?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This paper explores the use of Chitosan as sustainable agent to improve dyeing properties of cotton fabric dyed with Opuntia ficus-indica L fruit peel and the determination of the amount of anthocyanin pigments by the spectrophotometric method. The results of this study highlights that cotton fabrics treated with chitosan have better depth of shade (K/S = 12) than those untreated fabrics (K/S = 3.7) dyed with Opuntia ficus-indica L fruit peel. We have thoroughly investigated the effects of dye bath pH and temperature on the color properties of the aforementioned fabrics. UV protection of cotton fabrics increased after natural dyeing, and it was further improved after being treated with chitosan. Experimental results show that the fastness properties of dyed cotton fabrics treated with chitosan were improved from average to excellent.
摘要
本文探讨了用壳聚糖作为可持续剂, 改善仙人掌果皮染色棉织物的染色性能, 并用分光光度法测定花色苷色素的含量. 本研究结果表明, 经壳聚糖处理的棉织物比经仙人掌果皮染色的未处理棉织物 (K/S=3.7) 具有更好的色度深度 (K/S=12) . 我们已经彻底研究了染色浴pH值和温度对上述织物颜色特性的影响. 天然染色后棉织物的紫外线防护能力增强, 经壳聚糖处理后进一步提高. 实验结果表明, 经壳聚糖处理的染色棉织物的牢度性能由一般提高到优异.
Introduction
Synthetic dyes are highly used for textile coloration since these are expected to have a wide range of colors with high substantivity to textiles.
These dyes are soluble organic compounds (Rathi, Kumar, and Dai-Viet Citation2021), especially those classified as reactive, direct, basic and acids. They exhibit high solubility in water making it difficult to remove them by conventional methods (Danouche et al. Citation2021). One of its properties is the ability to impart color to a given substrate (Yusuf Citation2018) because of the presence of chromophoric groups in its molecular structures. However, the property of fixing the color to the material is related to the auxotrophic groups, which are polar and can bind to polar groups of textile fibers (Rathi, Kumar, and Dai-Viet Citation2021).
Textile dyes not only causes significant damage to the water bodies (Rathi, Kumar, and Dai-Viet Citation2021), but also prevents the penetration of light through water (Yusuf Citation2018) which leads to a reduction in the rate of photosynthesis. These dyes also act as toxic, mutagenic, and carcinogenic agents (Danouche et al. Citation2021).
Therefore, sustainable, greener, and cleaner productions have recently become important concerns in textile processes. That is why in nowadays researchers give more concern to the use of natural dyes in textile industry as they are recyclable, biodegradable, and available in nature.
During these recent years, many scientific researches carried in the area of natural dyes have successfully revealed the coloring and the bio-mordanting potential of a number of dye plants such as:
Vitis vinifera leaves (Mansour, Ezzili, and Mhenni Citation2013), Rubia cordifolia (Yusuf et al. Citation2013), Lawsonia inermis (Yusuf et al. Citation2012), Hibiscus sabdariffa L. (Mansour and Ben Ali Citation2019), Peganum harmala (Yusuf Citation2018), Crocus sativus Carthamus tinctorius ,Terminalia arjuna,Iresine herbstii,Alternanthera bettzickiana,Duranta erecta, Calendula officinalis, Asclepias, Azadirachta indica (Yusuf Citation2018), Beta Vulgaris L.(Benli Citation2020), Acorn and Oak Leaves (Quercus) .(Benli and Bahtiyari Citation2021), Momordica charantia L (Batool et al. Citation2022), Cocos nucifera (Adeel et al, CitationForthcoming, Citation2022), and Camelia sinensis(Hayat et al. Citation2022).
As far as Sustainability has become, also, important concern in polymer production, chitosan is one of most polymer used (Islam et al. Citation2014).
Chitosan is a natural biopolymer material produced from chitin biopolymer, which is the second natural abundant biopolymer found in nature after cellulose. Chitin has been found in many sources such as marine crustaceans, insects, some algae, and fungi (Alam et al. Citation2008). Chitosan is non-toxic, biodegradable, and antimicrobial material which has many beneficial applications in many fields such as food industry, water treatment, pharmacology industry, manufacturing of membranes and cosmetics (Draczynski et al. Citation2017).
