ABSTRACT
To compare the bicarbonate kinetics and gastrointestinal (GI) symptom responses between an equal dose of sodium bicarbonate and sodium citrate using delayed-release capsules. Thirteen active males (age 20.5 ± 2.1 y, height 1.8 ± 0.1 m and body mass [BM] 76.5 ± 9.6 kg) consumed either 0.3 g.kg−1 BM sodium bicarbonate, sodium citrate or a placebo, using a double-blind, randomized crossover design. Blood bicarbonate ion (HCO3−) concentration, pH and GI symptoms were measured pre-consumption and every 10 min for 180 min post-consumption. Blood HCO3− concentration (P < 0.001) and pH (P = 0.040) were significantly higher in the sodium bicarbonate condition compared with sodium citrate condition up to 3 h post-consumption. Peak blood HCO3– concentration was significantly higher with the sodium bicarbonate compared with citrate (P < 0.001). Mean GI symptom scores were lower (P = 0.037) for sodium citrate (1.5 ± 1.8 AU) than bicarbonate (2.6 ± 3.1 AU), with considerable inter-individual variability. No GI symptoms were reported following consumption of the placebo. Both substances increase HCO3− values significantly, with sodium bicarbonate causing significantly higher pH and HCO3− values than the same dose of sodium citrate, but results in slightly more severe GI symptoms.
Introduction
The use of exogenous extracellular buffering agents has been widely investigated in the literature (Matson & Tran, Citation1993; McNaughton, Citation1992; McNaughton et al., Citation2016) and across a range of sporting activities (Kumstát et al., Citation2018; Saunders et al., Citation2014; Shave et al., Citation2001). Typically, these agents have been used for their potential ergogenic effects on short duration, high-intensity exercise (Grgic, Citation2020). The most commonly used extracellular buffers are sodium bicarbonate and sodium citrate (Carr et al., Citation2011), since they have the potential to increase base excess by increasing blood bicarbonate ion (HCO3−) concentration and increasing blood pH. Many studies seeking to investigate the effects of sodium citrate and sodium bicarbonate on subsequent performance measures, often commence exercise at a standardized time of around 60–90 min post-ingestion (Kumstát et al., Citation2018; Schabort et al., Citation2000; Shave et al., Citation2001). This comes despite individual-level data supporting a minimum of 100–180+ minutes (Potteiger et al., Citation1996; Requena et al., Citation2005; Urwin et al., Citation2016, Citation2019) or 30–180 minutes (Gough et al., Citation2019, Citation2017; Jones et al., Citation2016; L.F. De Oliveira et al., Citation2020) in order to achieve peak alkalotic responses with sodium citrate and sodium bicarbonate, respectively. Heibel et al. (Citation2018) have previously suggested that this peak should result in blood HCO3− concentration increases of >5 mmol-L−1 for the greatest chance of an ergogenic effect. However, the different ingestion times for sodium bicarbonate and sodium citrate are due to the longer post-ingestion time for peak pH and bicarbonate (time-to-peak) changes to occur in the blood. This is likely to be a result of the higher molecular weight of sodium citrate compared to sodium bicarbonate (NaHCO3) and this too may influence total blood HCO3− changes.
What is clear, is that the timing of optimal pre-exercise ingestion has received considerable attention recently (Gough et al., Citation2019, Citation2017; Miller et al., Citation2016) and has been shown to exhibit considerable inter-individual variability (Jones et al., Citation2016; Sparks et al., Citation2017), suggesting that individual timing of ingestion is important. Indeed, Boegman et al. (Citation2020) have recently demonstrated that individualizing ingestion time provides an important competitive advantage in elite rowers compared to a standard ingestion time. Furthermore, what also appears to be important in the use of these buffering agents, is the potentially ergolytic effect for those individuals that suffer from gastrointestinal (GI) side-effects (Deb et al., Citation2018), suggesting that careful individual assessments are needed prior to ingesting them before exercise.
