ABSTRACT
Laccase-catalyzed coloration toward wool was carried out and the K/S of wool fabric dyed was much higher than raw wool, presenting a claybank color. On this basis, the reaction mechanism is elucidated using tyrosine, tryptophan and the mixture as substrates. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and LC-MS as well as FT-IR measurements were used to study the reactions. The results indicate that the laccase-catalyzed reaction of tyrosine monomer, tyrosine monomer and the mixture, respectively, generates free radicals, including phenoxy radical and benzene radicals, pyrrolidine radical. The coupling reaction can occur between two free radicals, and further the Michael addition reaction occurs between benzene radicals with monomer another tyrosine. In view of the tan polytyrosine and rufous polytryptophan, as well as the higher content of tyrosine than tryptophan, the oxidation coupling reaction of tyrosine residue predominates over that of tryptophan residue in the process of laccase-catalyzed coloration toward wool.
摘要
对羊毛进行了漆酶催化染色,染色后的羊毛织物的K/S远高于原毛,呈现粘土班克色. 在此基础上,以酪氨酸、色氨酸和混合物为底物阐明了反应机理. 使用基质辅助激光解吸/电离飞行时间质谱(MALDI-TOF MS)和LC-MS以及FT-IR测量来研究反应. 结果表明,漆酶催化酪氨酸单体、酪氨酸单体和混合物的反应分别产生自由基,包括苯氧基和苯自由基、吡咯烷自由基. 偶联反应可以发生在两个自由基之间,进一步的迈克尔加成反应发生在苯自由基与单体另一个酪氨酸之间. 考虑到棕褐色多酪氨酸和红褐色多隐球菌,以及酪氨酸含量高于色氨酸,在漆酶催化的羊毛染色过程中,酪氨酸残基的氧化偶联反应占主导地位.
Introduction
In recent years, tremendous efforts have been focused on saving water and energy in the textile industry due to environmental pressure. Some new dyeing methods have been developed such as microwave, ultrasonic, ultraviolet gamma, enzymes (Adeel et al. Citation2021, Citation2022, Citation2022; Kamran, Adeel, and Rehman Citation2022; Zuber et al. Citation2020). Enzymes have been widely used in textile processing owing to the comparatively mild conditions, effective and eco-friendly, resulting in the extensively potential for industrial applications (Enaud et al. Citation2010; Kaur, Chakraborty, and Dubey Citation2016; Madhu and Chakraborty Citation2017; Thakur et al. Citation2012). The commonly used enzymes in textile processes are amylases, catalase, cellulase and laccase (Baek et al. Citation2021; Schückel, Matura, and van Pée Citation2011). Among the interesting features of oxidative reactions catalyzed by laccases possess broad substrate specificity and involvement of molecular oxygen as the final electron acceptor, thus gaining interest as substitutes of chemicals. Laccases (EC 1.10.3.2), as a class of multi-copper oxidoreductases, are mainly obtained from bacteria and fungi. Owing to the high redox property, laccase was widely used as biocatalysts to degrade the organic pollutant (Claus, Faber, and König Citation2002) and oxidate the polymerization of organic molecule. For the latter, various radicals formed during the reactions can undergo coupling reactions, resulting in colorful polymers and achieving dye-free dyeing process. Although both bio-mordant (Adeel et al. Citation2019) and laccase in our work have a role in dyeing, the mechanisms of them are also very different. Laccase is used as a catalyst to catalyze the polymerization of phenols, which are further used to dyeing. While bio-mordants depend on chelating effect. In general, small colorless aromatic compounds such as diamines, aminophenols, aminonaphtols and phenols can be oxidized by laccase to aryloxy-radicals, which may undergo further non-enzymatic reactions resulting in colored oligomeric, dimeric and polymeric products (Bai et al. Citation2018; Nong et al. Citation2020; Polak et al. Citation2016, Citation2020; Sousa, Martins, and Robalo Citation2013). Phenols being the common substrates for laccase can be oxidized into reactive radicals that can further undergo polymerization (Bai et al. Citation2015, Citation2016; Hadzhiyska et al. Citation2006; Lam et al. Citation2019; Pezzella et al. Citation2016; Wang et al. Citation2018). Besides, many colorants can be produced through laccase-catalyzed oxidation of phenolic compounds or related colorless precursors followed by further polymerization, leading to the formation of a polymeric chromogen structure.
Considering most plant components containing phenolic hydroxyl groups, many studies have reported that natural plant phenols were oxidized by laccase, and further for the dyeing of textiles (Atav, Buğdaycı, and Yakın Citation2022; Montazer et al. Citation2009). Some of the most interesting natural plant compounds are flavonoids (Kim and Cavaco-Paulo Citation2012; Kim et al. Citation2008; Kim, Moldes, and Cavaco-Paulo Citation2007). In these researches, flavonoids could be polymerized under laccase-catalyzed oxidation to form generally dark brown in color and graft onto cotton fabric or linen fabric, thus improving the dyeing fastness (Fu et al. Citation2015). Therefore, the topic on the formation of various pigments by laccase-catalyzed and further applied to textile dyeing has always been the focus of ecological textile research. And the biological dyeing is carried out always by one-bath method, namely, laccase polymerization and fiber dyeing are performed at the same time.
