?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The main purpose of this study is to develop a green dyeing process using Corchorus olitorius dye extract. Corchorus olitorius leaves were used as a new dyestuff source that has never been used for cotton fabric dyeing. Dye extraction was performed using a non-conventional clean process containing ethanol in an ultrasound bath. The extracted dye was applied to cotton fabric in a bath exhaustion process. The effect of dyeing parameters on the color strength of dyed fabric was studied. A response surface methodology design was used to optimize the dyeing process. The results showed that pH, dyeing temperature, and dyeing time significantly affect the dyed fabric. The optimum dyeing parameters were found at 90°C in a bath pH of 9 for 90 min. Finally, to improve the color strength, and the color-fastness, three mordanting processes, and three different mordants were used. Results show that pre-mordanted dyed cotton fabric using aluminum potassium sulfate produces the best fastness properties.
摘要
本研究的主要目的是开发一种使用Corchore olitorius染料提取物的绿色染色工艺. 珊瑚叶被用作一种从未用于棉织物染色的新染料来源. 在超声波浴中使用含有乙醇的非常规清洁工艺进行染料提取. 将提取的染料应用于浴耗尽过程中的棉织物. 研究了染色参数对染色织物颜色强度的影响. 采用响应面方法设计优化染色工艺. 结果表明,pH值、染色温度和染色时间对染色织物有显著影响. 最佳染色参数为90°C,浴液pH值为9,染色时间为90 min. 最后,为了提高色强度和色牢度,使用了三种媒染工艺和三种不同的媒染剂. 结果表明,硫酸铝钾预媒染棉织物具有最佳的牢度性能.
Introduction
Textile materials are generally colored for fashion purposes or customers’ desires (Ashis Kumar and Konar Citation2011). The art of natural dyeing is one of the oldest techniques known by man and dates back to the dawn of civilization. Until the 19th century, dyes were natural. They mostly have plant origins such as madder, gaude, woad, indigo, etc., or animal origins such as the cochineal. They could also be mineral and microbial dyes. The use of natural dyes has significantly decreased after the appearance of synthetic dyes in the second half of the nineteenth century (Saxena and Raja Citation2014). The wide variety of colors available with good fastness properties at a low cost has been the main reason for the replacement of natural dyes in their synthetic alternatives (Bechtold et al. Citation2003). The production of synthetic dyes depends mainly on the petrochemical source and some of these dyes contain carcinogenic amines (Ashis Kumar and Konar Citation2011; Hunger Citation2003; Punyacharoennon, Deerattrakul, and Luepong Citation2021). The use of these dyes causes serious health risks and negatively influences the ecosystem’s stability (Amutha, Grace Annapoorani, and Sudhapriya Citation2020; Bruna, Camargo, and Aparecida Citation2013; Gulzar et al. Citation2015). In addition, many countries have already imposed strict environmental standards on these dyes, including Germany, which completely banned azo dyes (Goula et al. Citation2017; Uddin Citation2015).
Natural dyes have become more and more coveted textile dyeing products since they are renewable and biodegradable. They are eco-friendly and skin-friendly and can provide health benefits to the wearer (Kamboj, Jose, and Singh Citation2022; Mahdi, Zohra, and Ahmed Citation2021; Saxena and Raja Citation2014). Corchorus olitorius is an important green leafy vegetable in many tropical areas including Egypt, Sudan, Bangladesh, in tropical Asia such as Philippines and Malaysia, as well as in many tropical African countries. It belongs to the family of Tiliaceae. It is cultivated to provide bark for fiber production (jutes) and mucilaginous leaves utilized in food (Ben Yakoub, Benabderrahim, and Ferchichi Citation2016; Sengupta, Ghosh, and Mustafa Citation2022). The leaves (either fresh or dried) are cooked into a thick viscous soup or added to stew or soup and are rich sources of vitamins and minerals (Loumerem and Alercia Citation2016). It is considered as a source of carotenoids, vitamin C, vitamin E, fatty acids, and minerals and has demulcent, diuretic, lactagogue, purgative, and tonic properties (Handoussa et al. Citation2013). It is considered a folk remedy for aches and pains, managing diabetes, hypertension (Oboh et al. Citation2012) and swellings, becoming an ingredient of facial creams, lotions, hair tonics, and hand creams. Corchorus olitorius leaves are used for cystitis, dysuria, fever, and gonorrhea, while its cold infusion is said to restore appetite and strength (Ben Fadhel et al. Citation2021). In this study, the specific color of Corchorus olitorius leaves inspired us to explore this plant as a source of green dye for cotton fabrics. Indeed, according to available literature (Ben Fadhel et al. Citation2021), leaves were never being used previously in the dyeing of textile materials.
