ABSTRACT
Natural leather still represents great importance among the materials used by humans in modern times, although it is the oldest-known fiber used by humans. Adding new features to the leather, such as bacteria and fungi resistance, electrical conductivity and magnetic properties, is an ambitious goal for leather developers. In our study, production and characterization of anti-microbial sole leather coated with zinc oxide nanoparticles were achieved. For this purpose, the ZnONPs have been prepared through green synthesis and the prepared nanomaterial loaded on the sole leather by a padding mangle. The surface morphology of the nano coated sole leather was studied using a scanning electron microscope (SEM). Antimicrobial performance of the treated leather to some microorganisms was evaluated by applying the qualitative agar disc diffusion method. The SEM image of the treated leather samples showed successive leather coating, and the nanoparticles completely adhere to the leather surface. The nano-treated leather samples showed significant resistance to the tested bacteria and fungi. Results are promising for the utilization of zinc oxide nanoparticles as additives for sole leather coating. Accordingly, the study succeeded in producing a natural microorganism-resistant sole leather, which can be used in the production of healthy and microorganisms-resistant shoes.
摘要
天然皮革在现代人类使用的材料中仍然占有重要地位,尽管它是已知人类使用的最古老的纤维. 为皮革添加新的功能,如抗细菌和真菌、导电性和磁性,是皮革开发商的一个雄心勃勃的目标. 在我们的研究中,实现了用氧化锌纳米颗粒涂覆的抗微生物鞋底革的生产和表征. 为此,通过绿色合成制备了ZnONPs,并通过填充机将制备的纳米材料负载在鞋底皮革上. 采用扫描电子显微镜(SEM)对纳米涂层鞋底革的表面形貌进行了研究. 采用定性琼脂扩散法评价了处理后皮革对某些微生物的抗菌性能. 处理后的皮革样品的SEM图像显示连续的皮革涂层,并且纳米颗粒完全粘附在皮革表面. 纳米处理的皮革样品对测试的细菌和真菌表现出显著的耐药性. 研究结果对纳米氧化锌作为鞋底涂料添加剂的应用前景广阔. 因此,本研究成功地生产出了一种天然的抗微生物鞋底革,可用于生产健康、抗微生物的鞋.
Introduction
Hides and skins are the most important by-products of the massacres, representing a high economic value if it is converted into natural leather. Converting hides and skins into leather involves economic importance in addition to its environmental benefits. It well-known that leather goods are economic products and a source of hard currency for several countries around the world. Leather-based products are broadly traded goods across the globe. Therefore, the development of these products has a great economic benefit.
According to the international trade center, the global trade of raw skin, hides and leather for 2019 was 20,351,371 and 19,535,920 thousand US, respectively (Map Citation2020). Nano-biotics have been developed due to the quick development in nanotechnology especially nano-metal oxides which having special and important features. Several methods developed to prepare these nano oxides, including chemical, physical and biological methods. However, eco-friendly green methods are greatest preferred. In the leather industry, nanotechnology is in its infancy and has not reached wide applications. However, there is an important advantage to using nano metal oxides in leather goods such as iron nano oxide (Fe3O4) which imparts the leather paramagnetic properties (Thanikaivelan, Murali, and Krishnaraj Citation2016). Wilson, Ragothaman, and Palanisamy (Citation2021) synthesized a bimetallic Cu–Fe oxide nanoparticles and coated them over the leather surface using poly vinyl alcohol PVA to obtain electrically conductive and magnetically active bifunctional leather (Wilson, Ragothaman, and Palanisamy Citation2021). Velmurugan et al. (Citation2014) prepared silver and gold nanoparticles using a natural polymer pine gum solution as the reducing agent, they applied the synthesized nano-particles onto cotton fabrics and leather in order to develop antimicrobial fiber. Silver nano-particle coated cotton fabric and leather exhibited significant antibacterial activity against Brevibacterium linens (Velmurugan et al. Citation2014). Several nano-metal oxides (Ag2O, ZnO, MnO2, CuO, etc.) have great, causing cell wall distortion and the considerable binding affinity toward thiol -SH- containing groups. Consequently, it has a great ability antimicrobial effects (Muthukrishnan et al. Citation2019; Wang, Hu, and Shao Citation2017). Most nanomaterials exhibit significant inhibition of microbial growth via electrostatic attraction with the cell membrane to reduce enzymatic activity (Idrissa et al. Citation2022). On the other hand, the nanomaterial itself undergoes oxidation, consequently, increasing the oxidative stress and causing cell death (Muthukrishnan Citation2021).