In addition, the macromolecule peptide chain of protein fiber contains amino group, carboxyl group and phenolic hydroxyl group as well as side group. We focus on the modification and dyeing of protein fibers by laccase (Jia et al. Citation2018, Citation2018). Compared to the strategy mentioned above, the protein fabrics treated with laccase alone contribute to energy-saving and environmental protection. During the past few years, we have been targeting ecological dyeing of protein fibers with laccase in an attempt to optimize technology conditions. However, most studies on enzymatic coloration focus on process regulation. Although the pathway and mechanism of laccase-catalyzed oxidative cross-linking reaction have been investigated in the field of food chemistry (Li et al. Citation2021; Li, Su, and Cavaco-Paulo Citation2021; Mattinen et al. Citation2005, Citation2006; Niku-Paavola et al. Citation1988; Permana et al. Citation2018), the mechanism of dye fixation remains unclear and rather empirical, especially in the case of protein fiber. Therefore, it is crucial to understand enzymatic reactions on amino acid at the molecular level. Herein, inspired by previous outstanding work and the dyeing performance of wool in the presence of laccase, the reaction mechanism was elucidated using free amino acids and the mixture as substrates. The results indicated the dyeing of wool attribute to the coupling reaction and the Michael addition reaction between free radicals formed by laccase. Besides, the oxidation coupling reaction of tyrosine residue is the main and that of tryptophan residue is the secondary in the process of laccase-catalyzed coloration toward wool.
Materials and methods
Materials
All wool fabrics (100%, 220 g∙m−2) used in this study were available in the market. The fabrics were washed with ethanol and then rinsed with deionized water. Laccase (EC 1.10.3.2) from Trametes versicolor was supplied by Novozymes, Denmark. Tyrosine, tryptophan and ABTS were purchased from Sigma-Aldrich. All other chemicals used were of analytical grade and without further purification.
Characterizations
The colorimetric assay of the dyed wool fabrics was evaluated using a Datacolor 650 spectrophotometer having an illuminant that includes the UV component and excludes the specular component. CIELAB data of the dyed fabrics were recorded. Each fabric was measured four times, and the average value was used. The dyed wool fabric was prepared by the same method. Fiber slices with a certain thickness (10–30 µm) were prepared with a Y172 fiber micrograph and placed on glass slide. An optical microscope equipped with Nikon Optiphot-POL lens was used to observe the color uniformity of the dyed fibers. The infrared spectra of polymers were detected at a wavelength from 4000 to 600 cm−1 using a Thermo Nicolet iS10 spectrometer.
MALDI-TOF-MS
MALDI-TOF was used to detect the molecular mass of oxidative products using a Bruker ultraflextreme MALDI-TOF Mass Spectrometer (Bruker AXS Co., Germany). The oxidative products of tyrosine, tryptophan and the mixture of tyrosine/tryptophan catalyzed by laccase were dissolved in DMF resulting a 1 mg∙mL−1 solution. Using sinapic acid (the saturated solution dissolved in 30% acetonitrile and 0.1% tetrahydrofuran) as the matrix, the samples were mixed with it the volume ratio of 1:1 (1 uL each). The sample/matrix mixtures were deposited on a steel target matched with MALDI followed by air-drying at room temperature. The dried samples were conducted of positive-ion mass. An N2 laser with a wavelength of 337 nm was performed using an RP700–3500 reflection mode. Positive ion spectroscopy was resolved with flexControl software. The analysis software was flexAnalysis Version 3.3.
LC-MS
The product was dissolved in 5 mol∙L−1 ammonia solution and tested by liquid chromatography/mass spectrometry (LC-MS). The standard atmospheric pressure ionization (API) mass spectrometry experiments were performed using an Agilent 1290B, and the mass spectrometry was ABsciex QTRAP5500. Separation of samples was carried out on an Agilent C18 column (100 mm, 2.1 um) at the column temperature of 30°C, with a flow rate of 1.0 mL∙min−1. The elution solvents consisted of 15% methanol and acetic acid-triethylamine buffer (pH ≈ 7) (85%). The elution was monitored at λ 254 nm.