The extraction of natural dyes from plant material is a very specific process since the coloring matter is tightly bound with plant cell membranes (Sivakumar, Vijaeeswarri, and Lakshmi Anna Citation2011). There are many extraction methods that are suitable for the extraction of natural dyes from their source materials, which include maceration and Soxhlet extraction techniques, etc., as conventional techniques, and microwave-assisted extraction, ultrasonic extraction, etc., as non-conventional techniques (Tiwari Citation2015). Conventional extraction techniques are time-consuming and use large amounts of solvents. However, non-conventional techniques are more effective, nontoxic, and ecological. The use of ultrasound to extract the natural dye extracted from Corchorus olitorius leaves has been reported in this paper. The ultrasonic process is an eco-friendly green extraction technology. It is more efficient with low organic solvent consumption (cost-effective). It is easy to use and requires low investment compared to other innovative extraction techniques.
This study aims to develop a green process for dyeing cotton fabric using for the first time the natural dye extracted from Corchorus olitorius leaves. The extraction was performed using an ultrasonic process as a clean process. Parameters of the dyeing process were carried out to maximize the color strength. Efforts have also been deployed to optimize the mordanting method that improves the color strength and fastness properties using various mordants.
Materials & methods
Materials
A plain weave ready-to-wear clothing made of bleached cotton was purchased from a local textile store in Tunisia. It has a density of 107 g/m2. The yarn count around warp direction and weft direction were equal to 58 yarns/cm.
Corchorus olitorius leaves used in this paper were purchased from a local market in Tunisia. To ensure the homogeneity of the material, all the quantities used were purchased from the same supplier. It was conditioned and blended into a powder before storage in a dry and dark recipient to avoid alteration of their characteristics.
The metallic mordants, namely, copper sulfate (CuSO4), ferrous sulfate (FeSO4), and aluminum potassium sulfate (alum) (KAl (SO4)2) have been used. They were purchased from Sigma Aldrich Tunisia and used without further purification. These mordants did not fall into the heavy metal category (Adeel et al. Citation2017; Jordeva et al. Citation2020). Acetic acid and sodium hydroxide were used to adjust the pH of the dye solution. Ethanol was used as a solvent for dye extraction.
Methods
Dye extraction
The extraction of Corchorus olitorius was performed using ethanol ultrasonic bath. A liquor ratio of 1:10 was used (about 1 L of ethanol for 100 g plant material). The extraction temperature and the extraction time were fixed by preliminary tests in our laboratory according to previous literatures (Ghorpade, Darrekar, and Vankar Citation2000; Mansour and Heffernan Citation2011) at 70°C for 25 min.
UV/Vis analysis
A UV–visible spectrophotometer, type HACH DR 3900 was used for the current study. For all the measurements, we used a 1-cm-thick glass cell and all dye solutions were diluted in an appropriate solvent and analyzed in the visible range (380–780 nm).
Infrared spectrophotometer
Infrared spectroscopy of the dye extract was performed to identify the chemical bonds. The ATR-IR spectra were recorded using the FTIR-ATR spectrophotometer (Perkin-Elmer). Each spectrum was analyzed at a frequency range from 400 to 4000 cm−1 with a resolution of 4 cm−1.
Dyeing processes
To investigate the potential use of the extracted colorant, cotton fabrics were dyed at a liquor ratio of 40:1 by the exhaust dyeing method. The dyeing process () was performed with a lab dyeing machine (AHIBA Datacolour International USA). Then, the dyed fabrics were rinsed with water and dried at room temperature. Since the extracted colorant was not soluble in water, the use of a solvent to facilitate its dispersion in the bath was necessary. The nature and the concentration of the solvent were performed according to preliminary tests and some similar works on other natural dyes available in the literature (Baaka, Mahfoudhi, and Mhenni Citation2018; Moussa et al. Citation2018).
Figure 1. Dyeing process of pre-mordanted, meta-mordanted, post-mordanted, and non-mordanted cotton fabric with Corchorus olitorius dye extract and schematic representation of the dye interaction with the fabric.
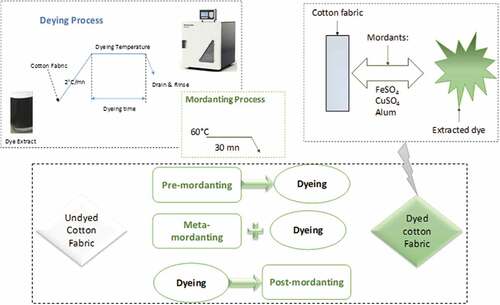
Three dyeing conditions were varied (dye bath pH, temperature, and dyeing time) (). All the experiments were designed according to Minitab 17 software. The pH of the dye baths was adjusted at the desired values by adding acetic acid and sodium hydroxide solutions.
