Klerck, K.; Mangelings, D.; Vander Heyden, Y. Supercritical Fluid Chromatography for the Enantioseparation of Pharmaceuticals. J. Pharm. Biomed. Anal. 2012, 69, 77–92. DOI: 10.1016/j.jpba.2012.01.021.
- Gross, M. S.; Olivos, H. J.; Butryn, D. M.; Olson, J. R.; Aga, D. S. Analysis of Hydroxylated Polybrominated Diphenyl Ethers (Oh-bdes) by Supercritical Fluid Chromatography/Mass Spectrometry. Talanta. 2016, 161, 122–129. DOI: 10.1016/j.talanta.2016.08.013.
- Yang, Y.; Liang, Y.; Yang, J.; Ye, F.; Zhou, T.; Gongke, L. Advances of Supercritical Fluid Chromatography in Lipid Profiling. J. Pharm. Anal. 2019, 9(1), 1–8. DOI: 10.1016/j.jpha.2018.11.003.
- Tyśkiewicz, K.; Dębczak, A.; Gieysztor, R.; Szymczak, T.; Rój, E. Determination of Fat- and Water-Soluble Vitamins by Supercritical Fluid Chromatography: A Review. J. Sep. Sci. 2018, 41(1), 336–350. DOI: 10.1002/jssc.201700598.
- Breitbach, A. S.; Lim, Y.; Xu, Q.-L.; Kürti, L.; Armstrong, D. W.; Breitbach, Z. S. Enantiomeric Separations of α-Aryl Ketones with Cyclofructan Chiral Stationary Phases via High Performance Liquid Chromatography and Supercritical Fluid Chromatography. J. Chromatogr. A. 2016, 1427, 45–54. DOI: 10.1016/j.chroma.2015.11.069.
- Yang, J.; Zhu, L.; Zhao, Y.; Xu, Y.; Sun, Q.; Liu, S.; Liu, C.; Ma, B. Separation of Furostanol Saponins by Supercritical Fluid Chromatography. J. Pharm. Biomed. Anal. 2017, 145, 71–78. DOI: 10.1016/j.jpba.2017.05.023.
- Teubel, J.; Wüst, B.; Schipke, C. G.; Peters, O.; Parr, M. K. Methods in Endogenous Steroid Profiling – A Comparison of Gas Chromatography Mass Spectrometry (GC–MS) with Supercritical Fluid Chromatography Tandem Mass Spectrometry (SFC-MS/MS). J. Chromatogr. A. 2018, 1554, 101–116. DOI: 10.1016/j.chroma.2018.04.035.
- Zheng, J.; Pinkston, J. D.; Zoutendam, P. H.; Taylor, L. T. Feasibility of Supercritical Fluid Chromatography/Mass Spectrometry of Polypeptides with up to 40-Mers. Anal. Chem. 2006, 78(5), 1535–1545. DOI: 10.1021/ac052025s.
- Berger, T.;. Supercritical Fluid Chromatography: Primer; Agilent Technologies: USA, 2015.
- Patel, M. A.; Riley, F.; Ashraf-Khorassani, M.; Taylor, L. T. Supercritical Fluid Chromatographic Resolution of Water Soluble Isomeric Carboxyl/Amine Terminated Peptides Facilitated via Mobile Phase Water and Ion Pair Formation. J. Chromatogr. A. 2012, 1233, 85–90. DOI: 10.1016/j.chroma.2012.02.024.
- Losacco, G. L.; Veuthey, J.-L.; Guillarme, D. Metamorphosis of Supercritical Fluid Chromatography: A Viable Tool for the Analysis of Polar Compounds? TrAC Trends Anal Chem. 2021, 141, 116304. DOI: 10.1016/j.trac.2021.116304.
- Desfontaine, V.; Nováková, L.; Ponzetto, F.; Nicoli, R.; Saugy, M.; Veuthey, J.-L.; Guillarme, D. Liquid Chromatography and Supercritical Fluid Chromatography as Alternative Techniques to Gas Chromatography for the Rapid Screening of Anabolic Agents in Urine. J. Chromatogr. A. 2016, 1451, 145–155. DOI: 10.1016/j.chroma.2016.05.004.
- Škerget, M.; Kotnik, P.; Knez, Ž. Supercritical Carbon Dioxide Sorption Processes on Various Sorbent Materials. In Proceedings of the 10th Europen meeting on supercritical fluids; International Society for the Advancement of Supercritical Fluids: Colmar, France, 2005.