Chitosan is, also, known by its sustainability and low toxicity. This latter was shown for chitosan (CH) with different molecular weight (MW) and deacetylation degree (DD) as well as for succinyl derivatives (CHs) and CH-based nanoparticles (Yusuf et al. Citation2019; Zubareva et al. Citation2017).
In this study, the use of chitosan was explored as an ecofriendly agent to improve dyeing properties of cotton fabric dyed with Opuntia ficus-indica L fruit peel ().
Opuntia (Opuntia ficus-indica L) that belongs to the cactus family (Cactaceae), which includes about 1500 species of cactuses, is a tropical or subtropical plant that grows in the areas of Mexico, Latin America, Africa, and the Mediterranean countries. It is used in medicine, food, and cosmetics in the form of tea, jam, juice, and oil from seeds (Diaz Medina, Rodriguez Rodriguez, and Romero Citation2007). Opuntia is an important source of pigmented bioactive substances such as anthocyanins, betaline, and betateinine (Yahia and Mondragon-Jacobo Citation2011).
The responsible compounds for the brilliant color and some of biological activities are anthocyanins. They are interestingly reported to be the major sources of antioxidants in Opuntia (Opuntia ficus-indica L) peel extracts (Tsai and Ou Citation1996).
Therefore, part of this study is to evaluate dyeing cotton with Opuntia (Opuntia ficus-indica L). Cotton can be easily dyed, but the cellulose–dye bond is not very strong. Natural cellulose fibers are negatively charged because of the presence of carboxyl groups (Izabela and Wei Citation2004). Using cationic agents in the pretreatment of cotton may improve dye absorption and can not only increase the dyeing color strength but also enhance wash fastness (Aranaz et al. Citation2009).
Chitosan is one of cationic agents; it is used as a plant growth booster in agriculture and used for eliminating oils, heavy minerals, and phosphorus from water in industrial companies (Sharif et al. Citation2018).
Chitosan also can speed up blood clotting and provide antibacterial properties and used for fabric modification (Vakhitova and Safonov Citation2003). It is a deacetylated of chitin produced from prawn (Penaeus indicus) shells, shrimp (Caridean Shrimp)shells, crab (Callinectes sapidus) shells, fly larva shells and squid pens. A number of authors reported a better dyeability for treated cotton by chitosan (Mansour and Ben Ali Citation2019; Vakhitova and Safonov Citation2003). Vakhitova and Safonov found that using chitosan in acid dye bath increased intensity and strength of color (Vakhitova and Safonov Citation2003).
Thus, in this study, the effect of chitosan on dyeing properties of cotton fabrics dyed with Opuntia (Opuntia ficus-indica L) peel is investigated. The results of fastness properties, shades, depths of shade and UV protection properties are also studied.
Experimental
Material and methods
Prickly pear (Opuntia ficus-indica)
Prickly pear (Opuntia ficus-indica) fruit were purchased from a Tunisian market, their peel were reduced to thick juice with blender.
Chitosan
Chitosan was extracted from mud crab shells by Hayet ben ali et al. The degree of deacetylation of the extracted chitosan from snow crabs which determined by potentiometric titration was reported as being about 82% (Ben Ali et al. Citation2011).
Characterization of the cotton fabrics
Commercially bleached cotton fabric with the following specifications was purchased from textile shop, Tunisia; plain weave (plain weave and weight, 150 g m −2), thickness was 0.261 cm mm −1, number of yarns cm −1 in warp direction was 43 and in weft direction was 42. Before dyeing, the fabric was treated with a solution containing 5 g L −1 nonionic detergent, at 50°C for 30 min. Then, the fabric was thoroughly rinsed with water and air-dried at room temperature.
Chitosan treatment
Oxidation of cotton fabric with potassium periodate
A piece of cotton fabric of 5 g is immersed in solution of potassium periodate in deionized water (400 ml) in concentration of 5.0 mg/ml. The solution is then stirred for 1 h at 60 C. The cotton fabric is washed with deionized water several time to remove the oxidant and soaked in deionized water (400 ml) with stirring for 24 h at ambient temperature. This oxidized fabric is used for the next reaction without drying.