Table 1. The range of experimental dyeing process parameters.
Mordanting process reported by Jabar et al. (Jabar, Ogunmokun, and Taleat Citation2020) was adopted with slight modifications (). A concentration of 1% was used for all mordants (Faidi et al. Citation2016; Jordeva et al. Citation2020; Özlenen and Yıldırım Citation2019). In fact, the cotton sample was impregnated in mordant solution for 30 min, with continuous stirring at a temperature of 60°C before dyeing in pre-mordanting process and after dyeing in post-mordanting process. Meta-mordanting was performed by dissolving mordant in the dye solution.
Color measurement
Instrumental color evaluation of the dyed samples was carried out using a Data Color 650®, spectrophotometer with 10° using D65 illuminant. An average of three measurements of color strength (K/S) was recorded. The relative color strength (K/S values) was assessed using the following Kubelka-Munk EquationEquation (1)(1)
(1) (Bhouri et al. Citation2022).
Where R is the reflectance of the dyed sutures at maximum absorption wavelength, S is the scattering coefficient and K is the absorption coefficient of dyed samples.
Colorfastness
The dyed fabric underwent different fastness tests such as light, washing, and rubbing. The test of colorfastness to light is carried out according to ISO 105-B02 standard. The dyed samples of 4.5 cm × 1 cm were fixed on cardboard in a way that all samples were half exposed to light and half covered. This sample is exposed to xenon light in Sunset apparel. After 24 h, the specimen was brought out, and the color fading was evaluated against the blue wool standards.
The test of colorfastness to washing was performed according to ISO 105-C06 standard. A piece of 10 × 4 cm was placed between two undyed fabrics (wool and cotton) of the same size and sewn all together. The washing was done by 4 g/l soap solution for 30 min using an Autowash machine (SDL) at 50°C. All samples were rinsed with running water and dried in the shade and then assessed using the grayscale.
Finally, the test of colorfastness to rubbing was accomplished as per the standard ISO 105-X12 method. A piece of cotton was rubbed 10 times on a dyed sample using a crock meter. Two types of rubbing were done, wet rubbing and dry rubbing. The color stained on the wet and dry control fabric is evaluated using a grayscale.
Results & discussion
Characterization of Corchorus olitorius leaves ethanol extract
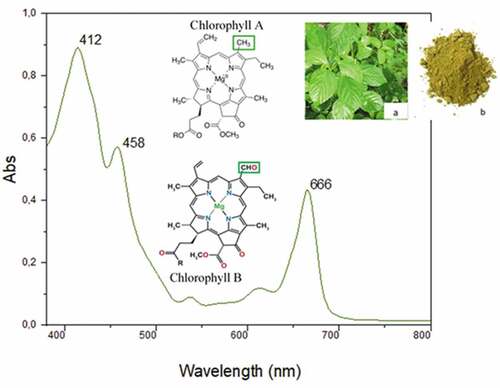
The ethanolic extract of Corchorus olitorius shows a dark green color. shows the visible spectrum of the obtained extract recorded at wavelength 380–780 nm. It was found that this spectrum showed three peaks in the visible region at 412, 458, and 666 nm corresponding, respectively, to purple, blue, and red-emitting wavelengths that characterize the green color extract. These peaks reveal the presence of carotenoids and chlorophylls in obtained ethanolic extract. Indeed, peaks at 412, 458, and 666 nm are characteristic peaks for chlorophyll A and B. These results are in accordance with those previously reported by Ikyo et al. (Ikyo et al. Citation2020) and Kang et al. (Kang et al. Citation2018) and prove successful extraction of chlorophylls. In addition, the broad absorption in the blue region could also hide the absorbance peak of carotenoids in the solutions, which is known to absorb in the visible spectrum in the range from 430 to 480 nm (Kang et al. Citation2018).
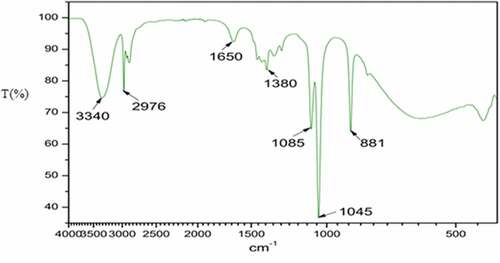
Infrared measurements were performed to identify the potential functional groups in the Corchorus olitorius leaves extract, which may be responsible for potential reactions during the dyeing process. displays bands at 3340 cm−1 which were assigned to the free OH stretching modes possibly from the phenolic components of Corchorus olitorius leaves extract. This observation agreed with finding made by Ismail et al. (Ismail et al. Citation2018) in synthesis of Corchorus olitorius extract. The band at 2976 cm−1 was assigned to the C-H stretching of the methyl or methylene group of the chlorophyll (Kang et al. Citation2018). The bonds at 1650 and 1380 were attributed to the methyl stretching vibration of C-H. A very strong band at 1045 cm−1 could be assigned to the C-OH vibrations of the carbonyl components in the Corchorus olitorius leaves. These findings are in agreement with those reported by Jabar et al. (Jabar, Ogunmokun, and Taleat Citation2020) in natural dyes for textile dyeing.