- Rajendran, A.;. Design of Preparative-Supercritical Fluid Chromatography. J. Chromatogr. A. 2012, 1250, 227–249. DOI: 10.1016/j.chroma.2012.05.037.
- Miller, L. M.; Pinkston, J. D., and Taylor, L. T. Modern Supercritical Fluid Chromatography: Carbon Dioxide Containing Mobile Phases. In Chemical Analysis: A Series of Monographs on Analytical Chemistry and Its Applications; pp 1–6 ; Hoboken, NJ, USA: John Wiley & Sons, 2019.
- Dispas, A.; Jambo, H.; André, S.; Tyteca, E.; Hubert, P. Supercritical Fluid Chromatography: A Promising Alternative to Current Bioanalytical Techniques. Bioanalysis. 2017, 10(2), 107–124. DOI: 10.4155/bio-2017-0211.
- Korany, M. A.; Mahgoub, H.; Haggag, R. S.; Ragab, M. A. A.; Elmallah, O. A. Green Chemistry: Analytical and Chromatography. J Liquid Chromatography Related Technol. 2017, 40(16), 839–852. DOI: 10.1080/10826076.2017.1373672.
- Grand-Guillaume Perrenoud, A.; Veuthey, J.-L.; Guillarme, D. Comparison of Ultra-High Performance Supercritical Fluid Chromatography and Ultra-High Performance Liquid Chromatography for the Analysis of Pharmaceutical Compounds. J. Chromatogr. A. 2012, 1266, 158–167. DOI: 10.1016/j.chroma.2012.10.005.
- Hubert, P.; Marini, R. D.; Dispas, A.; Andri, B. Overview of the Analytical Lifecycle of Supercritical Fluid Chromatography Methods. Am. J. Anal. Chem. 2016, 7(1), 720–726. DOI: 10.4236/ajac.2016.71008.
- Hicks, M. B.; Regalado, E. L.; Tan, F.; Gong, X.; Welch, C. J. Supercritical Fluid Chromatography for GMP Analysis in Support of Pharmaceutical Development and Manufacturing Activities. J Pharm Biomed Anal. 2016, 117, 316–324. DOI: 10.1016/j.jpba.2015.09.014.
- Akbal, L.; Hopfgartner, G. Hyphenation of Packed Column Supercritical Fluid Chromatography with Mass Spectrometry: Where are We and What are the Remaining Challenges? Anal. Bioanal. Chem. 2020, 412(25), 6667–6677. DOI: 10.1007/s00216-020-02715-4.
- Desfontaine, V.; Nováková, L.; Guillarme, D. SFC-MS versus RPLC-MS for Drug Analysis in Biological Samples. Bioanalysis. 2015, 7(10), 1193–1195. DOI: 10.4155/bio.15.41.
- Biba, M.; Regalado, E. L.; Wu, N.; Welch, C. J. Effect of Particle Size on the Speed and Resolution of Chiral Separations Using Supercritical Fluid Chromatography. J. Chromatogr. A. 2014, 1363, 250–256. DOI: 10.1016/j.chroma.2014.07.010.
- Regalado, E. L.; Welch, C. J. Pushing the Speed Limit in Enantioselective Supercritical Fluid Chromatography. J Separation Sci. 2015, 38(16), 2826–2832. DOI: 10.1002/jssc.201500270.
- Lesellier, E.; Fougere, L.; Poe, D. P. Kinetic Behaviour in Supercritical Fluid Chromatography with Modified Mobile Phase for 5μm Particle Size and Varied Flow Rates. J. Chromatogr. A. 2011, 1218(15), 2058–2064. DOI: 10.1016/j.chroma.2010.12.057.
- Berger, T. A.;. Separation of Polar Solutes by Packed Column Supercritical Fluid Chromatography. J. Chromatogr. A. 1997, 785(1), 3–33. DOI: 10.1016/S0021-9673(97)00849-2.
- DaSilva, J. O.; Coes, B.; Frey, L.; Mergelsberg, I.; McClain, R.; Nogle, L.; Welch, C. J. Evaluation of Non-Conventional Polar Modifiers on Immobilized Chiral Stationary Phases for Improved Resolution of Enantiomers by Supercritical Fluid Chromatography. J. Chromatogr. A. 2014, 1328, 98–103. DOI: 10.1016/j.chroma.2013.12.073.