The cited data explains the antimicrobial mechanism action and cytotoxicity of the nano materials. TiO2, CuO and Fe3O4 nanoparticles have a great ability to penetrate the membrane of the cell, MgO and CaO nanoparticles are high alkaline causing cell membrane damage (El Zowalaty et al. Citation2015; Ingle, Duran, and Rai Citation2014; Jin and He Citation2011; Tchamna and Lee Citation2017). In particular, ZnO nanoparticle, compared to other metal oxides, has gained more attention for their brilliant antimicrobial properties, easy obtainability and low-priced raw materials for synthesis are other significant advantages. It has excellent electrostatic attraction due to the accumulation on the bacterial membrane in addition to the formation of H2O2 which causing the generation of reactive oxygen species (Boroumand Moghaddam et al. Citation2017). Generally, the presence of nanomaterials on the surfaces of the treated leather resists the growth of microorganisms as well as self-cleaning (Habib and Mulchandani Citation2022). Moreover, it enhances the quality of the desired product and reduces the dependence on other chemicals used in the finished product (Dixit et al. Citation2015).
The use of nanotechnology in the leather industry represents hope for leather developers to manufacture high-quality leather products with unique characteristics. This goal can be achieved by manufacturing safe nanomaterials and applying them to leather in order to give them valuable properties. In this research, we applied zinc oxide nanoparticles, prepared by the green chemistry method, to the chrome-tanned leather and assessed the extent of the leather’s resistance to microorganisms in order to obtain microbial-resistant leather that can be used in various leather products, especially that are susceptible to fungal growth, such as shoes, horse accessories, etc.
Materials and methods
Materials
Chromium-tanned sole leather was delivered from a local medium tannery, Zn (NO3)2 (99.5%, from Panreac, Barcelona, Spain) and polyvinyl alcohol PVA (99% from Sigma-Aldrich, Germany) all reagents that were used in this work are fine grade and used without further purification.
Methods
Synthesis and characterization of the zinc oxide nanoparticles
The green synthesis of zinc oxide nano particle was prepared according to the method cited in our previous work (Habib and Mulchandani Citation2022). The morphological study of the nanoparticle surface was studied by Scanning Electron Microscope SEM (ZEISS-EVO 15 - UK). The structure of synthesized zinc oxide nanoparticles was tested by X-ray diffraction using a Bruker D8 Discover diffractometer operating with Cu Kα radiation (λ = 1.5406 Å), step 0.04º, time per step 1 s and 20–60 º 2θ interval.
Loading the synthesized Zinc Oxide Nanoparticles onto the sole leather surface
The sole leather pieces of thickness 0.4 mm and 1 × 2 cm 2 area were prepared to hold zinc oxide nanoparticles along with a solution containing 10 g of polymeric binder PVA, 10 g wax filler and 70 mL water and the solution was stirred at 120°C until complete dissolving. Five percent dose of the synthesized nano-oxide (based on the treated leather) was dispersed into ethanol under sonication and added dropwise to the binder solution. The samples were cut into small pieces (1 × 2 cm2) and immersed in a prepared solution for 60 min, and then the samples were passed through a padding mangle (the padding mangle is a machine used to squeeze the impregnated fiber so the chemical can be loaded in the treated fiber) and well dried in an electric oven at 70°C until the treated samples are free of moisture.
Treated leather characterization
UV–visible spectroscopy analysis
A UV–vis spectrophotometer (V-670 UV-VIS, JASCO, Japan) with a scanning range of 280 to 420 nm and a resolution of 1 nm was used to characterize zinc oxide nanoparticles.