Indeed, acute GI distress is a relatively common side-effect of ingesting large quantities of exogenous buffers, particularly when provided as an aqueous solution. Since GI distress appears partly attributable to interaction with acids in the stomach, contemporary research has focused on ways to reduce this discomfort (Hilton et al., Citation2019b, Citation2019a), while simultaneously attempting to make supplementation more palatable for application in a practical context (Urwin et al., Citation2019). To achieve this, a range of capsules have been utilized as a means of avoiding degradation of capsules in the stomach and exploiting the substantial pH differences across the GI tract, causing degradation to occur predominantly in the less acidic duodenum (pH 6–7 arbitrary units [AU]) (Ibekwe et al., Citation2008). Many of these capsules contain hydroxpropyl methylcullulose which resists degradation in acidic environments (pH ~1-2 AU). Whilst it was thought that reducing neutralization in the stomach may increase blood concentrations (L. F. De Oliveira et al., Citation2018), reductions in blood HCO3– concentration have been observed, potentially due to reductions in the time available for absorption (Hilton et al., Citation2019b, Citation2019a).
To date, no previous literature has assessed the time-to-peak of acid-base variables (HCO3− and pH) and GI distress following administration of sodium citrate, provided within delayed-release (DR) capsules. Successfully minimizing GI distress following sodium citrate/bicarbonate ingestion may improve the usability of these agents for athletes who would otherwise be discouraged by potential side-effects. Furthermore, no studies have directly compared blood acid-base responses following sodium citrate and sodium bicarbonate ingestion using an identical population and administration strategy. Whereas limited comparisons between buffering agents have been made to date, any observations made may further guide an athlete’s choice to ingest one buffering agent over the other. Therefore, the aim of this study was to determine the bicarbonate kinetic and gastrointestinal symptom responses to an orally ingested dose of sodium bicarbonate and sodium citrate, administered in DR capsules.
Methods
Participants
Thirteen recreationally active males (age 20.5 ± 2.1 y, height 1.8 ± 0.1 m and body mass 76.5 ± 9.6 kg) were recruited for the current study. All participants were familiar with high intensity or intermittent exercise and took part in exercise >3 h wk−1. Participants who had taken any nutritional supplements (e.g. beta-alanine) which may influence the response to sodium bicarbonate/citrate within the last 6 months (Baguet et al., Citation2009) were not eligible for the study. Written informed consent was obtained after verbal and written explanations of the benefits and potential side-effects associated with the investigation. The study was approved by the departmental research ethics committee of Edge Hill University (SPA-REC-2019-004).
Experimental design
Participants attended the laboratory on three separate occasions and consumed 0.3 g.kg−1 BM sodium bicarbonate, sodium citrate and a placebo (cornflour) in a double-blind, randomized crossover design. Experimental trials were counterbalanced in the order of administration and were completed under standard laboratory environmental conditions. All trials were separated by at least 48 h and took place at the same time of day (09:00) to minimize the physiological effects of circadian rhythms (Reilly, Citation1990).
Participants were asked to refrain from ingesting alcohol and undertaking any form of unaccustomed, intense exercise for at least 24 h prior to experimental testing. Within this 24 h period, participants were required to maintain their normal diet, and keep a record of dietary intake ensure intake. This was to was replicated in the 24 h period before each subsequent trial and minimize any changes this may have on acid-base balance (Bishop & Spencer, Citation2004; McNaughton, Citation1992). On the day of testing, to further minimize potential changes in acid-base balance and standardize the possible occurrence of GI symptoms, participants arrived at the laboratory in a fasted state (8 h).
Experimental procedures
Acid-base balance
On arrival to the laboratory, semi-nude BM was recorded after bladder evacuation and after completing a medical screening questionnaire. Participants consumed either the experimental supplements or a placebo (cornflour) within 10 min. All supplements were administered in size 00 opaque (white) DR (DRCaps, Lonza, France) capsules and the same number of capsules (mean ± SD = 36 ± 4 capsules) were provided. During the testing period, all participants remained seated. During this time, fingertip capillary blood samples (95 μL) were obtained pre-consumption and then every 10 min for 180 min post-consumption. Blood HCO3− concentration, and pH were measured using a blood gas analyser (Radiometer ABL800, Denmark) which was calibrated immediately prior to all testing sessions. This equipment has been used extensively in similar research studies and is deemed to provide valid and reliable measurements (Fagoni et al., Citation2018; Gough et al., Citation2017; Miller et al., Citation2016). The time to the first significant change in HCO3− concentration (Tlag), peak HCO3− concentration (Cmax), absolute change in HCO3− concentration (ΔCmax), time-to-peak HCO3− concentration (Tmax) and area under the concentration-time curve (AUC) were calculated.