This spectral analysis shows that the obtained dye exhibits a phenolic and flavonoid structure that has an acid tendency with carbonylic or hydroxylic groups. Thus, Corchorus olitorius dye extract shows the domination of the negative ions in the dye solution. These ions have the ability to bind to positive charges that have to be prepared on the textile support.
Factors affecting the dyeing process
To evaluate the effect of the ethanol concentration on color strength (K/S), samples were dyed in an aqueous bath of ethanol containing from 0% to 60% of the total bath volume. The dyeing process was carried out at a pH of 7 and a temperature of 70°C for 45 min. Results of the color strength measurements are plotted in . A gradual increase in color strength was observed, and the maximum was reached at a concentration of 40%, and then it remains constant at higher ethanol concentrations. This increase can be attributed to dye solubility. In fact, the extracted dye had shown very low solubility in water demonstrated by low fabric uptake. When increasing ethanol concentration in the dye bath, the dispersion of the dye increases, and subsequently the adsorption of the dye improves giving better K/S values. Consequently, we fixed the solvent quantity at 40% in all the experiments.
Figure 4. I: (a) effect of solvent quantity on the color strength (K/S) (at T = 70°C; pH = 7; dyeing duration = 45 min); (b) effect of the pH of dyeing bath on (K/S) (at solvent quantity = 40%; T = 70°C; dyeing duration = 45 min); (c) effect of dyeing temperature on K/S (at solvent quantity = 40%; pH = 8; dyeing duration = 45 min); (d) effect of dyeing duration on K/S (at solvent quantity = 40%; pH = 8; T = 110°C). II: main effect plot of experimental parameters on the color strength.
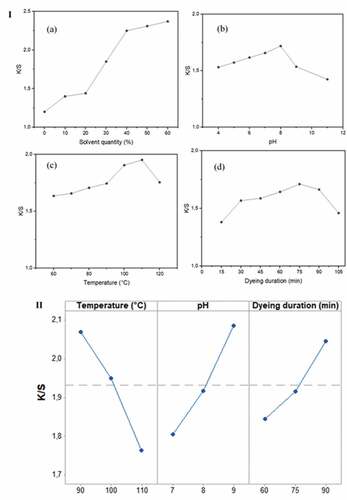
The effect of pH of the bath ranging from 4 to 11 on the evaluation of color strength was studied at an ethanol concentration of 40%, a temperature of 70°C for 45 min. shows that the color strength increases considerably from pH 4 to pH 8, these results can be attributed to the high affinity between dye structures and the fiber (Guesmi et al. Citation2013). At a pH higher than 8, an important decrease in the K/S values is observed indicating that the electrostatic interactions between the cotton fiber and the phenolic extract play an important role in the adsorption of the dyestuff onto cotton. This result can be explained, as mentioned by Friedman et al. (Friedman and Jürgens Citation2000) by the fact that phenolic compounds are not stable to high pH leading to a degradation of the dye resulting in poor color strength.
The evaluation of color strength was studied under dyeing temperature ranging from 60°C to 120°C for an ethanol concentration of 40%, a pH of 8, and a dyeing duration of 45 min. shows that the color strength increases with the increase in dyeing temperature to reach a maximum value of K/S at 110°C. Indeed, at high temperatures, cotton fiber swells and dye solubility improves subsequently, dye diffusion enhances.
The effects of dyeing durations ranging from 15 to 105 min on the evolution of the color strengths were investigated at an ethanol concentration of 40%, a pH of 8, and a dyeing temperature of 110°C. shows that the color strength was improved when the dyeing time increased until a maximum of 75 min. Above this value, a decrease in the K/S was observed. Indeed, this can be explained by the high exhaustion of the dye bath at 75 min. For longer periods and at high temperatures, the dyestuff is degraded and transformed into colorless substances. Also, this decrease could be explained by partial degradation of the colored cellulosic material (Bouatay et al. Citation2016; Rehman et al. Citation2022).