- Tarafder, A.; Hill, J. F. Scaling Rule in SFC. II. A Practical Rule for Isocratic Systems. J. Chromatogr. A. 2017, 1482, 65–75. DOI: 10.1016/j.chroma.2016.12.044.
- Hamman, C.; Schmidt, D. E.; Wong, M.; Hayes, M. The Use of Ammonium Hydroxide as an Additive in Supercritical Fluid Chromatography for Achiral and Chiral Separations and Purifications of Small, Basic Medicinal Molecules. J. Chromatogr. A. 2011, 1218(43), 7886–7894. DOI: 10.1016/j.chroma.2011.08.064.
- DaSilva, J. O.; Bennett, R.; Mann, B. F. Doing More with Less: Evaluation of the Use of High Linear Velocities in Preparative Supercritical Fluid Chromatography. J. Chromatogr. A. 2019, 1595, 199–206. https://doi.org/10.1016/j.chroma.2019.02.047.
- Oman, M.; Kotnik, P.; Škerget, M.; Knez, Ž. Supercritical Fluid Chromatography and Scale up Study. Acta Chim. Slov. 2014, 61(4), 746–758.
- Pirrone, G. F.; Mathew, R. M.; Makarov, A. A.; Bernardoni, F.; Klapars, A.; Hartman, R.; Limanto, J.; Regalado, E. L. Supercritical Fluid Chromatography-Photodiode Array Detection-Electrospray Ionization Mass Spectrometry as a Framework for Impurity Fate Mapping in the Development and Manufacture of Drug Substances. J. Chromatogr. B. 2018, 1080, 42–49. DOI: 10.1016/j.jchromb.2018.02.006.
- Mattrey, F. T.; Makarov, A. A.; Regalado, E. L.; Bernardoni, F.; Figus, M.; Hicks, M. B.; Zheng, J.; Wang, L.; Schafer, W.; Antonucci, V., et al. Current Challenges and Future Prospects in Chromatographic Method Development for Pharmaceutical Research. TrAC Trends Anal Chem 2017, 95, 36–46. DOI: 10.1016/j.trac.2017.07.021.
- Harps, L. C.; Joseph, J. F.; Parr, M. K. SFC for Chiral Separations in Bioanalysis. J Pharm Biomed Anal. 2019, 162, 47–59. DOI: 10.1016/j.jpba.2018.08.061.
- Lesellier, E.;. Usual, Unusual and Unbelievable Retention Behavior in Achiral Supercritical Fluid Chromatography: Review and Discussion. J. Chromatogr. A. 2020, 1614, 460582. DOI: 10.1016/j.chroma.2019.460582.
- Hofstetter, R. K.; Hasan, M.; Eckert, C.; Link, A. Supercritical Fluid Chromatography. ChemTexts. 2019, 5(3), 13. DOI: 10.1007/s40828-019-0087-2.
- Grand-Guillaume Perrenoud, A.; Hamman, C.; Goel, M.; Veuthey, J.-L.; Guillarme, D.; Fekete, S. Maximizing Kinetic Performance in Supercritical Fluid Chromatography Using State-of-the-Art Instruments. J. Chromatogr. A. 2013, 1314, 288–297. DOI: 10.1016/j.chroma.2013.09.039.
- Konya, Y.; Izumi, Y.; Bamba, T. Development of a Novel Method for Polar Metabolite Profiling by Supercritical Fluid Chromatography/Tandem Mass Spectrometry. J. Chromatogr. A. 2020, 1632, 461587. DOI: 10.1016/j.chroma.2020.461587.
- Chollet, C.; Boutet-Mercey, S.; Laboureur, L.; Rincon, C.; Méjean, M.; Jouhet, J.; Fenaille, F.; Colsch, B. A.; Touboul, D. Supercritical Fluid Chromatography Coupled to Mass Spectrometry for Lipidomics. J. Mass Spectrometry. 2019, 54(10), 791–801. DOI: 10.1002/jms.4445.
- Schulze, S.; Paschke, H.; Meier, T.; Muschket, M.; Reemtsma, T.; Berger, U.; Rapid, A. Method for Quantification of Persistent and Mobile Organic Substances in Water Using Supercritical Fluid Chromatography Coupled to High-Resolution Mass Spectrometry. Anal. Bioanal. Chem. 2020, 412(20), 4941–4952. DOI: 10.1007/s00216-020-02722-5.