Gastrointestinal symptoms
Symptoms of GI distress were recorded pre-consumption and every 10 min post-consumption for 180 min using a visual analogue scale (Miller et al., Citation2016) ranging from 0 (i.e. no symptom) to 10 (i.e. severe symptom). Participants were instructed to rate symptoms including nausea, flatulence, stomach cramping, belching, stomach ache, bowel urgency, diarrhoea, vomiting, and stomach bloating. Symptoms were described in lay terms to participants before the experimental trials commenced to ensure that symptoms were reported consistently. Aggregated GI symptom scores were calculated, as well as the highest GI symptom reported post-consumption.
Statistical analysis
Data normality was assessed using the Shapiro–Wilk test and by visual inspection of the normality plots (Grafen & Hails, Citation2002). Blood acid-base (HCO3 – and pH) profiles were analysed using two-way (trial × time) analysis of variance (ANOVA) with repeated-measures. A correction factor (Huynh-Feldt) was applied when Mauchly’s test indicated that the sphericity assumption was not plausible. One-way ANOVA with repeated measures were used to compare all other variables (GI symptom scores, Tlag, Cmax, ∆ Cmax, Tmax and AUC) between trials. Where a significant main-effect was shown, Sidak-adjusted post hoc tests were used for pairwise comparisons. Effect sizes were reported as partial eta-squared (ηp2) for one- and two-way ANOVA (Cohen, Citation1988), whereas Hedge’s g (± 95% confidence intervals [CI]) were calculated for paired comparisons (Lakens, Citation2013). The α-level of statistical significance was set at P < 0.05. Data were analysed using the Statistical Package for the Social Sciences (SPSS®) version 25 software.
Results
Acid–base balance
There were significant increases in blood HCO3 – concentration (F = 142, P < 0.001, ηp2 = 0.90) in the bicarbonate and citrate conditions compared with pre-consumption, with no change in placebo (). Blood HCO3 – concentration was highest at 140 min (P < 0.001) in the bicarbonate (mean difference = 5.3 mmol-L – 1; 95% CI 3.9–6.8 mmol-L–1) and 170 min (P < 0.001) in the citrate (mean difference = 3.5 mmol-L – 1, 95% CI 1.9–5.0 mmol.L–1) conditions compared with pre-consumption. HCO3− concentration was significantly higher in the bicarbonate condition (F = 142, P < 0.001, ηp2 = 0.92) compared with the citrate condition (P < 0.001), with a significant condition × time interaction (F = 31.7, P < 0.001, ηp2 = 0.73; ); blood HCO3− concentration was significantly higher for bicarbonate compared to citrate from 30 min to 170 min post-consumption.
Figure 1. Mean (±SD) A blood HCO3− concentration and B pH following the consumption of 0.3 g.kg–1 BM sodium bicarbonate, sodium citrate or a placebo (cornflower). *Denotes significant difference between sodium bicarbonate and sodium citrate (P < 0.05)
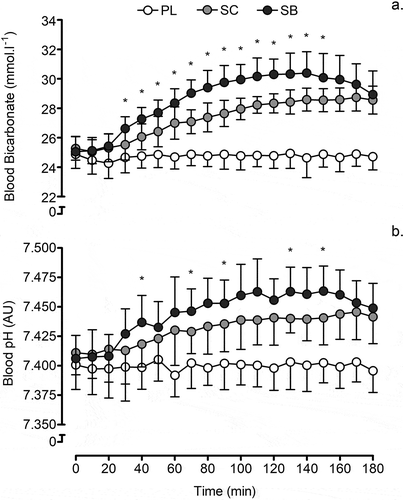
Blood pH increased in the bicarbonate and citrate conditions (F = 21.1, P < 0.001, ηp2 = 0.64) compared with pre-consumption (), with no change in placebo. The highest values occurred at 110 min (P < 0.001) in the bicarbonate and 170 min (P = 0.007) in the citrate conditions. The consumption of sodium bicarbonate or citrate had a significant effect on blood pH (F = 51.2, P < 0.001, ηp2 = 0.81) compared with placebo. Blood pH was significantly higher (P = 0.040) with sodium bicarbonate than with citrate (mean difference [MD] = 0.014 AU; 95% CI 0.001–0.027 AU), with a significant condition × time interaction (F = 7.0, P < 0.001, ηp2 = 0.37; ); blood pH was significantly higher for bicarbonate compared to citrate at several timepoints post-consumption.