Scanning Electron Microscopy (SEM)
The morphology of the surface of treated leather samples of the untreated leather was studied using a Scanning Electron Microscope SEM (TE-Scan – Vega 3) with an attached energy-dispersive X-ray spectrum (EDX) unit.
Fourier Transform Infrared Spectroscopy (FTIR)
The treated leather samples were analyzed using Fourier Transform Infrared (FTIR) to see if the composition of the leather remained unchanged after being coated with zinc oxide nanoparticles. A Bruker VERTEX 80/80 v (Boston, EUA) FTIR spectrometer was used. The spectrum was recorded at a wave range of 400–4000 cm−1 (Schauermann et al. Citation2013).
Antimicrobial activity of leather loaded with zinc oxide nanoparticles
Four bacterial species and four fungal species were used for the bioassay.
Antimicrobial activities of the sole leather pieces loaded with zinc oxide nanoparticles were screened by using the agar disc diffusion method (Sanghi and Verma Citation2008). One milliliter of bacterial (Escherichia coli, Salmonella enteritidis, Bacillus cereus and Staphylococcus aureus) or fungal (Aspergillus flavus, Fusarium oxysporum, F. solani and Trichophyton mentagrophytes) spore suspension was inoculated into petri dish containing 25 mL of sterile medium. Wells of 5 mm diameter were made on the agar medium, and each well was filled with a piece of a definite area of leather loaded with ZnO nanoparticles. The plates were incubated at 28°C for 5 days in the case of fungi and 37°C for 2 days in the case of bacteria. The diameters of the inhibitory zones around the wells were measured in triplicate, and the mean value for each species was calculated in millimeter and finally analyzed and photographed.
Results and discussion
Zinc oxide nanoparticles are physiochemically characterized to determine their properties such as shape, size, charge, surface, functional groups and purity. Some techniques were used to determine these properties such as ultraviolet- (UV-) visible spectroscopy, Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and X-ray diffraction (XRD) (Vaishnav et al. Citation2017). These chemical and structural characterization techniques were as follows
UV–Vis spectroscopy
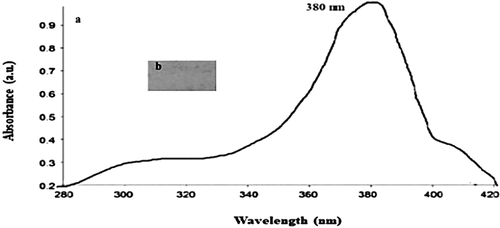
UV–visible spectroscopy in the 200–800 nm range is used to determine the optical properties of ZnONPs. ZnONPs UV Vis spectra were shown in (). The maximum absorption peak of the ZnONPs was found at 340 nm and 380 nm for sole leather coated with ZnONPs, which was similar to what was reported by Chandra et al. (Citation2019), who stated that ZnONPs typically show an absorption peak in the range of 340–380 nm, a characteristic band for pure ZnO. In UV–visible spectroscopy, a decrease in the intensity of the original extinction peak indicates particle destabilization and peak broadening or secondary peak formation at longer wavelengths can be caused by an increase in metal concentration above a threshold value or by the formation of aggregates. As a result, the stability and extent of NP aggregation can be observed (Rocha et al. Citation2018).
Fourier Transform Infrared (FT-IR) analysis
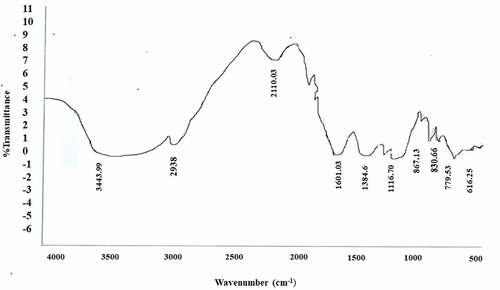
FTIR analysis was performed on the synthesized zinc oxide nanoparticles to detect the various characteristic functional groups responsible for their stabilization and coordination of the synthesized nanoparticles. The peaks represent the functional groups present in the synthesized zinc oxide nanoparticles. Metal oxide exhibits interatomic vibration absorption bands in the fingerprint region below 1000 cm−1. ZnO has vibrational peaks in the 400 to 600 cm−1 range (Abbasi et al. Citation2015). The phytochemical components present in the synthesis of ZnONPs are responsible for all of the current peaks. Different particle sizes can result in different wave numbers and frequencies.