Optimization of the dyeing process
The results were analyzed by the statistical software Minitab version 17 and the main effects and interactions between factors were determined. The analysis of regression and the variance (ANOVA), as well as the graphical optimization, was performed in order to determine the optimal conditions of the critical variables.
pH, dyeing temperature, and the dyeing duration were chosen as independent variables. The color strength parameter was defined as a dependent output response variable.
The regression analysis of the experimental plan led to the following equation:
K/S = -1,110 + 0,06639 Temperature - 0,2128 pH + 0,01360 Dyeing duration - 0,000288 Temperature*Temperature+0,02793 pH*pH + 0,000131 Dyeing duration*Dyeing duration - 0,000725 Temperature*pH - 0,000243 Temperature*Dyeing duration - 0,000283 pH*Dyeing duration
R2 the squared multiple correlation coefficient presents the percentage indicating the adequacy of the data with a statistical model. The R2 of the studied model is 97.99%. Therefore, the procured model had good predictability. Details of the regression equation are presented in . According to this table, the majority of the variables showed a significant effect with a p-value <.05 except for the interaction effect of Temperature *pH and pH*Dyeing duration having a respective p-value of 0.19 and 0.552.
Table 2. Estimated effects and coefficients for the color strength (K/S).
shows the main effect plot of each experimental parameter on the color strength response. The dyeing duration had the greatest effect on K/S values. Regarding the dyeing temperature, the response parameter has shown the greatest value at 90°C, beyond this value, the impact decreases progressively. Finally, the greatest value of color strength was found at a pH value of 9.
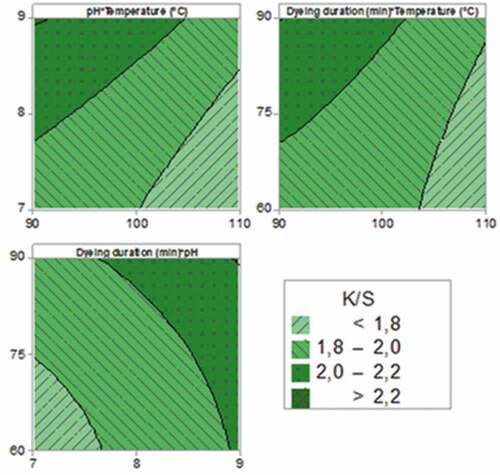
Response surface contour plots of experimental parameters on color strength are given . Contour plots illustrate the behavior of the measured characteristics as a function of two based factors. Indeed, the response surface is considered as a two-dimensional surface where all points that have the same response are connected to produce the contour lines of constant responses.
According to these plots, it can be seen that the highest color strength values were obtained when bath temperature is less than 100°C, the pH value is over than 7.8, and the dyeing duration exceeded 70 min the K/S can be higher than 2.
The optimum conditions of the Corchorus olitorius dyeing process were predicted using the response optimizer tool of Minitab17 software for a maximized response of the color strength.
Optimum operating conditions were found at a dyeing temperature of 90°C, a dyeing time of 90 min, and a pH of 9. At these conditions, the predicted theoretical color strength is approximately 2.3779 with an overall desirability value of 0.993.
To confirm the established model, further samples were performed under optimal conditions and color strengths were measured to compare experimental results with the predicted response given by the Minitab software. The color strengths of the obtained experiments are about 2.321 ± 0.05. These results confirm the validity of the established model.
Effect of mordanting process on dyeing properties
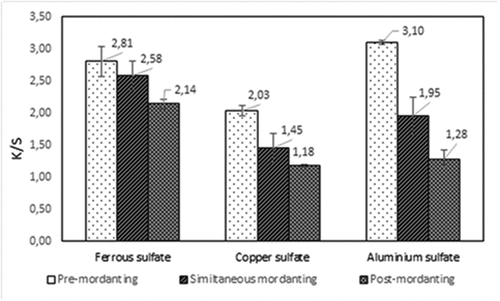
The effect of mordanting process with different mordants on the color strengths of the dyed cotton is shown in . It was observed that the K/S value of the dyed cotton fabrics increases considerably by using a pre-mordanting process. In fact, metal ions contribute to the coupling of the dyestuff groups to the cotton fiber, which leads to the formation of a stable complex structure. It can also be seen that the best results of the color strength were reached with aluminum sulfate mordant. Indeed, the use of a mordant in a pre-mordanting dyeing process allows the formation of an insoluble complex with both the cotton fiber and the phenolic groups of the dye extracted from Corchorus olitorius leaves. Thus, such coordination could change the shade of the dyed cotton fabrics (). Cotton is made up of polar hydroxyl (-OH) groups that provide multiple sites for hydrogen bonding with the polar groups in the dye molecule. The possible interactions of complexes formed by the metal salts of the mordant (Fe2+, Cu2+, Al3+) in the cotton dyeing process have been widely investigated (Ferda and Onal Citation2015). At basic pH, the oxygen atoms of cellulose units are easily bonded to the metal cations of mordant salt.