Bicarbonate kinetics
Consuming either sodium bicarbonate or sodium citrate had a significant and large effect on all bicarbonate kinetic variables (). Bicarbonate Tlag was significantly longer with citrate (MD = 15 ± 21 min; 95% CI 2–28 min) compared with the bicarbonate, as was Tmax (22 ± 26 min; 95% CI 6–38 min). Similarly, Cmax (1.9 ± 1.0 mmol∙L–1; 95% CI 1.2–2.5 mmol∙L–1), ∆Cmax (2.1 ± 1.2 mmol∙L–1; 95% CI 1.4–2.8 mmol∙L–1) and AUC (246 ± 141 mmol∙min–1∙L–1; 95% CI 161–331 mmol∙min–1∙L–1) were significantly higher for bicarbonate compared with the citrate condition.
Table 1. Mean (±SD) bicarbonate kinetic variables following the consumption of 0.3 g.kg–1 BM sodium bicarbonate and sodium citrate
Gastrointestinal symptoms
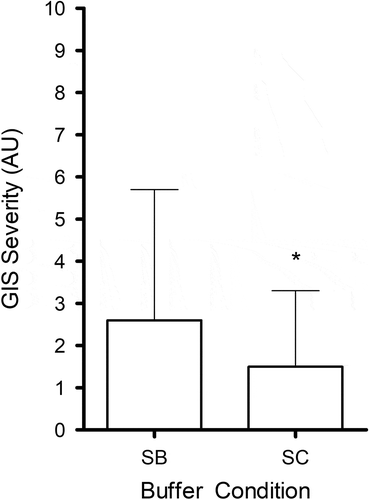
No GI symptoms were reported following consumption of the placebo. More participants reported GI symptoms in the bicarbonate (n = 8) compared with the citrate (n = 6) condition (). Furthermore, GI symptom scores were significantly higher (, P = 0.037) with sodium bicarbonate (2.6 ± 3.1 AU) than with sodium citrate (1.5 ± 1.8 AU).
Table 2. Overall and maximal GI symptoms experienced for each participant following the consumption of 0.3 g.kg–1 BM sodium bicarbonate and sodium citrate. Symptom severity scores are displayed in (). Time or time range of maximal symptom are displayed in []
Discussion
This is the first study to investigate and compare the blood-acid base responses and GI symptoms to the same dose of sodium bicarbonate and sodium citrate ingested in DR capsules. The outcomes of this work indicate that both sodium bicarbonate and sodium citrate significantly increase blood HCO3− concentration using the DR capsule delivery method; however, this response is greater following sodium bicarbonate ingestion when both buffers were ingested using a 0.3 g-kg−1 BM dose. This study also showed that concentration kinetics are prolonged with sodium citrate, potentially due to its larger molecular weight. This larger molecular weight is likely to slow the digestion and absorption of sodium citrate and lead to the delayed blood HCO3− response which is of a lower magnitude. The majority of the blood bicarbonate kinetic responses (Cmax, ΔCmax, Tmax and AUC) were also greater with sodium bicarbonate, except Tlag, which was greater for sodium citrate. These data suggest that sodium bicarbonate may be the more favourable buffering supplement to ingest, considering measures of acid-base balance, when ingested using a buffering agent at 0.3 g-kg−1 BM, since it induces larger increases in blood HCO3− concentration (>5 mmol-L−1), more likely to result in ergogenic effects (Heibel et al., Citation2018). This notion is supported by meta-analytical data which showed a significant effect of sodium bicarbonate on exercise outcomes (+1.7% [90% CL ±2.0%]) with a typical dose of 0.3 g·kg−1, but no overall effect of sodium citrate (0.0% [–1.3%]) albeit with a typical dose of 0.3 g·kg−1 (Carr et al., Citation2011).