shows that the absorption peaks correspond to metal-oxygen (ZnO stretching vibrations) vibrational mode; the peak at 3447 cm−1 indicate the presence of O–H stretch bonding, and aldehydic C–H stretching was recorded at 2938 cm−1. The peak at 1601 cm−1 indicate the presence of C=C aromatic stretch bonding, and 1484 cm−1 is ascribed to the vibrational modes of aromatic nitro compounds and alkyl C=C stretch, amide and open-chain imino. The peak at 1116 cm−1 was ascribed to the stretching vibration of C-N bond of the primary amine or to the stretching vibration of the C-O bond of the primary alcohol. C-O-C stretch was recorded at 867 cm−1, 830 cm−1, 780 cm−1 and 618 cm−1 with aromatic C-H bending. The presence of these functionals makes the synthesized zinc oxide nanoparticles as an effective antimicrobial agent. Our results for zinc oxide nanoparticles were in accordance with the results cited by (Ahmad et al. Citation2003).
This result suggests that protein molecules not only act as reducing agents but also by binding ZnONPs via free amino groups or cysteine residues or by negatively charged carboxylate groups in extracellular enzyme filtrates from fungal mycelium. Showed that it also acts as a stabilizing agent due to the electrostatic attraction of (Noorjahan et al. Citation2015).
X-ray Diffraction Technique (XRD(
XRD is a powerful technique which gives information about the structure, average size and crystalline nature of a sample. Different lattice planes cause simultaneous reflections of the X-ray beam incident on a crystal. This may lead to constructive or destructive interference depending on the angle of incidence of X-rays and wavelength of X-rays. Crystalline solid has its unique characteristic X-ray diffraction pattern which is referred to as a “fingerprint” for its identification. Sharp and narrow diffraction peaks imply high crystallinity and small size of the biosynthesized NPs (Bunaciu, Udriştioiu, and Aboul-Enein Citation2015).
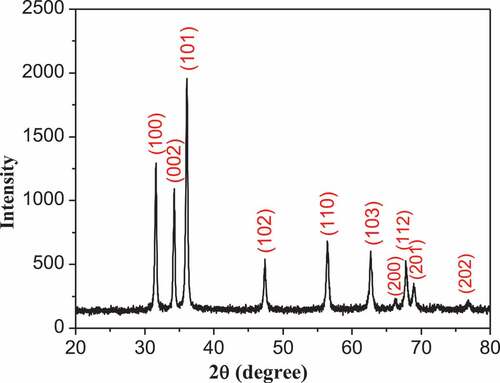
XRD pattern of ZnO nanoparticles is shown in . The formed peaks confirmed the formation of zinc oxide nanoparticles, and the formation of narrow peaks confirmed the formation of pure nanomaterials with high crystallinity and good fine size. The XRD pattern confirms the hexagonal ZnO wurtzite structure, which is the predominant crystal structure according to (JCPDS No. 89–0510). The structure shows lines with 2θ values indicating a hexagonal structure.
The diffraction peaks at 31.8°, 34.4°, 36.3°, 47.5°, 57°, 62.9°, 66.4°, 67.9°, 69° and 77°Correspond to the (100), (002), (101), (102), (110), (103), (200), (112), (201) and (202) lattice planes, respectively. These results with high intensity of (101), (100) and (002) direction planes indicate the hexagonal wurtzite ZnO crystal (in the space group P63mc, with lattice constants of a = b = 0.323 nm, c = 0.521 nm) (JCPDS 36–1451) (Ibupoto et al. Citation2013; Lin et al. Citation2014; Soni et al. Citation2013)
The nanoparticle size of ZnO could be calculated using Scherrer’s formula (Wang et al. Citation2010)
χ = K (λ/bcosθ)
where χ is the grain size, K is the Scherrer constant equal to 0.89, λ is the x-ray wavelength equal to 0.154 nm, b is the FWHM of the XRD for the observed peaks and θ is the Bragg angle [26]. The calculated nanoparticle is found to be 60 nm.