Coated by chitosan
A 2% solution of chitosan in acetic acid solution is prepared by stirring a dispersion of chitosan (8.0 g) in aqueous acetic acid solution (400 ml) for 1 h at 60 C. The oxidized cotton fabric is immersed in the chitosan solution with constant stirring for 2 h at 60 C, washed with deionized water several times, and soaked in deionized water (400 ml) with stirring for 24 h at ambient temperature. The resulting cotton fabric is dried at 60°C under vacuum for 6 h to produce the modified cotton fabric.
Extraction
Opuntia ficus-indica L peels are boiled in 100 mL of distilled water for 60 min at the temperature of 95°C at a bath ratio of 1/20 (1 g of plant material extracted by 20 mL of distilled water). The dye baths were filtered and cooled.
Fourier transform infrared spectroscopic analysis
Fourier transform infrared (FTIR) spectra are recorded on a Nexus 470 FTIR Spectrometer, Nicolet Company, USA, using potassium bromide disks. A total of 32 scans for each sample are taken with a resolution of 4 cm −1, with a range of 4000–400 cm −1.
Dyeing process
Dyeing is performed by the exhaustion method using a liquor ratio of 20:1, with the cotton fabric dyed in laboratory dyeing machine (Ahiba Datacolor International, USA) at different pH values (2–8) and at different temperatures (45–95°C). The pH values are recorded with Eutech pH-meter instruments and adjusted with acetic acids and dilute solution of sodium hydroxide 0.1 N. The temperature is raised at constant value (45–95°C) over 30 min and held at this level for 90 min. The dyeing is carried out in the laboratory dyeing machine (Ahiba Datacolour International, USA). Then, the dyed fabrics are removed, rinsed with water, and followed by a soaping step using 3 g L −1 of a nonionic detergent (4-Nonylphenyl-polyethylene glycol nonionic) at 60°C during 5 min. Finally, the fabric samples are washed thoroughly with cold water, squeezed, and dried at room temperature. The dyeing experiment is performed in duplicate.
Evaluation of dyeing quality
The reflectance and the L*, a*, b* coordinates of the dyed samples are measured with a Spectro Flash SF300 spectrophotometer (illuminant D65/10o observer) with data.
Master 23 software (Datacolor International, USA). Relative color strengths (K/S values) are determined using the Kubelk and Munk equations (1).
where R is the decimal fraction of the reflectance of dyed fabric, R0 is the decimal fraction of the reflectance of undyed fabric, K is the absorption coefficient and S is the scattering coefficient.
Evaluation of functional finishing properties
The UV protection ability of dyed cotton fabrics was measured on YG912E Textile Anti-ultraviolet Performance Tester (MEBON Co. Ltd., China) according to the EU standard EN13758-2001.
% UV transmittance (TUVA and TUVB) and UPF are calculated using the following relations (2):
where Sλ is the erythema reference action spectrum, Eλ is solar irradiance (w m −2 nm −1), Δλ is wavelength increment, and Tλ is spectral transmittance of the specimen.
Fastness testing
The dyed samples were evaluated according to NF and ISO standard methods: ISO 105-X12:2002 for color fastness to rubbing; ISO 105-C10:2006 for color fastness to washing, and ISO 105-B02:1994 for color fastness to light.