In order to achieve higher blood bicarbonate responses, a larger dose of sodium citrate of 0.5 g-kg−1 has been suggested to be suitable and potentially tolerated by the GI system (Urwin et al., Citation2019). These desirable >5 mmol-L−1 increases in blood HCO3− may explain the efficacy of coinciding exercise with Tmax, and subsequently therefore with ΔCmax (Miller et al., Citation2016; Gough et al., Citation2017; Boegman et al.Citation2020). Sodium bicarbonate is generally considered a more effective ergogenic supplement than sodium citrate (Carr et al., Citation2011). However, discrepancies may occur due to differences in timing of ingestion relative to exercise (De Salles Painelli & Lancha Junior, Citation2018). Studies commonly request participants ingest sodium bicarbonate and sodium citrate 90 min prior to exercise. While this appears to be suitable for sodium bicarbonate since peak HCO3− concentration occurs around this time (L.F. De Oliveira et al., Citation2020), it likely takes up to 3 h to reach peak values following sodium citrate ingestion (Urwin et al., Citation2016; Urwin et al., Citation2019). Our data support these assertions, since peak HCO3− concentration occurred much earlier for sodium bicarbonate, while HCO3− concentration only peaked at 170 min with sodium citrate. The bicarbonate kinetics here suggest that HCO3− concentration was decreasing after reaching its peak with sodium bicarbonate, although it remained elevated above concentrations with sodium citrate from 30 min until 170 min post-ingestion. This suggests that although sodium bicarbonate might be considered the more effective supplement when taken 30–170 min prior to exercise, if taken 3 h pre-exercise, there may not be differences between sodium citrate and sodium bicarbonate. In fact, had we continued data collection beyond 180 min, blood HCO3− concentration may have remained elevated for an extended period with sodium citrate (Urwin et al., Citation2019).
It is important however, to acknowledge that many previous performance comparison studies investigating both sodium citrate and sodium bicarbonate have not used an individualized ingestion strategy, nor have they suitably quantified the GI symptoms. Establishing which is the most effective ingestion strategy is likely to be highly individualized. Individuals experiencing severe GI symptoms are unlikely to use exogenous buffers prior to competition, and yet most studies have failed to account for this in their evaluation of the ergogenicity of their supplement, which may reduce the overall estimate of the effectiveness of these buffering agents (Deb et al., Citation2018). Indeed, this work is the first study to compare GI symptoms for both sodium bicarbonate and sodium citrate in the same participants using DR capsules. More GI discomfort was apparent with sodium bicarbonate compared to sodium citrate, although absolute levels of discomfort were relatively low, compared to more traditional ingestion methods (Hilton et al., Citation2019a, Citation2019b). Whilst lower levels of discomfort with both supplements were reported than is typically reported with gelatine capsules (Hilton et al., Citation2019a, Citation2019b) there were some participants that still experienced quite severe GI symptoms. This variability in GI response is consistent with previous work that has observed considerable inter-individual variability (Deb et al., Citation2018; Gough et al., Citation2017) even when DR capsules are employed (Hilton et al., Citation2019a). Clearly, those individuals susceptible to GI symptoms that may be so pronounced as to be ergolytic, would be unlikely to use such a pre-exercise ingestion strategy.