Scanning Electron Microscopy (SEM) analysis of Zinc Oxide Nanoparticles
SEM analysis is shown in . The images in the figure confirmed the formation of zinc oxide nanoscale, the image well confirmed the homogeneity of the nanoparticles, as it appears clearly that the particles are in nano-size and there are no impurities or by-products, which means that the sample has a high degree of purity.
SEM Scanning Electron Microscopy (SEM) analysis of the treated leather
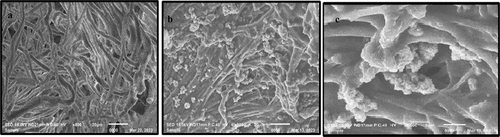
The SEM micrographs in show the surface of treated, un-treated leather sample and the cross section of the treated leather sample. The surface appears in the untreated samples as a rougher and more porous surface, and this appears clearly when compared to the surface of the samples that have been treated, where the particles appear clearly on the treated surface, which indicates the success of the process of loading them, as the images show the homogeneity of the material on the treated surface. In addition, the pores are clearly visible on the surface of the treated leather proposing that the coating formed by finishing is thin. Also, SEM images of treated leather revealed that the dimensions of pores were reduced with the finishing of the leather.
Antimicrobial activity of leather pieces loaded with zinc oxide nanoparticles
showed the antibacterial and antifungal activity of sole leather loaded with zinc oxide nanoparticles. Generally, no clear zones were observed in the case of untreated leather piece. Zone of clearance was observed in all the bacterial and fungal species. From its values, it is inferred that synthesized ZnONPs have great antimicrobial activities.
Table 1. The antimicrobial activity (IZ: inhibition zone in mm) of the leather pieces saturated with zinc oxide nanoparticles against different bacterial and fungal species.
In the case of bacteria, its strongest activity was observed against Bacillus cereus, Staphylococcus aureus, Escherichia coli and Salmonella enteritidis with inhibition zones reached 20, 18, 15 and 13 mm, respectively, while it reached 36, 33, 30 and 28 mm against the growth of Fusarium oxysporum, Aspergillus flavus, Trichophyton mentagrophytes and Fusarium solani, respectively. These findings reveal the excellent antimicrobial potential of zinc oxide nanoparticles against pathogenic bacteria and fungi.
The detailed mechanism of the bioactivity of ZnONPs is still under discussion. It is proposed by many researchers that ZnONPs show their promising antimicrobial activities via several mechanisms; ZnONPs caused damage of the permeability of the plasma membrane and loss of proton motive force as well as the release of Zn2+ ions have a significant inhibition of active transport causing damage to amino acid metabolism and the enzyme systems. Another possible mechanism for the antibacterial activity of ZnONPs is the attachment of NPs to the microbial cell membrane by electrostatic forces. The positive zeta potential of ZnONPs promotes attachment to negatively charged microbial cells, leading to entry of ZnONPs into cells, leakage of intracellular contents and ultimately cell death (Jayaseelan et al. Citation2012).
Another possible mechanism of ZnONPs antibacterial activity is induced oxidative stress which occurs in microbial cells through the interaction of Zn2+ ions with thiol groups of bacterial respiratory enzymes (Fontecha-Umaña et al. Citation2020; Sirelkhatim et al. Citation2015). Respiratory oxidative stress has been reported to damage cellular components such as proteins, lipids and nucleic acids. In addition, these free radicals can disrupt mitochondrial function, damage the electron transport chain and oxidative phosphorylation, furthermore, increased oxidative stress initiates gene expression for the production of apoptotic markers, ultimately leading to cell death (Swain et al. Citation2014). Various evidences suggest that zinc oxide nanoparticles act as promising antimicrobial agents. Similarly, the present study also supports the above fact. Interestingly, zinc oxide nanoparticles exhibited good antimicrobial activity against all the tested pathogens even at low concentration and this is because of the stability and oligo dynamic nature of the synthesized zinc oxide nanoparticles to inhibit the microbes as well as due to their large surface area-to-volume ratio (Premanathan et al. Citation2011; Rai, Yadav, and Gade Citation2009).