Measurement of laccase activity
The activity of laccases was determined spectrophotometrically at 420 nm with ABTS as substrate. 0.5 mmol∙L−1 ABTS solution was prepared and preheated at a certain temperature. 2.9 mL ABTS solution was fully mixed with 0.1 mL enzyme solution. The absorbance of ABTS was measured at 420 nm, and every 0.05 absorbance value recorded. In order to ensure the accuracy, it is necessary to try different concentrations of laccase solutions. One unit of laccase (U) is defined as the amount of laccase required to oxidize 1 μmol of ABTS per min.
Chromogenic in-situ of wool catalyzed by laccase
The dyeing process of wool fabric is consistent with the literature (Jia et al. Citation2018), and the details are as follows: wool fabrics were added into conical flask, and 50 mL 0.1 mol∙L−1 acetate buffer (pH 5.0) was added with bath ratio of 50:1. Then, laccase or inactivated laccase was added, respectively. Then, the clean wool fabric was immersed into the solution and kept for 24 h at 50°C. After the reaction, the samples were taken out and washed twice at 50°C, then washed with cold water and finally dried for testing. In addition, the inactivation laccase was formed by boiling laccase for 10 min.
Amino acid catalyzed by laccase
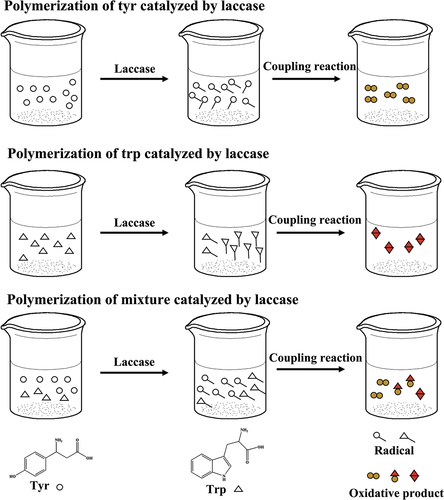
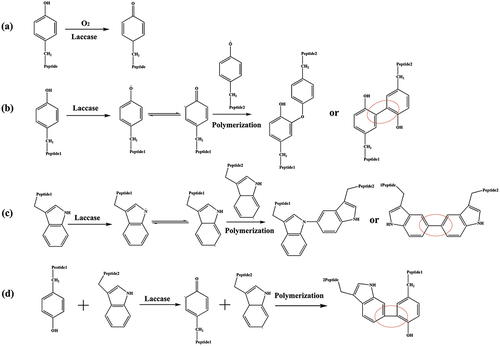
The 83 mL 20 g∙L−1 prepared laccase solution was added to conical flask containing 17 mL amino acid solution (0.5 g∙L−1 tyrosine, 6 g∙L−1 tryptophan or 1:1 tyrosine-tryptophan mixture, respectively). The solution was kept for 24 h at 50°C. After the reaction, the precipitation generated was washed by buffer solution repeatedly to remove the unreacted tyrosine or tryptophan monomer, and then dried it for testing ().
Results and discussion
Measurement of laccase activity
According to different absorbance value of 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) radical solution, the standard curve of ABTS radical concentration and absorbance value is diagrammed. The standard curve equation is Y = 0.0492×-0.0022, R2 is 0.9991. According to the equation of laccase activity (LC), the laccase activity was 49.0 U∙g−1.
k = 0.0492; ΔA (A) represents changes in absorbance value. V0 (mL) represents the volumes of ABTS and enzyme solution. ΔT (s) represents the times required for the absorbance value to change. V1 (mL) represents the volumes of enzyme solution. C represents concentration of laccase.
Laccase-catalyzed coloration of wool fabrics
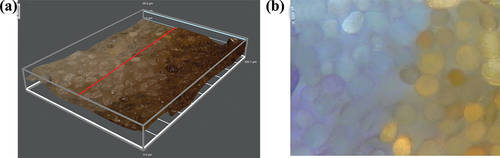
The coloration of wool fabrics treated by laccase is shown in Table S1. The color of wool fabrics is claybank after treatment. Compared to the raw wool fabrics, the C value became bigger while the h value became smaller, indicating the color changed of wool fabrics after enzymatic treatment. In fact, the laccase aqueous solution is red. In order to rule out the influence of the color of laccase solution, the wool fabric was treated by inactivation laccase, the solution of which was red. The result showed that the color parameters of wool fabric treated by deactivated laccase and untreated were similar, proving the color of wool fabric has nothing to do with the color of enzyme solution. Comparing to untreated wool fiber, the cross section has obvious brown-yellow color, showing good color uniformity (). The results indicated that the color of wool fibers not only acted on the surface of the fibers, but also penetrated into the interior of the fibers, resulting in the uniform color of the fibers.