For those that can use the DR capsule effectively, it likely avoids degradation of the capsule in the stomach, reducing interaction with stomach acids, in turn minimizing the uncomfortable bloating in particular. Hilton et al., (Citation2019a, Citation2019b) have previously shown reduced GI symptoms using DR compared to gelatine capsules following ingestion of 0.3 g.kg-1 BM of sodium bicarbonate in over 90% of participants. DR capsules have also previously been shown to lead to lower GI symptoms with sodium bicarbonate ingestion when compared to aqueous solution (Hilton et al., Citation2019a, Citation2019b). Greater discomfort with sodium bicarbonate might also be associated with the different molecular masses of the respective compounds; the lighter sodium bicarbonate compound (84 g.mol-1) might breakdown quicker in the gut compared to the heavier sodium citrate (258 g.mol-1), causing this discomfort. These differences must be taken with caution, however, since the literature suggests that a 0.5 g.kg-1 BM dose of sodium citrate might be considered more effective to increase bicarbonate concentration and improve performance (McNaughton, Citation1992). Future studies should investigate a variety of doses in order to determine if the use of DR capsules to deliver sodium citrate alters the pharmokinetic responses.
A limitation of this study is that we only analysed the bicarbonate kinetics of blood HCO3− concentration and pH changes following sodium bicarbonate and sodium citrate supplementation in a fasted state. Whilst we see this as an important first study in determining the likely effects of these exogenous buffers consumed in DR capsules, it is likely that ingestion with a high carbohydrate meal would further reduce the severity of GI symptoms (Carr et al., Citation2011). Furthermore, the ingestion of a pre-exercise meal is also likely to more accurately reflect the pre-competition/training routine of athletes. We also wanted to determine the bicarbonate kinetics following administration of identical 0.3 g·kg−1 BM doses of sodium bicarbonate and sodium citrate but appreciate that a 0.5 g·kg−1 BM dose of sodium citrate might be considered optimal for HCO3− and performance changes (McNaughton, Citation1992). Therefore, future work should investigate the effects of these buffers delivered in DR capsules, at a variety of doses, following the ingestion of a pre-exercise meal strategy more likely to be used by athletes.
Conclusion
This study investigated the pharmokinetic and GI symptom effects of ingesting sodium bicarbonate and sodium citrate contained in delayed-release capsules at the same 0.3 g-kg−1 BM dose. Ingestion of sodium bicarbonate using this ingestion strategy resulted in a greater change in blood HCO3− compared to sodium citrate, but both exogenous buffering substances significantly alter HCO3− values. Ingestion of sodium citrate resulted in a smaller number of GI issues albeit at a dose that is smaller than previously reported to be tolerable and ergogenic. Future work should now focus on implementing dose responses studies, following feeding, prior to exercise.
Disclosure statement
The authors declare that they have no competing interests.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Baguet, A., Reyngoudt, H., Pottier, A., Everaert, I., Callens, S., Achten, E., & Derave, W. (2009). Carnosine loading and washout in human skeletal muscles. Journal of Applied Physiology, 106(3), 837–842. https://doi.org/10.1152/japplphysiol.91357.2008
- Bishop, D., & Spencer, M. (2004). Determinants of repeated-sprint ability in well-trained team-sport athletes. Journal of Sports Medicine and Physical Fitness, 44(1), 1–7.
- Boegman, S., Stellingwerff, T., Shaw, G., Clarke, N., Graham, K., Cross, R., & Siegler, J. (2020). The influence of sodium bicarbonate ingestion timing on world-class rowing performance. Frontiers in Nutrition, 7, 138. https://doi.org/10.3389/fnut.2020.00138
- Carr, A. J., Hopkins, W. G., & Gore, C. J. (2011). Effects of acute alkalosis and acidosis on performance: A Meta-analysis. Sports Medicine, 41(10), 801–814. https://doi.org/10.2165/11591440-000000000-00000
- Cohen, J. (1988). Statistical power analysis for the behavioural sciences (2nd ed. ed.). Lawrence Erlbaum Associates.