Few studies have shown that zinc oxide nanoparticles can destroy fungal spores by disrupting membrane integrity. Other studies have shown that zinc oxide nanoparticles interact with sulfur-containing compounds, leading to DNA and protein damage and eventual cell death. This study suggests that zinc oxide nanoparticles may have important applications and could be used as effective antifungal against harmful pathogens by inhibiting bacterial enzymes such as dehydrogenases (Suwanboon et al. Citation2013).
Conclusion
Using of nanoparticles as a friendly and sustainable alternative to control leather microbes is a promising topic for inhibiting the growth of leather fungi. ZnONPs have gained significant interest due to their unique physicochemical properties and the wide applications in diverse areas. The green synthesis methods of ZnONPs have recently gained much importance because it is simple, cheap, biocompatible, eco-friendly and easily scaled up compared to other physical and chemical methods. A sole leather loaded with nano-zinc oxide has been developed and the treated leather samples showed significant resistance against fungus and bacteria
Consent for publication
The participant has consented to the submission of this article to the journal. We confirm that the manuscript, or part of it, has neither been published nor is currently under consideration for publication. This work and the manuscript were approved by all coauthors.
Author contributions
All authors contributed in writing, editing and revising the manuscript and agreed to the published version of the manuscript.
Highlights
In our study, production and characterization of anti-microbial sole-leather loaded with zinc oxide nanoparticles were achieved.
For this purpose, the nano-zinc oxide has been prepared through green synthesis and the prepared nanomaterial loaded on the sole leather by a padding mangle.
The surface morphology of the nano coated sole leather was studied using a scanning electron microscope (SEM).
The antimicrobial performance of the treated leather to some microorganisms was evaluated by applying qualitative agar disc diffusion method. The nano-treated leather samples showed significant resistance to the tested bacteria and fungi.
The SEM image of the treated leather samples showed successive leather coating, and the nanoparticles completely adhere to the leather surface.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University for funding this work through Research Group no. RG-21-09-82.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
References
- Abbasi, T., J. Anuradha, S. U. Ganaie, and S. A. Abbasi. 2015. Gainful utilization of the highly intransigent weed ipomoea in the synthesis of gold nanoparticles. Journal of King Saud University - Science 27 (1):15–11. doi:10.1016/j.jksus.2014.04.001.
- Ahmad, A., S. Senapati, M. I. Khan, R. Kumar, R. V. Ramani, and S. M. Sastry. 2003. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 14 (7):824–28. doi:10.1088/0957-4484/14/7/323.
- Boroumand Moghaddam, A., M. Moniri, S. Azizi, R. Abdul Rahim, A. Bin Ariff, W. Zuhainis Saad, F. Namvar, M. Navaderi, and R. J. M. Mohamad. 2017. Biosynthesis of ZnO nanoparticles by a new Pichia kudriavzevii yeast strain and evaluation of their antimicrobial and antioxidant activities. Molecules 22 (6):872. doi:10.3390/molecules22060872.
- Bunaciu, A. A., E. Udriştioiu, and H. Y. Aboul-Enein. 2015. X-ray diffraction: Instrumentation and applications. Critical Reviews in Analytical Chemistry 45 (4):289–99. doi:10.1080/10408347.2014.949616.
- Chandra, H., D. Patel, P. Kumari, J. S. Jangwan, and S. Yadav. 2019. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Materials Science & Engineering 102:212–20. doi:10.1016/j.msec.2019.04.035.