- Desfontaine, V.; Guillarme, D.; Francotte, E.; Nováková, L. Supercritical Fluid Chromatography in Pharmaceutical Analysis. J Pharm Biomed Anal. 2015, 113, 56–71. DOI: 10.1016/j.jpba.2015.03.007.
- Nováková, L.; Desfontaine, V.; Ponzetto, F.; Nicoli, R.; Saugy, M.; Veuthey, J.-L.; Guillarme, D. Fast and Sensitive Supercritical Fluid Chromatography – Tandem Mass Spectrometry Multi-Class Screening Method for the Determination of Doping Agents in Urine. Anal. Chim. Acta. 2016, 915, 102–110. DOI: 10.1016/j.aca.2016.02.010.
- Periat, A.; Perrenoud, A.-G.-G.; Guillarme, D. Evaluation of Various Chromatographic Approaches for the Retention of Hydrophilic Compounds and MS Compatibility. J Separation Sci. 2013, 36(19), 3141–3151. DOI: 10.1002/jssc.201300567.
- West, C.;. Enantioselective Separations with Supercritical Fluids - Review. Current Anal Chem. 2014, 10(1), 99–120. DOI: 10.2174/1573411011410010009.
- Tarafder, A.;. Metamorphosis of Supercritical Fluid Chromatography to SFC: An Overview. TrAC Trends Anal Chem. 2016, 81, 3–10. DOI: 10.1016/j.trac.2016.01.002.
- West, C.; Melin, J.; Ansouri, H.; Mengue Metogo, M. Unravelling the Effects of Mobile Phase Additives in Supercritical Fluid Chromatography. Part I: Polarity and Acidity of the Mobile Phase. J. Chromatogr. A. 2017, 1492, 136–143. DOI: 10.1016/j.chroma.2017.02.066.
- West, C.; Lesellier, E. Effects of Mobile Phase Composition on Retention and Selectivity in Achiral Supercritical Fluid Chromatography. J. Chromatogr. A. 2013, 1302, 152–162. DOI: 10.1016/j.chroma.2013.06.003.
- Pilařová, V.; Plachká, K.; Khalikova, M. A.; Svec, F.; Nováková, L. Recent Developments in Supercritical Fluid Chromatography – Mass Spectrometry: Is It a Viable Option for Analysis of Complex Samples? TrAC Trends Anal Chem. 2019, 112, 212–225. DOI: 10.1016/j.trac.2018.12.023.
- Nováková, L.; Perrenoud, A.-G.-G.; Francois, I.; West, C.; Lesellier, E.; Guillarme, D. Modern Analytical Supercritical Fluid Chromatography Using Columns Packed with Sub-2μm Particles: A Tutorial. Anal. Chim. Acta. 2014, 824, 18–35. DOI: 10.1016/j.aca.2014.03.034.
- Smith, R. M.;. Supercritical Fluids in Separation Science – The Dreams, the Reality and the Future. J. Chromatogr. A. 1999, 856(1), 83–115. DOI: 10.1016/S0021-9673(99)00617-2.
- Blackwell, J. A.; Stringham, R. W.; Weckwerth, J. D. Effect of Mobile Phase Additives in Packed-Column Subcritical and Supercritical Fluid Chromatography. Anal. Chem. 1997, 69(3), 409–415. DOI: 10.1021/ac9608883.
- Losacco, G. L.; Veuthey, J.-L.; Guillarme, D. Supercritical Fluid Chromatography – Mass Spectrometry: Recent Evolution and Current Trends. TrAC Trends Anal Chem. 2019, 118, 731–738. DOI: 10.1016/j.trac.2019.07.005.
- Berger, T. A.; Berger, B.; Majors, R. A Review of Column Development for Supercritical Fluid Chromatography. LCGC North Am. 2010, 28(5), 344–357.
- Zheng, J.; Taylor, L. T.; Pinkston, J. D.; Mangels, M. L. Effect of Ionic Additives on the Elution of Sodium Aryl Sulfonates in Supercritical Fluid Chromatography. J. Chromatogr. A. 2005, 1082(2), 220–229. DOI: 10.1016/j.chroma.2005.04.086.