- De Oliveira, L. F., Saunders, B., & Artioli, G. G. (2018). Is bypassing the stomach a means to optimize sodium bicarbonate supplementation? A case study with a postbariatric surgery individual. International Journal of Sport Nutrition and Exercise Metabolism, 28(6), 660–663. https://doi.org/10.1123/ijsnem.2017-0394
- De Oliveira, L. F., Saunders, B., Guilherme Yamaguchi, G., Swinton, P., & Artioli, G. G. (2020). Is individualization of sodium bicarbonate ingestion based on time to peak necessary? Medicine Science Sports Exercise, 52(8), 1801–1808. https://doi.org/10.1249/MSS.0000000000002313
- De Salles Painelli, V., & Lancha Junior, A. H. (2018). Thirty years of investigation on the ergogenic effects of sodium citrate: Is it time for a fresh start? British Journal of Sports Medicine, 52(14), 942–943. https://doi.org/10.1136/bjsports-2016-096516
- Deb, S. K., Gough, L. A., Sparks, S. A., & McNaughton, L. R. (2018). Sodium bicarbonate supplementation improves severe-intensity intermittent exercise under moderate acute hypoxic conditions. European Journal of Applied Physiology, 118(3), 607–615. https://doi.org/10.1007/s00421-018-3801-7
- Fagoni, N., Breenfeldt Andersen, A., Oberholzer, L., Haider, T., Meinild Lundby, A. K., & Lundby, C. (2018). Reliability and validity of non‐invasive determined haemoglobin mass and blood volumes. Clinical Physiology and Functional Imaging, 38(2), 240–245. https://doi.org/10.1111/cpf.12406
- Gough, L. A., Deb, S. K., Brown, D., Sparks, S. A., & McNaughton, L. R. (2019). The effects of sodium bicarbonate ingestion on cycling performance and acid base balance recovery in acute normobaric hypoxia. Journal of Sports Sciences, 37(13), 1464–1471. https://doi.org/10.1080/02640414.2019.1568173
- Gough, L. A., Deb, S. K., Sparks, A. S., & McNaughton, L. R. (2017). The reproducibility of blood acid base responses in male collegiate athletes following individualised doses of sodium bicarbonate: A randomised controlled crossover study. Sports Medicine, 47(10), 2117–2127. https://doi.org/10.1007/s40279-017-0699-x
- Grafen, A., & Hails, R. (2002). Modern statistics for the life sciences (pp. 368). Oxford University Press.
- Grgic, J.(2020). Effects of sodium bicarbonate ingestion on measures of Wingate test performance: A meta-analysis. Journal of the American College of Nutrition, 14 Dec 2020, Online Ahead of Print. 1–10. https://doi.org/10.1080/07315724.2020.1850370.
- Heibel, A. B., Perim, P. H. L., De Oliveira, L. F., McNaughton, L. R., & Saunders, B. (2018). Time to optimize supplementation: Modifying factors influencing the individual responses to extracellular buffering agents. Frontiers in Nutrition, 5, 1–12. https://doi.org/10.3389/fnut.2018.00035
- Hilton, N. P., Leach, N. K., Sparks, S. A., Gough, L. A., Craig, M. M., Deb, S. K., & McNaughton, L. R. (2019a). A novel ingestion strategy for sodium bicarbonate supplementation in a delayed-release form: A randomised crossover study in trained males. Sports Medicine-Open, 5(1), 4. https://doi.org/10.1186/s40798-019-0177-0
- Hilton, N. P., Leach, N. K., Craig, M. M., Sparks, S. A., & McNaughton, L. R. (2019b). Enteric-coated sodium bicarbonate attenuates gastrointestinal side-effects. International Journal of Sports Nutrition and Exercise Metabolism, 21(1), 1–7. https://doi.org//10.1123/ijsnem.2019-0151
- Ibekwe, V. C., Fadda, H. M., McConnell, E. L., Khela, M. K., Evans, D. F., & Basit, A. W. (2008). Interplay between intestinal pH, transit time and feed status on the in vivo performance of pH responsive ileo-colonic release systems. Pharmaceutical Research, 25(8), 1828–1835. https://doi.org/10.1007/s11095-008-9580-9