FT-IR
The IR spectra of tyrosine and corresponding oxidative product are shown in . The peaks in the range of 3128–3462 cm−1 are assigned to the stretching vibration peak of phenolic hydroxyl group of tyrosine, while the intensity of peaks of oxidation product is weaker and the peak is wider, indicating that phenolic hydroxyl group of tyrosine structure is polymerized. The peaks at 1639, 1704 cm−1 are assigned to ν(C=O) of aromatic ketone, which indicates the phenolic hydroxyl group is oxidized to quinone structure. The absorptions at 1022 cm−1 and 1131 cm−1 attribute to ν(C-O-C) (Faix Citation1991). The formation of ether bonds belongs to coupling reaction between phenoxy radical and benzoyl carbon radical. The IR spectra of tryptophan and corresponding oxidative product are shown in . The peaks in the range of 3200–3425 cm−1 are assigned to ν(N-H) of tryptophan. After the reaction, the absorption peak at about 3200 cm−1 disappears, showing that the imine group in the tryptophan structure is involved in the formation of tertiary amine. The peaks in the range of 1585–1605 cm−1 belong to δ(N-H) in-plane bending vibration, and the double peaks become single at 1599 cm−1. The results indicate that the secondary amine in the structure of tryptophan is changed into tertiary amine, that is, the amino groups of tryptophan participate in the free radical coupling reaction because of laccase-catalyzed. The intensity of peak at 2849 cm−1 increasing indicates the benzene ring in the tryptophan structure also participates in the radical coupling reaction. In addition, the obvious absorption peaks at 1132 and 1341 cm−1 because of the C-N bond forming between benzpyrole and benzene ring.
Figure 3. (a) FT-IR spectra of tyrosine and oxidation products of tyrosine with laccase; (b) FT-IR spectra of tryptophan and corresponding oxidative product; (c) the IR spectra of the oxidative product catalyzed by laccase with different ratios of tyrosine/tryptophan mixtures.
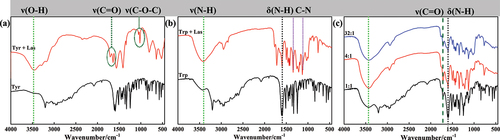
In order to further clarify if phenoxy radical generated by tyrosine can react with pyrrolinitrogen radical generated by tyrosine by coupling, the IR spectra of the oxidative products catalyzed by laccase with different ratios of tyrosine/tryptophan mixtures are shown in . The absorptions in the range of 1340–1600 cm−1 attribute to ν(C-N), while the peak at 966 cm−1 attribute to ν(C-O-O). The results show that the tyrosine and tryptophan are self-polymerized by laccase, respectively.
MALDL-TOF MS and LC-MS
MALDI-TOF MS was used to analyze the reaction products in laccase catalyzed reactions. The polymerization of the tyrosine, tryptophan and their mixture as well as the covalent linkages formed among different residues in the catalysis was studied by MALDI-TOF MS. As shown in , the main molecular weights of tyrosine oxidation products are between 200 Da and 1100 Da, representing the oligomer structures with different degree of polymerization catalyzed by laccase from the dimer to the tetramer. The molecular weight of the dimer is in the range of 200–361 Da, while the molecular weight of the trimer is in the range of 368–540 Da and the molecular weight of the tetramer is in the range of 550–718 Da. The molecular weight of tyrosine and oligomers indicates that the polymeric products with different degrees of polymerization were formed under the catalysis of laccase (Lopez-Llorca and Fry Citation1989; Mikolasch and Schauer Citation2009). We speculate that the connection modes between tyrosine monomers included Ph-Ph connection and Ph-O connection (Simat and Steinhart Citation1998).
Figure 4. (a) MALDI-TOF mass spectra of tyrosine samples treated by laccase; (b) Mass spectrogram of dityrosine samples.