Table 3. Caption of different mordanting samples.
shows the dyeing figures for the different processes. From this table, it can be seen that unmordanted dyed fabric does not exhibit a uniform appearance, although, the shade of mordanted dyed fabric is quite different. This is mainly due to the nature of the bonding between the textile, the mordant and the dye extract of Corchorus olitorius. Pre-mordanted dyed cotton has a deeper color than meta and post-mordanted fabric. This confirms that the pre-mordanted process prepares the reactive groups on the material before introducing the dye to the bath, which ensures uniform distribution and avoids flattening on surface.
Fastness properties of dyed fabrics
Untreated cotton and pre-mordanting cotton samples were investigated in terms of fastness to washing, light, and rubbing. The obtained results are given in . This table shows that washing fastness and light fastness were improved, respectively, from 2 (very weak solidity) to 4/5 and from 1 to 4 for the cotton treated with a ferrous sulfate mordant. This result can be explained by the formation of strong bonds between the dye, the mordant, and the cotton fiber.
Table 4. Evaluation of fastness properties of untreated and treated cotton.
The dry rub dye fastness test shows very little variation depending on the used mordant. The values of fastness range between 3 and 4 (). Indeed, a good rubbing fastness indicates that the dye molecules are well fixed on the textile. Wet rubbing fastness values are lower than dry fastness values. These results are similar to those found in the work of Dhanania et al. (Dhanania, Singhee, and Samanta Citation2022) which have shown that the addition of water facilitates the dissolution of the poorly fixed dye resulting in a decrease in the wet rubbing fastness.
Conclusion
The extracted dye from Corchorus olitorius leaves was used for cotton fabric dyeing. The influence of different experimental conditions on dyeing properties was examined. A regression equation of the color strength was established. Optimal operating conditions were found at a dyeing temperature of 90°C, a dyeing time of 90 min, and a pH of 9. The theoretical color strength of dyed fabric was estimated to be near 2.3779 with an overall desirability value of 0.993. The dyed samples, especially alum pre-mordanted dyed cotton fabric, showed better fastness properties and color strength. In fact, wash fastness and light fastness were improved and rubbing fastness was globally good with an effective K/S of 3.10. Therefore, the obtained natural dye from Corchorus olitorius leaves can be used as a potential alternative to conventional synthetic dyes to make a green dyeing industry.
Highlights
An ecological process of the dye extraction from Corchorus olitorius leaves was developed using ultrasonic methods.
The dyeing conditions of cotton fabrics using corchorus olitorius extracts were optimized using an experimental plan.
The color strength model was well predicted using pH, dyeing temperature and the dyeing duration.
The best dyeing performance improving the fastness properties was achieved using aluminum sulfate as a mordant.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adeel, S., S. Rafi, M. Salman, F. Rehman, and S. Abrar. 2017. Potential resurgence of natural dyes in applied fields. In Plant-based natural products: Derivatives and applications, ed. Shahid Ul-Islam, 1–14. Wiley-Scrivener. doi: 10.1002/9781119423898.ch1.
- Amutha, K., S. Grace Annapoorani, and N. Sudhapriya. 2020. Dyeing of textiles with natural dyes extracted from Terminalia arjuna and thespesia populnea fruits. Industrial Crops and Products 148:112303. doi:10.1016/j.indcrop.2020.112303.
- Ashis Kumar, S., and A. Konar. 2011. Dyeing of textiles with natural dyes. Natural Dyes: 15-28 Academic Editor: InTech. doi:10.5772/21341.
- Baaka, N., A. Mahfoudhi, and M. F. Mhenni. 2018. Tannin-rich natural dye extracted from kermes oak (Quercus coccifera L.): Process optimization using response surface methodology (RSM). Journal of Natural Fibers 16 (8):1209–20. doi:10.1080/15440478.2018.1458685.
- Bechtold, T., A. Turcanu, E. Ganglberger, and S. Geissler. 2003. Natural dyes in modern textile dyehouses — how to combine experiences of two centuries to meet the demands of the future? Journal of Cleaner Production 11 (5):499–509. doi:10.1016/s0959-6526(02)00077-x.
- Ben Fadhel, A., W. Miled, W. Haddar, and N. Meksi. 2021. Clean printing process of cotton with natural dyes: Effect of paste formulation components on printing performances. Chemical Industry and Chemical Engineering Quarterly 27 (1):1–13. doi:10.2298/ciceq191004019b.