- Cazenave-Gassiot, A.; Boughtflower, R.; Caldwell, J.; Hitzel, L.; Holyoak, C.; Lane, S.; Oakley, P.; Pullen, F.; Richardson, S.; Langley, G. J. Effect of Increasing Concentration of Ammonium Acetate as an Additive in Supercritical Fluid Chromatography Using CO2–Methanol Mobile Phase. J. Chromatogr. A. 2009, 1216(36), 6441–6450. DOI: 10.1016/j.chroma.2009.07.022.
- Pinkston, J. D.; Stanton, D. T.; Wen, D. Elution and Preliminary Structure-Retention Modeling of Polar and Ionic Substances in Supercritical Fluid Chromatography Using Volatile Ammonium Salts as Mobile Phase Additives. J Separation Sci. 2004, 27(1–2), 115–123. DOI: 10.1002/jssc.200301672.
- Coan, C. R.; King, A. D., Jr. Solubility of Water in Compressed Carbon Dioxide, Nitrous Oxide, and Ethane. Evidence for Hydration of Carbon Dioxide and Nitrous Oxide in the Gas Phase. J. Amer. Chem. Soc. 1971, 93(8), 1857–1862. DOI: 10.1021/ja00737a004.
- Ashraf‐Khorassani, M.; Taylor, L. T. Subcritical Fluid Chromatography of Water Soluble Nucleobases on Various Polar Stationary Phases Facilitated with Alcohol-Modified CO2 and Water as the Polar Additive. J Separation Sci. 2010, 33(11), 1682–1691. DOI: 10.1002/jssc.201000047.
- Płotka, J. M.; Biziuk, M.; Morrison, C.; Namieśnik, J. Pharmaceutical and Forensic Drug Applications of Chiral Supercritical Fluid Chromatography. TrAC Trends Anal Chem. 2014, 56, 74–89. DOI: 10.1016/j.trac.2013.12.012.
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Cifuentes, A. Chiral Analysis in Food Science. TrAC Trends Anal Chem. 2020, 123, 115761. DOI: 10.1016/j.trac.2019.115761.
- Galea, C. M.; Vander Heyden, Y., and Mangelings, D. 2017. Chapter 12 - Separation of Stereoisomers. In Supercritical Fluid Chromatography, Poole, C. F., Ed., 345–379. Amsterdam, Netherlands: Elsevier: doi:10.1016/B978-0-12-809207-1.00012-4.
- Sekhon, B. S.;. Enantioseparation of Chiral Drugs - an Overview. Int. J. Pharm. Tech. Res. 2010, 2(2), 1584–1594.
- Nováková, L.; Douša, M.; Losacco, G. L.; Veuthey, J.-L.; Guillarme, D. General Screening and Optimization Strategy for Fast Chiral Separations in Modern Supercritical Fluid Chromatography. Anal. Chim. Acta. 2017, 950, 199–210. DOI: 10.1016/j.aca.2016.11.002.
- West, C.;. Recent Trends in Chiral Supercritical Fluid Chromatography. TrAC Trends Anal Chem. 2019, 120, 115648. DOI: 10.1016/j.trac.2019.115648.
- Ilisz, I.; Bajtai, A.; Lindner, W.; Péter, A. Liquid Chromatographic Enantiomer Separations Applying Chiral Ion-Exchangers Based on Cinchona Alkaloids. J. Pharm. Biomed. Anal. 2018, 159, 127–152. DOI: 10.1016/j.jpba.2018.06.045.
- Hoffmann, C. V.; Pell, R.; Lämmerhofer, M.; Lindner, W. Synergistic Effects on Enantioselectivity of Zwitterionic Chiral Stationary Phases for Separations of Chiral Acids, Bases, and Amino Acids by HPLC. Anal. Chem. 2008, 80(22), 8780–8789. DOI: 10.1021/ac801384f.
- Orosz, T.; Grecsó, N.; Lajkó, G.; Szakonyi, Z.; Fülöp, F.; Armstrong, D. W.; Ilisz, I.; Péter, A. Liquid Chromatographic Enantioseparation of Carbocyclic β-Amino Acids Possessing Limonene Skeleton on Macrocyclic Glycopeptide-Based Chiral Stationary Phases. Journal of Pharmaceutical and Biomedical Analysis. 2017, 145, 119–126. DOI: 10.1016/j.jpba.2017.06.010.