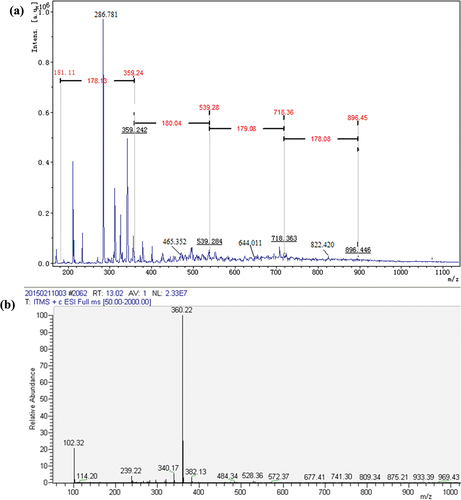
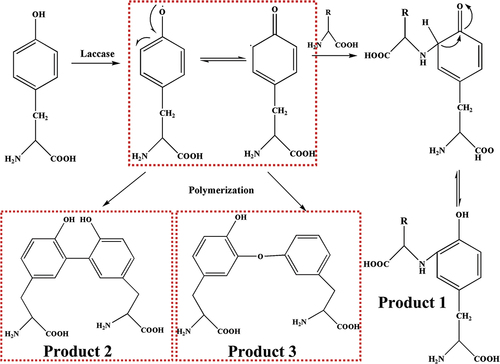
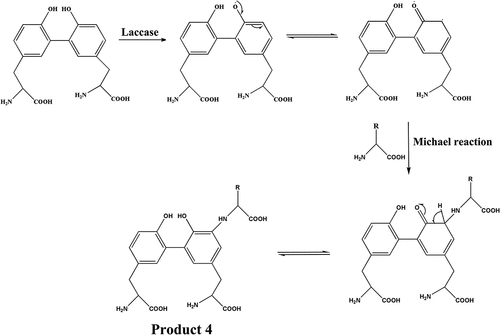
The MS analyses of tyrosine and its oxidation products were carried out. The mass-to-charge (m/z) ratio at 182 is attributed to tyrosine (Figure S1). Tyrosine oxidized by laccase shows two peaks at m/z = 360.22, probably contributing to dimer tyrosine (), namely, the phenolic hydroxyl group of tyrosine monomer is dehydrogenated in the presence of laccase to form phenoxy radical. And then the radical transfers to benzene ring to form benzene ring carbon radical (Product 1). Radical polymerization occurs between two types of radical intermediates, forming dimeric tyrosine in the form of benzene link (Product 2) and benzene oxygen link (Product 3) () (Irebo et al. Citation2007). In addition, Michael addition reactions may also occur between benzene ring carbon radicals and primary amino groups in the tyrosine structure (Product 4, ). In Figure S2, there is an obvious molecular ion peak at m/z = 541.19, which can be identified as the trityrosine (m/z = 539.60), that is, the product containing trityrosine. The reaction process can be inferred as follows: the phenol hydroxyl of a tyrosine monomer and dimer undergoes dehydrogenation forming phenolic oxygen free radicals and may be transferred to the benzene ring forming benzene carbon free radicals. The tyrosine monomer phenol oxygen radicals, monomer benzene carbon radicals, dimer phenol oxygen radicals, dimer benzene carbon radical are polymerized among them. The trityrosine is formed by C-C bonds and C-O bonds as well as Michael addition reaction (Products 5–9, Figures S3, S4) (Cannatelli and Ragauskas Citation2017; Thakur et al. Citation2012). In Figure S5, there is an obvious peak at m/z = 718.21, which can be identified as the tetratyrosine and the structural formula of the tetramer product are shown in Figure S6 (Products 10–15).
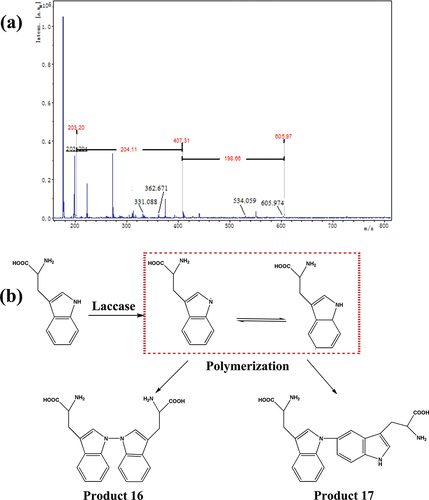
When the substrate is tryptophan, the molecular weight of polymerization products ranges from 200 to 700 Da (). The peak at m/z = 407 is assigned to ditryptophan. The peaks at m/z = 332 and 361 attribute to the dimer by elimination of -CH(NH2)(COOH) and -COOH, respectively. The peaks at m/z = 534 are assigned to tritryptophan by elimination of -CH(NH2)(COOH). Therefore, we speculate that the tryptophan can be oxidized to pyrrole nitrogen radicals in the presence of laccase, and further transfers to benzene radicals. Furthermore, the different radicals are polymerized, forming dimers (, Produces 16,17) and trimers (Produces 18–21). The connection modes include C-C connection between benzene ring carbon radical, N-N connection between pyrrole nitrogen radical, C-N connection between pyrrole nitrogen radical and benzene ring carbon radical. The possible molecular formulas are shown in Figure S7.
Figure 7. (a) MALDI-TOF mass spectra of tryptophan samples treated by laccase; (b) the proposed pathway of the enzymatic oxidation reaction of tryptophan.
When the substrate is the mixture with tyrosine : tryptophan = 1:1, MALDI-TOF mass spectra of the mixture after laccase treatment are shown in Figure S8. The m⁄z values of the main polymerization products with the corresponding presumed molecular formula are listed in Table S2. The results show that tyrosine and tryptophan could be oxidized and further polymerizate under the catalysis of laccase, respectively (Produces 22–29, Figures S9, S10). Besides, they can also have coupling reactions with each other ().