- Dixit, S., A. Yadav, P. D. Dwivedi, and M. J. J. Das. 2015. Toxic hazards of leather industry and technologies to combat threat: A review. Journal of Cleaner Production 87:39–49. doi:10.1016/j.jclepro.2014.10.017.
- El Zowalaty, M. E., S. H. Ali, M. I. Husseiny, B. M. Geilich, T. J. Webster, and M. Z. Hussein. 2015. The ability of streptomycin-loaded chitosan-coated magnetic nanocomposites to possess antimicrobial and antituberculosis activities. International Journal of Nanomedicine 10:3269. doi:10.2147/IJN.S74469.
- Fontecha-Umaña, F., A. G. Ríos-Castillo, C. Ripolles-Avila, and J. J. Rodríguez-Jerez. 2020. Antimicrobial activity and prevention of bacterial biofilm formation of Silver and Zinc Oxide Nanoparticle-Containing polyester surfaces at various concentrations for use. Foods 9 (4):442. doi:10.3390/foods9040442.
- Habib, M. A., and N. J. Mulchandani. 2022. Adding value to sheep leather via coating with green synthesized Zinc Oxide Nanoparticles. Approaches for Advanced Leather Products. Journal of the Society of Leather Technologists and Chemists106 6:264–70.
- Ibupoto, Z. H., K. Khun, M. Eriksson, M. AlSalhi, M. Atif, A. Ansari, and M. Willander. 2013. Hydrothermal growth of vertically aligned ZnO nanorods using a biocomposite seed layer of ZnO nanoparticles. Materials 6 (8):3584–97. doi:10.3390/ma6083584.
- Idrissa, H., M. Habibb, A. Alakhrasa, H. J. El Khaira, and B. M. Vol. 2022. ZnO nanoparticles and their properties as surface coating materials against coronavirus. Journal of Optoelectronic and Biomedical Materials 14 (2):53–61. doi:10.15251/JOBM.2022.142.53.
- Ingle, A. P., N. Duran, and M. Rai. 2014. Rai and biotechnology 2014. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Applied Microbiology and Biotechnology 98 (3):1001–09. doi:10.1007/s00253-013-5422-8.
- Jayaseelan, C., A. A. Rahuman, A. V. Kirthi, S. Marimuthu, T. Santhoshkumar, A. Bagavan, K. Gaurav, L. Karthik, and K. V. B. Rao. 2012. Spectrochim. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 90:78–84. doi:10.1016/j.saa.2012.01.006.
- Jin, T., and Y. He. 2011. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. Journal of Nanoparticle Research 13 (12):6877–85. doi:10.1007/s11051-011-0595-5.
- Lin, J.-H., R. A. Patil, R. S. Devan, Z.-A. Liu, Y.-P. Wang, C.-H. Ho, Y. Liou, and Y.-R. Ma. 2014. Photoluminescence mechanisms of metallic Zn nanospheres, semiconducting ZnO nanoballoons, and metal-semiconductor Zn/ZnO nanospheres. Scientific Reports 4 (1). doi:10.1038/srep06967.
- Map, T. 2020. Trade Map‐Trade statistics for international business development.
- Muthukrishnan, L. J. 2021. Nanotechnology for cleaner leather production: A review. Environmental Chemistry Letters 19 (3):2527–49. doi:10.1007/s10311-020-01172-w.
- Muthukrishnan, L., M. Chellappa, A. J. Nanda, and P. B. Biology. 2019. Bio-engineering and cellular imaging of silver nanoparticles as weaponry against multidrug resistant human pathogens. Journal of Photochemistry and Photobiology B: Biology 194:119–27. doi:10.1016/j.jphotobiol.2019.03.021.
- Noorjahan, C. M., S. K. Jasmine Shahina, T. Deepika, and R. Summera. 2015. Green synthesis and characterization of Zinc Oxide Nanoparticles from Neem (Azadirachta indicia). International Journal of Science Engineering Technology 4 (30):5751–53. doi:10.1088/1402-4896/abda6c.
- Premanathan, M., K. Karthikeyan, K. Jeyasubramanian, and G. Manivannan. 2011. Selective toxicity of ZnO nanoparticles toward gram positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine: Nanotechnology, Biology and Medicine 7 (2):184–92. doi:10.1016/j.nano.2010.10.001.