- Gordillo, R.;. Supercritical Fluid Chromatography Hyphenated to Mass Spectrometry for Metabolomics Applications. J Separation Sci. 2021, 44(1), 448–463. DOI: 10.1002/jssc.202000805.
- Bastings, J. J. A. J.; van Eijk, H. M.; Olde Damink, S. W.; Rensen, S. S. D-Amino Acids in Health and Disease: A Focus on Cancer. Nutrients. 2019, 11(9), 2205. DOI: 10.3390/nu11092205.
- Ashraf‐Khorassani, M.; Fessahaie, M. G.; Taylor, L. T.; Berger, T. A.; Deye, J. F. Rapid and Efficient Separation of PTH-Amino Acids Employing Supercritical CO2 and an Ion Pairing Agent. J High Resolution Chromatography. 1988, 11(4), 352–353. DOI: 10.1002/jhrc.1240110414.
- Dobashi, A.; Dobashi, Y.; Ono, T.; Hara, S.; Saito, M.; Higashidate, S.; Yamauchi, Y. Enantiomer Resolution of D- and L-Alpha-Amino Acid Derivatives by Supercritical Fluid Chromatography on Novel Chiral Diamide Phases with Carbon Dioxide. J. Chromatogr. 1989, 461, 121–127. DOI: 10.1016/s0021-9673(00)94281-x.
- Lajkó, G.; Ilisz, I.; Tóth, G.; Fülöp, F.; Lindner, W.; Péter, A. Application of Cinchona Alkaloid-Based Zwitterionic Chiral Stationary Phases in Supercritical Fluid Chromatography for the Enantioseparation of Nα-Protected Proteinogenic Amino Acids. J. Chromatogr. A. 2015, 1415, 134–145. DOI: 10.1016/j.chroma.2015.08.058.
- Lou, X.; Sheng, Y.; Zhou, L. Investigation of Parameters in the Separation of Amino Acid Enantiomers by Supercritical Fluid Chromatography. J. Chromatogr. A. 1990, 514, 253–257. DOI: 10.1016/S0021-9673(01)89396-1.
- Liu, Y.; Berthod, A.; Mitchell, C. R.; Xiao, T. L.; Zhang, B.; Armstrong, D. W. Super/Subcritical Fluid Chromatography Chiral Separations with Macrocyclic Glycopeptide Stationary Phases. J. Chromatogr. A. 2002, 978(1), 185–204. DOI: 10.1016/S0021-9673(02)01356-0.
- Stringham, R. W.;. Chiral Separation of Amines in Subcritical Fluid Chromatography Using Polysaccharide Stationary Phases and Acidic Additives. J. Chromatogr. A. 2005, 1070(1), 163–170. DOI: 10.1016/j.chroma.2005.02.044.
- West, C.; Bouet, A.; Gillaizeau, I.; Coudert, G.; Lafosse, M.; Lesellier, E. Chiral Separation of Phosphine-Containing Alpha-Amino Acid Derivatives Using Two Complementary Cellulosic Stationary Phases in Supercritical Fluid Chromatography. Chirality. 2010, 22(2), 242–251. DOI: 10.1002/chir.20735.
- Payagala, T.; Wanigasekara, E.; Armstrong, D. W. Synthesis and Chromatographic Evaluation of New Polymeric Chiral Stationary Phases Based on Three (1s,2s)-(−)-1,2-diphenylethylenediamine Derivatives in HPLC and SFC. Anal. Bioanal. Chem. 2011, 399(7), 2445–2461. DOI: 10.1007/s00216-010-4615-6.
- Wang, R.-Q.; Ong, -T.-T.; Ng, S.-C. Chemically Bonded Cationic β-Cyclodextrin Derivatives and Their Applications in Supercritical Fluid Chromatography. J. Chromatogr. A. 2012, 1224, 97–103. DOI: 10.1016/j.chroma.2011.12.053.
- Sánchez-Hernández, L.; Bernal, J. L.; Nozal, M. J.; Del;toribio, L. Chiral Analysis of Aromatic Amino Acids in Food Supplements Using Subcritical Fluid Chromatography and Chirobiotic T2 Column. J Supercritical Fluids. 2016, 107, 519–525. DOI: 10.1016/j.supflu.2015.06.027.