Conclusions
In this work, the chromogenic reaction mechanism of wool was elucidated using free amino acids and the mixture as substrates. The oxidized products were characterized by IR, MALDI-TOF MS and LC-MS. The results indicate that the tyrosine or tryptophan could be oxidized by laccase, generating different radicals. Besides, the radicals were coupled by free radical reaction and Michael addition reaction, isolating the dimer, trimer and tetramer. Furthermore, given the tan polytyrosine and rufous polytryptophan, as well as the higher content of tyrosine than tryptophan, the oxidation coupling reaction of tyrosine residue predominates over that of tryptophan residue in the process of laccase-catalyzed coloration toward wool.
Highlights
Laccase-catalyzed coloration toward wool was carried out.
The reaction mechanism was elucidated using tyrosine, tryptophan and the mixture as substrates.
The results indicate that the laccase-catalyzed reaction of tyrosine monomer, tyrosine monomer and the mixture, respectively, generate free radicals, further forming the polymers.
Acknowledgements
We Gratefully acknowledge financial support from Science and Technology Program of Nantong, China (JC2020126), Nantong University Doctoral priming fund (03082153), Jiangsu Province industry-university-research cooperation project (BY2020556).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adeel, S., N. Habib, F. Batool, N. Amin, T. Ahmad, S. Arif, and M. Hussaan. 2022. Environmental friendly exploration of cinnamon bark (Cinnamomum verum) based yellow natural dye for green coloration of bio-mordanted wool fabric. Environmental Progress & Sustainable Energy 41:e13794. doi:10.1002/ep.13794.
- Adeel, S., F.U. Rehman, M. K. Khosa, S. Rajab, K. M. Zia, M. Zuber, and F. Batool. 2022. Eco-friendly isolation of colorant from Arjun Bark for dyeing of bio-mordanted cotton fabric. short title: Dyeing of bio-mordanted cotton with Arjun bark colorant. Journal of Natural Fibers 19 (12):4684‒4695. doi:10.1080/15440478.2020.1870618.
- Adeel, S., F.U. Rehman, K. M. Zia, M. Azeem, S. Kiran, M. Zuber, M. Irfan, and M. A. Qayyum. 2021. Microwave-supported green dyeing of mordanted wool fabric with Arjun bark extracts. Journal of Natural Fibers 18 (1):136‒150. doi:https://doi.org/10.1080/15440478.2019.1612810.
- Adeel, S., K. M. Zia, M. Abdullah, F.U. Rehman, M. Salman, and M. Zuber. 2019. Ultrasonic assisted improved extraction and dyeing of mordanted silk fabric using neem bark as source of natural colourant. Natural Product Research 33 (14):2060‒2072. doi:10.1080/14786419.2018.1484466.
- Atav, R., B. Buğdaycı, and İ. Yakın. 2022. Laccase-catalyzed simultaneous dye synthesis and cotton dyeing by using plant extracts as dye precursor. The Journal of the Textile Institute 113 (4):628–13. doi:https://doi.org/10.1080/00405000.2021.1896159.
- Baek, N., X. Fan, J. Yuan, J. Xu, and Q. Wang. 2021. Polymerization and dyeing properties of gallic acid on silk fabric catalyzed by horseradish peroxidase. Fibers and Polymers 22 (8):2145–55. doi:https://doi.org/10.1007/s12221-021-0673-y.
- Bai, R., Y. Yu, Q. Wang, J. Yuan, and X. Fan. 2016. Effect of laccase on dyeing properties of polyphenol-based natural dye for wool fabric. Fibers and Polymers 17 (10):1613–20. doi:https://doi.org/10.1007/s12221-016-5598-5.
- Bai, R., Y. Yu, Q. Wang, J. Yuan, and X. Fan. 2018. Laccase-mediated in situ polymerization of pyrrole for simultaneous coloration and conduction of wool fabric. Textile Research Journal 88 (1):27–35. doi:https://doi.org/10.1177/0040517516673336.
- Bai, R., Y. Yu, Q. Wang, J. Yuan, X. Fan, and J. Shen. 2015. Laccase-catalyzed in-situ dyeing of wool fabric. The Journal of the Textile Institute 1:1–9. doi:10.1080/00405000.2015.1077039.
- Cannatelli, M. D., and A. J. Ragauskas. 2017. Two decades of laccases: Advancing sustainability in the chemical industry. Resource Policy 17 (1):122–40. doi:https://doi.org/10.1002/tcr.201600033.
- Claus, H., G. Faber, and H. König. 2002. Redox-mediated decolorization of synthetic dyes by fungal laccases. Applied Microbiology and Biotechnology 59 (6):672–78. doi:https://doi.org/10.1007/s00253-002-1047-z.
- Enaud, E., Trovaslet, M. Coauthors, F. Bruyneel, L. Billottet, R. Karaaslan, M. E. Sener, P. Coppens, A. Casas, I. J. Jaeger, et al. 2010. A novel azoanthraquinone dye made through innovative enzymatic process. Dyes and Pigments 85 (3):99–108. doi:10.1016/j.dyepig.2009.10.010.