- Ben Yakoub, A. R., M. A. Benabderrahim, and A. Ferchichi. 2016. Physiological and agromorphological responses of tossa jute (Corchorus olitorius L.) to drought stress. Journal of Plant Physiology & Pathology 4 (3). doi:10.4172/2329-955x.1000152.
- Bhouri, N., F. Debbabi, A. Merghni, E. Rohleder, B. Mahltig, and S. Ben Abdessalem. 2022. New manufacturing process to develop antibacterial dyed polyethylene terephthalate sutures using plasma functionalization and chitosan immobilization. Journal of Industrial Textiles 51 (4):6353S–76S. doi:10.1177/15280837211050525.
- Bouatay, F., N. Meksi, S. Adeel, F. Salah, and F. Mhenni. 2016. Dyeing behavior of the cellulosic and jute fibers with cationic dyes: Process development and optimization using statistical analysis. Journal of Natural Fibers 13 (4):423–36. doi:10.1080/15440478.2015.1043685.
- Bruna, D. C., V. Camargo, and M. M. M. Aparecida. 2013. Azo dyes: Characterization and toxicity– a review. Textiles and Light Industrial Science and Technology 2 (2):85–103.
- Dhanania, Y., D. Singhee, and A. K. Samanta. 2022. Optimization of dyeing process variables for eco-friendly dyeing of cotton fabric with babul bark extract as a natural dye and gallnut extract as a bio-mordant. Journal of Natural Fibers 19 (13):5478–95. doi:10.1080/15440478.2021.1875955.
- Faidi, K., N. Baaka, S. Hammami, R. El-Mokni, Z. Mighri, and M. F. Mhenni. 2016. Extraction of carotenoids from lycium ferocissimum fruits for cotton dyeing: Optimization survey based on a central composite design method. Fibers and Polymers 17 (1):36–43. doi:10.1007/s12221-016-5424-0.
- Ferda, E., and A. Onal. 2015. Dyeing of wool and cotton with extract of the nettle (Urtica dioica L.) leaves. Journal of Natural Fibers 12 (3):222–31. doi:10.1080/15440478.2014.918008.
- Friedman, M., and H. S. Jürgens. 2000. Effect of pH on the stability of plant phenolic compounds. Journal of Agricultural and Food Chemistry 48 (6):2101–10. doi:10.1021/jf990489.
- Ghorpade, B., M. Darrekar, and P. S. Vankar. 2000. Ecofriendly cotton dyeing with sappan wood dye using ultrasound energy. Colourage 47 (1):27–30.
- Goula, A. M., M. Ververi, A. Adamopoulou, and K. Kaderides. 2017. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrasonics Sonochemistry 34:821–30. doi:10.1016/j.ultsonch.2016.07.022.
- Guesmi, A., N. Ladhari, N. Ben Hamadi, M. Msaddek, and F. Sakli. 2013. First application of chlorophyll-a as biomordant: Sonicator dyeing of wool with betanin dye. Journal of Cleaner Production 39:97–104. doi:10.1016/j.jclepro.2012.08.029.
- Gulzar, T., S. Adeel, I. Hanif, F. Rehman, R. Hanif, M. Zuber, and N. Akhtar. 2015. Eco-friendly dyeing of gamma ray induced cotton using natural quercetin extracted from acacia bark (A. nilotica). Journal of Natural Fibers 12 (5):494–504. doi:10.1080/15440478.2014.964445.
- Handoussa, H., R. Hanafi, I. Eddiasty, M. El-Gendy, A. El Khatib, M. Linscheid, L. Mahran, and N. Ayoub. 2013. Anti-inflammatory and cytotoxic activities of dietary phenolics isolated from Corchorus olitorius and Vitis vinifera. Journal of Functional Foods 5 (3):1204–16. doi:10.1016/j.jff.2013.04.003.
- Hunger, K. 2003. Industrial dyes: Chemistry, properties, applications. Wiley-VCH: Verlag GmbH & Co. doi:10.1002/352760201.
- Ikyo, B. A., D. E. Enenche, S. Omotosho, M. Ofeozo, and T. T. Rotimi. 2020. Spectroscopic analysis of the effect of organic and inorganic fertilizers on the chlorophylls pigment in amaranth and jute mallow vegetables. Nigerian Annals of Pure and Applied Sciences: COVID-19 and Nigeria Scientists 3 (1):115–21. doi:10.46912/napas.168.
- Ismail, E. H., A. M. A. Saqer, E. Assirey, A. Naqvi, and R. M. Okasha. 2018. Successful green synthesis of gold nanoparticles using a Corchorus olitorius extract and their antiproliferative effect in cancer cells. International Journal of Molecular Sciences 19 (9):2612. doi:10.3390/ijms19092612.