- Wolrab, D.; Frühauf, P.; Gerner, C.; Kohout, M.; Lindner, W. Consequences of Transition from Liquid Chromatography to Supercritical Fluid Chromatography on the Overall Performance of a Chiral Zwitterionic Ion-Exchanger. J. Chromatogr. A. 2017, 1517, 165–175. DOI: 10.1016/j.chroma.2017.08.022.
- Vera, C. M.; Shock, D.; Dennis, G. R.; Farrell, W.; Shalliker, R. A. Comparing the Selectivity and Chiral Separation of D- and L- Fluorenylmethyloxycarbonyl Chloride Protected Amino Acids in Analytical High Performance Liquid Chromatography and Supercritical Fluid Chromatography; Evaluating Throughput, Economic and Environmental Impact. J. Chromatogr. A. 2017, 1493, 10–18. DOI: 10.1016/j.chroma.2017.02.017.
- Khater, S.; Canault, B.; Azzimani, T.; Bonnet, P.; West, C. Thermodynamic Enantioseparation Behavior of Phenylthiohydantoin-Amino Acid Derivatives in Supercritical Fluid Chromatography on Polysaccharide Chiral Stationary Phases. J. Sep. Sci. 2018, 41(6), 1450–1459. DOI: 10.1002/jssc.201701196.
- Lipka, E.; Dascalu, A.-E.; Messara, Y.; Tsutsqiridze, E.; Farkas, T.; Chankvetadze, B. Separation of Enantiomers of Native Amino Acids with Polysaccharide-Based Chiral Columns in Supercritical Fluid Chromatography. J. Chromatogr. A. 2019, 1585, 207–212. DOI: 10.1016/j.chroma.2018.11.049.
- Raimbault, A.; Dorebska, M.; West, C. Chromatography–Mass Spectrometry Method to Analyze Free Amino Acids. Anal. Bioanal. Chem. 2019, 411(19), 4909–4917. DOI: 10.1007/s00216-019-01783-5.
- Miller, L.; Yue, L. Chiral Separation of Underivatized Amino Acids in Supercritical Fluid Chromatography with Chiral Crown Ether Derived Column. Chirality. 2020, 32(7), 981–989. DOI: 10.1002/chir.23204.
- Lemasson, E.; Bertin, S.; West, C. Use and Practice of Achiral and Chiral Supercritical Fluid Chromatography in Pharmaceutical Analysis and Purification. J Separation Sci. 2016, 39(1), 212–233. DOI: 10.1002/jssc.201501062.
- Berger, T. A.; Deye, J. F.; Ashraf-Khorassani, M.; Taylor, L. T. Gradient Separation of PTH-Amino Acids Employing Supercritical CO2 and Modifiers. J. Chromatogr. Sci. 1989, 27(3), 105–110. DOI: 10.1093/chromsci/27.3.105.
- Veuthey, J. L.; Caude, M.; Rosset, R. Separation of Some Amino Acids by Supercritical Fluid Chromatography after a Pre-Derivatization Step with Classical Reagents. Chromatographia. 1989, 27(3), 105–108. DOI: 10.1007/BF02265859.
- Wolrab, D.; Frühauf, P.; Gerner, C. Direct Coupling of Supercritical Fluid Chromatography with Tandem Mass Spectrometry for the Analysis of Amino Acids and Related Compounds: Comparing Electrospray Ionization and Atmospheric Pressure Chemical Ionization. Anal. Chim. Acta. 2017, 981, 106–115. DOI: 10.1016/j.aca.2017.05.005.
- Huang, Y.; Wang, T.; Fillet, M.; Crommen, J.; Jiang, Z. Simultaneous Determination of Amino Acids in Different Teas Using Supercritical Fluid Chromatography Coupled with Single Quadrupole Mass Spectrometry. J Pharm. Anal. 2019, 9(4), 254–258. DOI: 10.1016/j.jpha.2019.05.001.
- Blackwell, J.; Stringham, R. Effect of Mobile Phase Components on the Separation of Polypeptides Using Carbon Dioxide‐Based Mobile Phases. J High Resolution Chromatography. 1999, 22(2), 74–78. DOI: 10.1002/(SICI)1521-4168(19990201)22:2<74::AID-JHRC74>3.0.CO;2-9.
- Tognarelli, D.; Tsukamoto, A.; Caldwell, J.; Caldwell, W. Rapid Peptide Separation by Supercritical Fluid Chromatography. Bioanalysis. 2009, 2(1), 5–7. DOI: 10.4155/bio.09.165.