- Faix, O. 1991. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 45 (s1):21–28. doi:https://doi.org/10.1515/hfsg.1991.45.s1.21.
- Fu, J., J. Su, P. Wang, Y. Yu, Q. Wang, and A. Cavaco-Paulo. 2015. Enzymatic processing of protein-based fibers. Applied Microbiology and Biotechnology 99 (24):10387–97. doi:https://doi.org/10.1007/s00253-015-6970-x.
- Hadzhiyska, H., M. Calafell, J. M. Gibert, J. M. Dagà, and T. Tzanov. 2006. Laccase-assisted dyeing of cotton. Biotechnology Letters 28 (10):755–59. doi:https://doi.org/10.1007/s10529-006-9043-5.
- Irebo, T., S. Y. Reece, M. Sjödin, D. G. Nocera, and L. Hammarström. 2007. Proton-coupled electron transfer of tyrosine oxidation: Buffer dependence and parallel mechanisms. Journal of the American Chemical Society 129 (50):15462–64. doi:https://doi.org/10.1021/ja073012u.
- Jia, W., Q. Wang, X. Fan, A. Dong, P. Wang, and J. Yuan. 2018. Laccase-mediated dye-free coloration of wool fabric. Indian Journal of Fibre & Textile Research 43:224–30. doi:10.56042/ijftr.v43i2.12902.
- Jia, W., Q. Wang, X. Fan, A. Dong, Y. Yu, and P. Wang. 2018. Mechanism and analysis of laccase-mediated coloration of silk fabrics. Fibers and Polymers 19 (4):868–76. doi:https://doi.org/10.1007/s12221-018-7553-0.
- Kamran, M., S. Adeel, and F.U. Rehman. 2022. Eco-friendly Pretreatment and dyeing of cotton with vat yellow 46. Journal of Natural Fibers 19 (14):9765‒9775. doi:10.1080/15440478.2021.1993406.
- Kaur, A., J. N. Chakraborty, and K. K. Dubey. 2016. Enzymatic Functionalization of wool for felting shrink-resistance. Journal of Natural Fibers 13 (4):437–50. doi:https://doi.org/10.1080/15440478.2015.1043686.
- Kim, S., and A. Cavaco-Paulo. 2012. Laccase-catalysed protein–flavonoid conjugates for flax fibre modification. Applied Microbiology and Biotechnology 93 (2):585–600. doi:https://doi.org/10.1007/s00253-011-3524-8.
- Kim, S., C. López, G. Güebitz, and A. Cavaco‐paulo. 2008. Biological coloration of flax fabrics with flavonoids using laccase from trametes hirsuta. Engineering in Life Sciences 8 (3):324–30. doi:https://doi.org/10.1002/elsc.200700061.
- Kim, S., D. Moldes, and A. Cavaco-Paulo. 2007. Laccases for enzymatic colouration of unbleached cotton. Enzyme and Microbial Technology 40 (7):1788–93. doi:https://doi.org/10.1016/j.enzmictec.2007.01.002.
- Lam, Y., Y. Ho, L. He, X. Wang, and J. H. Xin. 2019. Laccase-catalyzed biomimetic coloration of wool fabrics with phenols. AATCC Journal of Research 6 (1_suppl):41–44. doi:https://doi.org/10.14504/ajr.6.S1.9.
- Li, M., L. Liu, S. Kermasha, and S. Karboune. 2021. Laccase-catalyzed oxidative cross-linking of tyrosine and potato patatin- and lysozyme-derived peptides: Molecular and kinetic study. Enzyme and Microbial Technology 143:109694. doi:10.1016/j.enzmictec.2020.109694.
- Li, Y., J. Su, and A. Cavaco-Paulo. 2021. Laccase-catalyzed cross-linking of BSA mediated by tyrosine. International Journal of Biological Macromolecules 166:798–805. doi:10.1016/j.ijbiomac.2020.10.237.
- Lopez-Llorca, L. V., and S. C. Fry. 1989. Dityrosine, trityrosine and tetratyrosine, potential cross-links in structural proteins of plant-parasitic nematodes. Nematologica 35 (2):165–79. doi:https://doi.org/10.1163/002825989X00304.
- Madhu, A., and J. N. Chakraborty. 2017. Developments in application of enzymes for textile processing. Journal of Cleaner Production 145:114–33. doi:10.1016/j.jclepro.2017.01.013.
- Mattinen, M. -L., M. Hellman, P. Permi, K. Autio, N. Kalkkinen, and J. Buchert. 2006. Effect of protein structure on laccase-catalyzed protein oligomerization. Journal of Agricultural and Food Chemistry 54 (23):8883–90. doi:https://doi.org/10.1021/jf062397h.