Analysis of monomeric anthocyanins
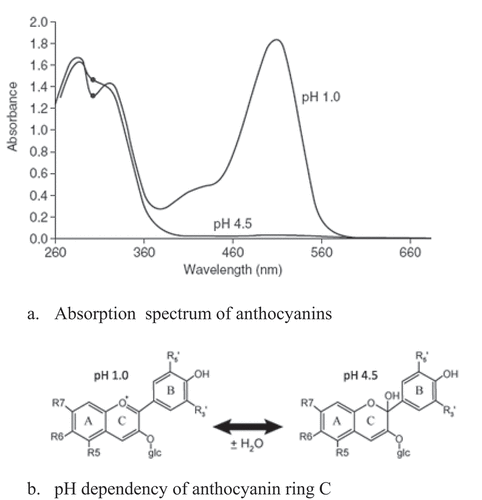
The monomeric anthocyanins contents of samples are determined using the pH-differential method as described by Giusti and Wrolstad (Lee et al. Citation2005). The wavelength of maximum absorption is 520 nm for anthocyanins. Spectrophotometric measurements are carried out using a double-beam spectrophotometer at 420 nm, 524 nm, and 700 nm. Calculations are based on cyanidin-3-glucoside () with extinction coefficient of 26,900 and molecular weight of 449.2. Quartz cuvettes of 1 cm pathlength are used and all measurements are carried out at room temperature (±25°C).
Absorbance readings are made against distilled water as a blank. Anthocyanin pigment was calculated with the equation:
Where A = (A524 nm – A700 nm) pH 1.0– (A524 nm – A700 nm) pH 4.5
DF = dilution factor; l = pathlength in cm; ε = molar extinction coefficient; 103 =factor for conversion from g to mg.
Results and discussions
Anthocyanin analysis
Monomeric anthocyanins content are determined using the pH-differential method that relies on the structural transformation of the anthocyanin chromophores () as a function of pH (Zubareva et al. Citation2017). Prickly pear (Opuntia ficus-indica) peel contains (7.64 mg/100 ml) 152,8 mg/kg of monomeric anthocyanins which is a significant amount compared to raspberry Rubus idaeus 100 mg/kg, raddish Raphanus sativus 110 mg/kg and plum 19 mg/kg.
Transform infrared (FTIR)
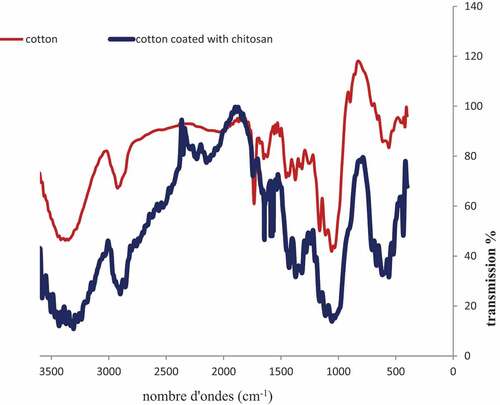
To confirm the variation of fabric surface after coating, chitosan-coated fabrics are characterized by FTIR-ATR spectroscopy.
shows spectra of cotton (control) and cotton with chitosan. The broad band in the range 3330–3260 cm−1 corresponds to the – OH stretching vibration of cellulose, and the asymmetric C – H stretching is observed in the range 2900 cm−1. The complex absorption in the range 1250–900 cm−1 is associated with C-O-C stretching groups of cellulose () pure cotton fabric; coated cotton with chitosan). The characteristic absorbance band of chitosan is amide I, C = O stretching and amide II. The absorbance band of N – H appears at 1640–1550 cm−1 normally, depending on the groups it connects, while the C = O stretching vibration usually shows absorbance band between 1680 and 1630 cm−1 (Yusuf et al. Citation2012).
Therefore, the peak in the range 1550–1645 cm−1 (), coated cotton with chitosan), which can be attributed to bounds between chitosan and cotton proves that the cotton is coated successfully.
The new peak at 1645 cm−1 (C = 0 stretching) and the extension at 1550 cm−1 resulting from possible -NH bending vibration of the amine groups of chitosan (, coated cotton with chitosan).
Morphological properties
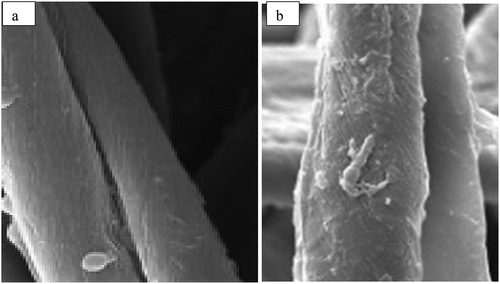
For the sake of exploring the effect of chitosan coating on cotton fabric, SEM is used to analyze these chitosan coated fabrics surface morphological properties. SEM images of chitosan coated fabrics are shown in which shows the surfaces of fibers in pure cotton fabric (.) are smooth while cotton fibers coated with chitosan () are slightly rough.