- Jabar, J. M., A. I. Ogunmokun, and T. A. A. Taleat. 2020. Color and fastness properties of mordanted ridelia ferruginea B dyed cellulosic fabric. Fashion and Textiles 7 (1):1–13. doi:10.1186/s40691-019-0195-z.
- Jordeva, S., M. Kertakova, S. Zhezhova, S. Golomeova Longurova, and K. Mojsov. 2020. Dyeing of textiles with natural dyes. Tekstilna industrija 68 (4):12–21. doi:10.5937/tekstind2004012J.
- Kamboj, A., S. Jose, and A. Singh. 2022. Antimicrobial activity of natural dyes – a comprehensive review. Journal of Natural Fibers 19 (13):5380–94. doi:10.1080/15440478.2021.1875378.
- Kang, Y., R. J. Park, S. K. Jung, and Y. H. Chang. 2018. Synthesis, characterization, and functional properties of chlorophylls, pheophytins, and Zn-pheophytins. Food Chemistry 245 (15):943–50. doi:10.1016/j.foodchem.2017.11.079.
- Loumerem, M., and A. Alercia. 2016. Descriptors for jute (Corchorus olitorius L.). Genetic Resources and Crop Evolution 63 (7):1103–11. doi:10.1007/s10722-016-0415-y.
- Mahdi, M. M., F. T. Zohra, and S. Ahmed. 2021. Dyeing of shoe upper leather with extracted dye from acacia nilotica plant bark-an eco-friendly initiative. Progress in Color, Colorants and Coatings 14:241–58. doi:10.30509/PCCC.2020.166673.1074.
- Mansour, H. F., and S. Heffernan. 2011. Environmental aspects on dyeing silk fabric with sticta coronata lichen using ultrasonic energy and mild mordants. Clean Technologies Environmental Policy 13 (1):207–13. doi:10.1007/s10098-010-0296-2.
- Moussa, I., N. Baaka, R. Khiari, A. Moussa, G. Mortha, and M. F. Mhenni. 2018. Application of Prunus amygdalus by-products in eco-friendly dyeing of textile fabrics. Journal of Renewable Materials 6 (1):55–67. doi:10.7569/jrm.2017.634141.
- Oboh, G., A. O. Ademiluyi, A. J. Akinyemi, T. Henle, A. Saliu, and U. Schwarzenbolz. 2012. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. Journal of Functional Foods 4 (2):450–58. doi:10.1016/j.jff.2012.02.003.
- Özlenen, E., and İ. L. Yıldırım. 2019. Metal mordants and biomordants. The impact and prospects of green chemistry for textile technology. In The textile institute book series, ed. Shahid-Ul-Islam and B. S. Butola, 57–82. Elsevier Ltd. doi: 10.1016/C2017-0-01957-2.
- Punyacharoennon, P., V. Deerattrakul, and K. Luepong. 2021. The influence of pH, temperature and time on dyeing of silk fabric by black bean anthocyanin-rich extract as colorant. Progress in Color, Colorants and Coatings 14 (3):179–86. doi:10.30509/PCCC.2021.81715.
- Rehman, F., S. Adeel, A. T. Ahmad, A. Mateen, and N. Amin. 2022. Statistical optimization of parameters for eco-friendly dyeing of cotton using direct red 31 dye. Journal of Natural Fibers 19 (14):7491–501. doi:10.1080/15440478.2021.1951420.
- Saxena, S., and A. Raja. 2014. Natural dyes: Sources, chemistry, application and sustainability issues. In Roadmap to sustainable textiles and clothing. Textile science and clothing technology, ed. S. Muthu, 37–80. Singapore: Springer. doi:10.1007/978-981-287-065-0_2.
- Sengupta, S., P. Ghosh, and I. Mustafa. 2022. Properties of poly-vinyl alcohol bonded jute (Corchorus olitorius) nonwoven fabric and its performance as disposable carry bag. Journal of Natural Fibers 19 (6):2034–52. doi:10.1080/15440478.2020.1798842.
- Sivakumar, V., J. Vijaeeswarri, and J. Lakshmi Anna. 2011. Effective natural dye extraction from different plant materials using ultrasound. Industrial Crops and Products 33 (1):116–22. doi:10.1016/j.indcrop.2010.09.007.
- Tiwari, B. K. 2015. Ultrasound: A clean, green extraction technology. Trends in Analytical Chemistry 71:100–09. doi:10.1016/j.trac.2015.04.013.
- Uddin, M. G. 2015. Extraction of eco-friendly natural dyes from mango leaves and their application on silk fabric. Textiles and Clothing Sustainability 1 (1):1–8. doi:10.1186/s40689-015-0007-9.