- Jones, R. L., Stellingwerff, T., Artioli, G. G., Saunders, B., Cooper, S., & Sale, C. (2016). Dose-response of sodium bicarbonate ingestion highlights individuality in time course of blood analyte responses. International Journal of Sport Nutrition and Exercise Metabolism, 26(5), 445–453. https://doi.org/10.1123/ijsnem.2015-0286
- Kumstát, M. M., Hlinský, T., Struhár, I., & Thomas, A. (2018). Does sodium citrate cause the same ergogenic effect as sodium bicarbonate on swimming performance? Journal of Human Kinetics, 65(1), 89. https://doi.org/10.2478/hukin-2018-0022
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t tests and ANOVAs. Frontiers in Psychology, 4, 1–12. https://doi.org/10.3389/fpsyg.2013.00863
- Matson, L. G., & Tran, Z. V. (1993). Effects of sodium bicarbonate ingestion on anaerobic performance: A metaanalytic review. International Journal of Sport Nutrition, 3(1), 2–28. https://doi.org/10.1123/ijsn.3.1.2
- McNaughton, L. R. (1992). Bicarbonate ingestion: Effects of dosage on 60 s cycle ergometry. Journal of Sports Sciences, 10(5), 415–423. https://doi.org/10.1080/02640419208729940
- McNaughton, L. R., Gough, L., Deb, S., Bentley, D., & Sparks, S. A. (2016). Recent developments in the use of sodium bicarbonate as an ergogenic aid. Current Sports Medicine Reports, 15(4), 233–244. https://doi.org/10.1249/JSR.0000000000000283
- Miller, P., Robinson, A. L., Sparks, S. A., Bridge, C. A., Bentley, D. J., & McNaughton, L. R. (2016). The effects of novel ingestion of sodium bicarbonate on repeated sprint ability. The Journal of Strength & Conditioning Research, 30(2), 561–568. https://doi.org/10.1519/JSC.0000000000001126
- Potteiger, J. A., Nickei, G. L., Webster, M. J., Haub, M. D., & Palmer, R. J. (1996). Sodium citrate ingestion enhances 30 km cycling performance. International Journal of Sports Medicine, 17(1), 7–11. https://doi.org/10.1055/s-2007-972800
- Reilly, T. (1990). Human circadian rhythms and exercise. Critical Reviews in Biomedical Engineering, 18(3), 165–180.
- Requena, B., Zabala, M., Padial, P., & Feriche, B. (2005). Sodium bicarbonate and sodium citrate: Ergogenic aids? The Journal of Strength & Conditioning Research, 19(1), 213–224. https://doi.org/10.1519/13733.1
- Saunders, B., Sale, C., Harris, R. C., & Sunderland, C. (2014). Sodium bicarbonate and high-intensity-cycling capacity: Variability in responses. International Journal of Sports Physiology and Performance, 9(4), 627–632. https://doi.org/10.1123/ijspp.2013-0295
- Schabort, E. J., Wilson, G., & Noakes, T. D. (2000). Dose-related elevations in venous pH with citrate ingestion do not alter 40-km cycling time-trial performance. European Journal of Applied Physiology, 83(4–5), 320–327. https://doi.org/10.1007/s004210000264
- Shave, R., Whyte, G., Siemann, A., & Doggart, L. (2001). The effects of sodium citrate ingestion on 3,000-meter time-trial performance. Journal of Strength and Conditioning Research, 15(2), 230–234.
- Sparks, S. A., Williams, E., Robinson, A., Miller, P., Bentley, D. J., Bridge, C., & McNaughton, L. R. (2017). Sodium bicarbonate ingestion and individual variability in time-to-peak pH. Research in Sports Medicine, 25(1), 58–66. https://doi.org/10.1080/15438627.2016.1258645
- Urwin, C. S., Dwyer, D. B., & Carr, A. J. (2016). Induced alkalosis and gastrointestinal symptoms after sodium citrate ingestion: A dose-response investigation. International Journal of Sport Nutrition and Exercise Metabolism, 26(6), 542–548. https://doi.org/10.1123/ijsnem.2015-0336
- Urwin, C. S., Snow, R. J., Orellana, L., Condo, D., Wadley, G. D., & Carr, A. J. (2019). Sodium citrate ingestion protocol impacts induced alkalosis, gastrointestinal symptoms, and palatability. Physiological Reports, 7(19), e14216. https://doi.org/10.14814/phy2.14216