Effect of pH
The Prickly pear natural dye is applied for dyeing the treated fabrics using constant dyeing condition except the pH level which is changed, noting that, the pH of Prickly pear (Opuntia ficus-indica L) extract, before pH adjustment, is 4.5.
The color strength of the dyed fabrics is measured and obtained data are cited in the .
Table 1. Effect of dye bath pH on the color strength of coated and non-coated cotton fabrics with chitosan, dyed at 95°C.
In fact, the shows that the pH values of the dye bath have a significant effect on the dyeability of chitosaned and non-chitosaned cotton fabrics with Prickly pear (Opuntia ficus-indica L).
The dye behavior during dyeing process of Prickly pear (Opuntia ficus-indica L) peel, exhaustion and fixation of natural dye on the chitosaned fabrics depend on the ionic attraction between dye anions and fiber cations, therefore the color yield will be affected with magnitude of ionic attraction which may be influenced with the pH of the medium.
The acidic pH dye bath improves the dyeability of cotton fabric especially chitosaned one with Prickly pear (Opuntia ficus-indica L), extract. This result may be explained on the fact that chitosan behaves, in acid solutions, as a cationic polyelectrolyte due to protonation of the amino groups.
Therefore, under acidic condition, most of the amino groups of chitosan will be existed in protonated form (-NH3+), which enhance the ionic attraction between the anionic dyes and cationic groups of the chitosan and fibers. Thus, the degree of protonation of amino groups of chitosan will define the degree of dye exhaustion on the fabrics treated with chitosan. On the other hand, in absence of electrostatic attraction (at neutral and alkaline medium) van der Waals forces and hydrophobic interaction as well as hydrogen bonding would provide attraction between dye molecules and both fiber and chitosan.
Noting that in conventional dyeing processes, treatments at acidic pH are not suitable for cotton fabric since they damage mechanical properties. However garment processes, especially enzyme treatments, are treating denim fabrics at pH 4 and 5.
Effect of dyeing temperature
Dyeing is usually an exothermic process; however, the dyeing equilibrium reacts to an increase in temperature by absorbing more heat energy; The dyeing rate increases with an increase in temperature.
Temperature is an important parameter in the dyeing process; it plays a very effective role in dyeing fabrics and in determining the state of molecular polymer chains. In fact, by rising the temperature the movement speed of segment polymer chain is increased therefore the diffusion of dye molecules will be easy to get into fiber and give high color strength. Also, the stability of the anthocyanin pigments largely depends on temperature among other factors such as pH, light and its structure (Lee et al. Citation2005). shows the effect of the temperature of dyeing on CIE LAB (L*a*b*) coordinates and K/S values of coated and Non-coated cotton fabrics with chitosan. Notifying that L* () is corresponding to the brightness (100 = white, 0 = black), a* is corresponding to the red/green coordinate (positive sign = red, negative sign = green), and b* is corresponding to the yellow/blue coordinate (positive sign = yellow, negative sign = blue). When dyeing temperature increases, the values of L* decreases and the values of K/S increase.
Table 2. Effect of the dyeing temperature on L, a*, b*, and depth of shades values (K/S) of dyed coated with chitosan and non- coated with chitosan cotton fabrics dyed with Prickly pear peel (Opuntia ficus-indica L.).
Thus, the highest value of L* and lowest value of K/S showed lighter shades, while both the lowest values of L* and the highest values of K/S signify deeper shades of dyed samples. Positive a* and positive b* represent red and yellow, respectively.