- Mattinen, M. -L., K. Kruus, J. Buchert, J. H. Nielsen, H. J. Andersen, and C. L. Steffensen. 2005. Laccase-catalyzed polymerization of tyrosine-containing peptides. The FEBS Journal 272 (14):3640–50. doi:https://doi.org/10.1111/j.1742-4658.2005.04786.x.
- Mikolasch, A., and F. Schauer. 2009. Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Applied Microbiology and Biotechnology 82 (4):605–24. doi:https://doi.org/10.1007/s00253-009-1869-z.
- Montazer, M., F. Dadashian, N. Hemmatinejad, and K. Farhoudi. 2009. Treatment of wool with laccase and dyeing with madder. Applied Biochemistry Biotechnology 158 (3):685–93. doi:10.1007/s12010-008-8403-0.
- Niku-Paavola, M. L., E. Karhunen, P. Salola, and V. Raunio. 1988. Ligninolytic enzymes of the white-rot fungus Phlebia radiata. The Biochemical Journal 254 (3):877–84. doi:https://doi.org/10.1042/bj2540877.
- Nong, Y., Z. Zhou, J. Yuan, P. Wang, Y. Yu, Q. Wang, and X. Fan. 2020. Bio-inspired coloring and functionalization of silk fabric via laccase-catalyzed graft polymerization of arylamines. Fibers and Polymers 21 (9):1927–37. doi:https://doi.org/10.1007/s12221-020-1044-9.
- Permana, D., K. Minamihata, M. Goto, and N. Kamiya. 2018. Laccase-catalyzed bioconjugation of tyrosine-tagged functional proteins. Journal of Bioscience and Bioengineering 126 (5):559–66. doi:https://doi.org/10.1016/j.jbiosc.2018.05.013.
- Pezzella, C., S. Giacobbe, V. G. Giacobelli, L. Guarino, S. Kylic, M. Sener, G. Sannia, and A. Piscitelli. 2016. Green routes towards industrial textile dyeing: A laccase based approach. Journal of Molecular Catalysis B, Enzymatic 134:274–79. doi:10.1016/j.molcatb.2016.11.016.
- Polak, J., A. Jarosz-Wilkołazka, K. Szałapata, M. Grąz, and M. Osińska-Jaroszuk. 2016. Laccase-mediated synthesis of a phenoxazine compound with antioxidative and dyeing properties – the optimisation process. New Biotechnology 33 (2):255–62. doi:https://doi.org/10.1016/j.nbt.2015.09.004.
- Polak, J., K. Wlizło, R. Pogni, E. Petricci, M. Grąz, K. Szałapata, M. Osińska-Jaroszuk, J. Kapral-Piotrowska, B. Pawlikowska-Pawlęga, and A. Jarosz-Wilkołazka. 2020. Structure and bioactive properties of novel textile dyes synthesised by fungal Laccase. International Journal of Molecular Sciences 21 (6):2052. doi:10.3390/ijms21062052.
- Schückel, J., A. Matura, and K. -H. van Pée. 2011. One-copper laccase-related enzyme from marasmius sp.: Purification, characterization and bleaching of textile dyes. Enzyme and Microbial Technology 48 (3):278–84. doi:https://doi.org/10.1016/j.enzmictec.2010.12.002.
- Simat, T. J., and H. Steinhart. 1998. Oxidation of free tryptophan and tryptophan residues in peptides and proteins. Journal of Agricultural and Food Chemistry 46 (2):490–98. doi:https://doi.org/10.1021/jf970818c.
- Sousa, A. C., L. O. Martins, and M. P. Robalo. 2013. Laccase-catalysed homocoupling of primary aromatic amines towards the biosynthesis of dyes. Advanced Synthesis & Catalysis 355 (14–15):2908–17. doi:https://doi.org/10.1002/adsc.201300501.
- Thakur, S., H. Patel, S. Gupte, and A. Gupte. 2012. Laccases: The biocatalyst with industrial and biotechnological applications. In Microorganisms in Sustainable Agriculture and Biotechnology, ed. P. Anil, T. Satyanarayana, and B. N. Johri, 309–42. Springer Netherlands.
- Wang, F., J. Gong, X. Zhang, Y. Ren, and J. Zhang. 2018. Preparation of biocolorant and eco-dyeing derived from polyphenols based on laccase-catalyzed oxidative polymerization. Polymers 10 (2):196. doi:https://doi.org/10.3390/polym10020196.
- Zuber, M., S. Adeel, F.U. Rehman, F. Anjum, M. Muneer, M. Abdullah, and K. M. Zia. 2020. Influence of microwave radiation on dyeing of bio-mordanted silk fabric using Neem Bark (Azadirachta indica)-based tannin natural dye. Journal of Natural Fibers 17 (10):1410‒1422. doi:https://doi.org/10.1080/15440478.2019.1576569.