- Patel, M. A.; Riley, F.; Wang, J.; Lovdahl, M.; Taylor, L. T. Packed Column Supercritical Fluid Chromatography of Isomeric Polypeptide Pairs. J. Chromatogr. A. 2011, 1218(18), 2593–2597. DOI: 10.1016/j.chroma.2011.03.005.
- Liu, J.; Regalado, E.; Mergelsberg, I.; Welch, C. Extending the Range of Supercritical Fluid Chromatography by Use of Water-Rich Modifiers. Org. Biomol. Chem. 2013, 11(30), 4925. DOI: 10.1039/c3ob41121d.
- Shao, Y.; Wang, C.; Apedo, A.; McConnell, O. Rapid Separation of Five Cyclosporin Analogs by Supercritical Fluid Chromatography. J Anal. Sci. Methods Inst. 2016, 06(2), 23–32. DOI: 10.4236/jasmi.2016.62004.
- Enmark, M.; Glenne, E.; Leśko, M.; Langborg Weinmann, A.; Leek, T.; Kaczmarski, K.; Klarqvist, M.; Samuelsson, J.; Fornstedt, T. Investigation of Robustness for Supercritical Fluid Chromatography Separation of Peptides: Isocratic Vs Gradient Mode. J. Chromatogr. A. 2018, 1568, 177–187. DOI: 10.1016/j.chroma.2018.07.029.
- Desfontaine, V.; Losacco, G. L.; Gagnebin, Y.; Pezzatti, J.; Farrell, W. P.; González-Ruiz, V.; Rudaz, S.; Veuthey, J.-L.; Guillarme, D. Applicability of Supercritical Fluid Chromatography – Mass Spectrometry to Metabolomics. I – Optimization of Separation Conditions for the Simultaneous Analysis of Hydrophilic and Lipophilic Substances. J. Chromatogr. A. 2018, 1562, 96–107. DOI: 10.1016/j.chroma.2018.05.055.
- Schiavone, N. M.; Bennett, R.; Hicks, M. B.; Pirrone, G. F.; Regalado, E. L.; Mangion, I.; Makarov, A. A. Evaluation of Global Conformational Changes in Peptides and Proteins following Purification by Supercritical Fluid Chromatography. J. Chromatogr. B. 2019, 1110–1111, 94–100. DOI: 10.1016/j.jchromb.2019.02.012.
- Molnár-Perl, I.;. Role of Chromatography in the Analysis of Sugars, Carboxylic Acids and Amino Acids in Food. J. Chromatogr. A. 2000, 891(1), 1–32. DOI: 10.1016/S0021-9673(00)00598-7.
- Thippeswamy, R.; Gouda, K. G. M.; Rao, D. H.; Martin, A.; Gowda, L. R. Determination of Theanine in Commercial Tea by Liquid Chromatography with Fluorescence and Diode Array Ultraviolet Detection. J. Agric. Food Chem. 2006, 54(19), 7014–7019. DOI: 10.1021/jf061715.
- Gopaliya, P.; Kamble, P. R.; Kamble, R.; Chauhan, C. S.; Review, A. Article on Supercritical Fluid Chromatography. Int. J. Pharma Res. Rev. 2014, 3(5), 59–66.
- Rossé, G.;. Supercritical Fluid Chromatography; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2018; Vol. 1. DOI: 10.1515/9783110500776
- Forgács, E.; Cserháti, T. Chromatography| Principles. In Encyclopedia of Food Sciences and Nutrition, (Second ed.; Caballero, B., Ed.; Academic Press: Oxford, 2003; pp 1259–1267. DOI: 10.1016/B0-12-227055-X/00230-3.
- Berger, T. A.;. Chromatography: Supercritical Fluid | Instrumentation. In Encyclopedia of Separation Science; Wilson, I. D., Ed.; Academic Press: Oxford, 2007; pp 1–8. DOI: 10.1016/B0-12-226770-2/00471-3.
- Berger, T. A. Chromatography: Supercritical Fluid | Theory of Supercritical Fluid Chromatography. In Encyclopedia of Separation Science; Wilson, I. D., Ed.; Academic Press: Oxford, 2007; pp 1–9. DOI: 10.1016/B0-12-226770-2/00451-8.