Therefore, samples dyed at 45°C and 60°C have higher value of a* than those dyed at 75°C, 85°C, and 95°C. Also, it is worth noting that cotton fabrics dyed at 75°C, 85°C, and 95°C have a higher value of than cotton fabrics dyed at 45°C and 60°C (). These results correlate with red and brown shades of dyed fabrics, which could be explained by the temperature effect on anthocyanins (Wang, Cao, and Prior Citation1997). As it expected, the dye uptake of chitosan-treated cotton fabrics (CC) () is better than non-treated ones (CNC) which is maybe due to two facts. First, the fiber swelling enhances the dye diffusion. Second, it can be attributed to the correlation between the dye structure and treated cotton fibers. Since the anthocyanins act as an acid dye, it therefore would interact ionically with the protonated terminal amino groups of the chitosan-treated cotton fibers under acidic medium (pH 4) via electrostatic dye attraction.
UVP activity
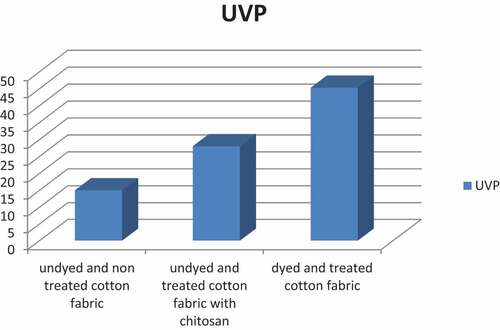
shows the UV effect of dyed cotton fabrics treated and non-treated with chitosan.
The results of UV protection show that cotton fabric treated with chitosan dyed with Prickly pear (Opuntia ficus-indica L) peel extract, shows the highest UV protection compared to those non-treated with chitosan.
According to AS/NZ 4399:1996 standard, it has to be considered that the analyzed fabric has an acceptable UV protection from UPF 15 value, the fabric is thought to have good protection between the range of 25 to 39 and excellent when values are over 40. (Alebeid and Zhao Citation2015).
Thus it should be highlighted that all analyzed samples give UV protection to cotton fabrics, being it excellent in the sample treated with chitosan and dyed with Prickly pear (Opuntia ficus-indica L) peel extract. On the contrary, the samples non treated with chitosan and dyed with Prickly pear (Opuntia ficus-indica L) peel extract and undyed, show 28 and 15 values, considered good and acceptable, respectively.
Fastness properties
The light, wash, and rubbing fastness values of chitosan treated and non-treated cotton fabrics dyed with Prickly pear peel (Opuntia ficus-indica L), aqueous extract are shown in . The control sample shows good rubbing and wash fastness properties of chitosaned dyed fabrics (3/4–5 units on the grayscale whereas 5 is the higher rating).
Table 3. Values of light, wash, and rubbing fastness of dyed coated with chitosan and non- coated with chitosan cotton fabrics, at T = 95°C.
This result can be explained by the affinity of chitosaned cotton fiber to anthocyanins. The light fastness of chitosaned cotton fabrics is good (5/6 units on the standard blue scale whereas 8 is the highest rating) which is better than those obtained with non-chitosaned cotton fabric. The chitosaned cotton fabric dyed with Prickly pear (Opuntia ficus-indica L) peel, extract shows higher color strength than the non-chitosaned cotton fabric because chitosaned cotton fabric contains more functional groups than the non-chitosaned one. In fact, the treatment of cotton with chitosan fabrics enhances the good penetration of the color in the fiber matrix. The value results obtained () illustrate that non-chitosaned cotton shows a yellowish-brown color; however, chitosaned cotton fabric shows reddish brown color.
Conclusion
The results have shown that the dyeing potential of the aqueous extracts of Prickly pear (Opuntia ficus-indica L) peel, as sustainable source of natural dye, is of considerable value. In this study results show that (Opuntia ficus-indica L) peel contains 152.8 mg/kg of monomeric anthocyanins which is an important amount.
Laboratory experiences indicate chitosan pretreatments have a good effect on the dye uptake which is greatly increased. Also, UV protection of cotton fabrics increased after natural dyeing, and it was further improved after being treated with chitosan. As shown in this study, chitosan-treated cotton fabrics, dyed with the extract, have better fastness dyeing properties than non-treated ones.
Highlights
The aqueous extract of Opuntia ficus-indica peels was used to dye chitosan-modified cotton fabric.
The modification of cotton fabrics with chitosan has improved their dyeing properties.
The dyed samples treated with chitosan showed excellent UV resistance.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adeel, S., S. Kiran, M. Shahid, S. Habib, N. Habib, and M. Hussaan. 2022. Ecofriendly application of coconut coir (Cocos nucifera) extract for silk dyeing. Environmental Science and Pollution Research 29 (1):564–12. doi:https://doi.org/10.1007/s11356-021-15669-6.
- Adeel, S., F. Rehman, A. Amin, N. Amin, F. Batool, A. Hassan, and M. Ozomay. Forthcoming. Sustainable exploration of coffee extracts (coffea arabica L.) for dyeing of microwave-treated bio-mordanted cotton fabric. Pigment & Resin Technology.
- Alam, R., M. Khan, R. Khan, S. Ghoshal, and M. Mondal. 2008. Study on the physico-mechanical properties of photo-cured chitosan films with oligomer and acrylate monomer. Journal of Polymers and the Environment 16 (3):213–19. doi:10.1007/s10924-008-0099-2.
- Alebeid, O., and T. Zhao. 2015. Anti-ultraviolet treatment by functionalizing cationized cotton with TiO2 nano-sol and reactive dye. Textile Research Journal 85 (5):449–57. doi:10.1177/0040517514549989.
- Aranaz, I., M. Mengibar, R. Harris, I. Panos, B. Miralles, N. Acosta, and A. Heras. 2009. Functional characterization of chitin and chitosan. Current Chemical Biology 3 (2):203–30. doi:10.2174/187231309788166415.
- Batool, F., S. Adeel, N. Iqbal, M. Azeem, and M. Hussaan. 2022. Sustainable natural coloring potential of bitter gourd (Momordica charantia L.) residues for cotton dyeing: Innovative approach towards textile industry. Environmental Science and Pollution Research 29 (23):34974–83. doi:10.1007/s11356-021-17803-w.
- Ben Ali, H., W. Tahri, N. Rahmouni, and S. Besbes-Hentati. 2011. Elaboration of tea polyphenols-chitosan complexes with antibacterial and antioxidant properties through adsorption. International Journal of Engineering Research 3 (11):632–36. doi:10.17950/ijer/v3s11/1102.
- Benli, H. 2020. Coloration of cotton and wool fabric by using bio-based red beetroot (Beta vulgaris L.). Journal of Natural Fibers 19 (10):3753–69. doi:10.1080/15440478.2020.1848725.
- Benli, H., and M. Bahtiyari. 2021. Testing Acorn and Oak leaves for the UV protection of wool fabrics by dyeing. Journal of Natural Fibers 1–14. Advance online publication. doi:10.1080/15440478.2021.1958423.
- Danouche, M., H. El Arroussi, W. Baha?d, and N. El Ghachtouli. 2021. An overview of the biosorption mechanism for the bioremediation of synthetic dyes using yeast cells. Environmental Technology Reviews 10 (1):58–76 32. doi:10.1080/21622515.2020.1869839.
- Diaz Medina, E. M., E. Rodriguez Rodriguez, and C. Romero. 2007. Chemical characterization of Opuntia dillenii and Opuntia ficus indica fruits. Food Chemistry 103 (1):38–45. doi:10.1016/j.foodchem.2006.06.064.
- Draczynski, Z., F. Flinčec Grgac, T. Dekanic, A. Tarbuk, and M. Bogun. 2017. Implementation of chitosan into cotton fabric. Tekstilec 60 (4):296–300. doi:10.14502/Tekstilec2017.60.296-301.
- Hayat, T., S. Adeel, F. Rehman, F. Batooland, N. Amin, T. Ahmad, and M. Ozomay. 2022. Waste black tea leaves (Camelia sinensis) as a sustainable source of tannin natural colorant for bio-treated silk dyeing. Environmental Science and Pollution Research 29 (16):24035–48. doi:10.1007/s11356-021-17341-5.
- Islam, M., W. Islam, A. Hoque, and M. Mondal. 2014. In Proceedings of the International Conference on Chemical Engineering. Paper presented at ICChE2014, Dhaka, Bangladesh, December 29–30.
- Izabela, K., and Z. Wei. 2004. Anthocyanins—more than nature’s colours. Journal of Biomedicine & Biotechnology 5 (5):239–42. doi:10.1155/S1110724304407013.
- Lee, G., D. Robert, E. Ronalad, K. Barnes, T. Eisele, and M. Giusti. 2005. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the ph differential method: Collaborative study. Journal AOAC International 88 (5):1269–72. doi:10.1093/jaoac/88.5.1269.
- Mansour, R., and H. Ben Ali. 2019. Investigating the use of chitosan: Toward improving the dyeability of cotton fabrics dyed with Roselle (Hibiscus sabdariffa L.). Journal of Natural Fibers 18 (7):1–10. doi:10.1080/15440478.2019.1675217.
- Mansour, R., B. Ezzili, and F. Mhenni. 2013. Dyeing properties of wool fabrics dyed with Vitis vinifera L. (black grenache) leaves extract. Fibers and Polymers 14 (5):786–92. doi:10.1007/s12221-013-0786-z.
- Rathi, B. S., P. -S. Kumar and N. Dai-Viet. 2021. Critical review on hazardous pollutants in water environment: Occurrence, monitoring, fate, removal technologies and risk assessment. The Science of the Total Environment 797:149134. doi:10.1016/j.scitotenv.2021.149134.
- Sharif, R., M. Mujtaba, M. Ur Rahman, A. Shalmani, H. Ahmad, T. Anwar, D. Tianchan, and X. Wang. 2018. The multifunctional role of chitosan in horticultural crops: A review. Molecules 23 (4):872–76. doi:10.3390/molecules23040872.
- Tsai, J., and M. Ou. 1996. Colour degradation of dried roselle during storage. Food Science 23:629–40.
- Vakhitova, N., and V. Safonov. 2003. Effect of chitosan on the efficiency of dyeing textiles with active dyes. Fibre Chemistry 35 (1):27–28. doi:10.1023/A:1023819521538.
- Wang, H., G. Cao, and R. Prior. 1997. Oxygen radical absorbing capacity of anthocyanins. Journal of Agriculture of Food Chemistry 45 (2):302–09. doi:10.1021/jf960421t.
- Yahia, E.-M., and C. Mondragon-Jacobo. 2011. Nutritional components and anti-oxidant capacity of ten cultivars and lines of cactus pear fruit (Opuntia spp.). Food Research International 44 (7):2311–18. doi:http://dx.doi.org/10.1016/j.foodres.2011.02.042.
- Yusuf, M. 2018. Mechanical and chemical structure of natural protein fibers: Wool and silk. In Handbook of renewable materials for coloration and finishing, M. Yusuf, 19–40. Hoboken, NJ: John Wiley and Sons.
- Yusuf, M. 2019. Biomedical applications of chitosan. In Handbook of ecomaterials, L.-M. Torres Martínez, O.-V. Kharissova, and B.-I. Kharisov, 3473–84. Cham: Springer Nature.
- Yusuf, M., A. Aijaz, S. Mohammad, I. Mohd, A. Shafat, M. Nikhat, and M. Faqeer. 2012. Antifungal activity of different natural dyes against traditional products affected fungal pathogens. Journal of Cleaner Production 27:42–50. doi:10.1016/j.jclepro.2012.01.005.
- Yusuf, M., S. Mohammad, A. Shafat, I. Mohd, I. Shahid-Ul, M. Faqeer, and A. Mohd. 2013. Eco-dyeing of wool using aqueous extract of the roots of indian madder (Rubia cordifolia) as natural dye. Journal of Natural Fibers 10 (1):14–28. doi:10.1080/15440478.2012.738026.
- Zubareva, A., B. Shagdarova, V. Varlamov, E. Kashirina, and E. Svirshchevskaya. 2017. Penetration and toxicity of chitosan and its derivatives. European Polymer Journal 93:743–49. doi:10.1016/j.eurpolymj.2017.04.021.