- Rai, M., A. Yadav, and A. Gade. 2009. Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances 27 (1):76–83. doi:10.1016/j.biotechadv.2008.09.002.
- Rocha, F. S., A. J. Gomes, C. N. Lunardi, S. Kaliaguine, and G. S. Patience. 2018. Experimental methods in chemical engineering: Ultraviolet visible spectroscopy—UV-Vis. The Canadian Journal of Chemical Engineering 96 (12):2512–17. doi:10.1002/cjce.23344.
- Sanghi, R., and P. Verma. 2008. Biomimetic synthesis and characterization of protein Capped Silver nanoparticles. Bioresource Technology 100 (1):501–04. doi:10.1016/j.biortech.2008.05.048.
- Schauermann, S., N. Nilius, S. Shaikhutdinov, and H. Freund. 2013. Nanoparticles for heterogeneous catalysis: New mechanistic insights. Accounts of Chemical Research 46 (8):1673–81. Epub 2012 Dec 19. doi:10.1021/ar300225s.
- Sirelkhatim, A., S. Mahmud, A. Seeni, N. H. M. Kaus, L. C. Ann, S. K. M. BakhoriHasan, and H. Mohamad. 2015. Review on Zinc Oxide Nanoparticles: Antibacterial activity and toxicity mechanism. Nano Micro Letters 7 (3):219–42. doi:10.1007/s40820-015-0040-x.
- Soni, B., M. Deshpande, S. Bhatt, N. Garg, and S. Chaki. 2013. Studies on ZnO nanorods synthesized by hydrothermal method and their characterization. Journal of Nano-And Electronic Physics 5:4077–4071.
- Suwanboon, S., P. Amornpitoksuk, A. Sukolrat, and N. Muensit. 2013. Optical and photocatalytic properties of La-doped ZnO nanoparticles prepared via precipitation and mechanical milling method. Ceramics International 39 (3):2811–19. doi:10.1016/j.ceramint.2012.09.050.
- Swain, P., S. K. Nayak, A. Sasmal, T. Behera, S. K. Barik, S. K. Swain, S. S. Mishra, A. K. Sen, J. K. Das, and P. Jayasankar. 2014. Antimicrobial activity of metal-based nanoparticles against microbes associated with diseases in aquaculture. World Journal of Microbiology & Biotechnology 30 (9):2491–502. doi:10.1007/s11274-014-1674-4.
- Tchamna, R., and M. J. Lee. 2017. Constraint handling optimal PI control of open-loop unstable process: Analytical approach. Korean Journal of Chemical Engineering 34 (12):3067–76. doi:10.1007/s11814-017-0219-6.
- Thanikaivelan, P., R. Murali, and K. J. R. A. Krishnaraj. 2016. Magnetic leathers. RSC Advances 6 (8):6496–503. doi:10.1039/C5RA21909D.
- Vaishnav, J., V. Subha, S. Kirubanandan, M. Arulmozhi, S. Renganthan, and J. Optoelectron. 2017. Green synthesis of zinc oxide nanoparticles by celosia argentea and its characterization. Journal of Optoelectronics and Biomedical Materials 9 (1):59–71.
- Velmurugan, P., S. M. Lee, M. Cho, J. H. Park, S. K. Seo, H. Myung, K. S. Bang, and B.T. Oh and biotechnology. 2014. Antibacterial activity of silver nanoparticle-coated fabric and leather against odor and skin infection causing bacteria. Bioprocess and Biosystems Engineering 98 (19):8179–89. doi:10.1007/s00253-014-5945-7.
- Wang, D., Z. Chen, D. Wang, N. Qi, J. Gong, C. Cao, and Z. Tang. 2010. Tang, Positron annihilation study of the interfacial defects in ZnO nanocrystals: Correlation with ferromagnetism. Journal of Applied Physics 107 (2):023524. doi:10.1016/j.jallcom.2019.152799.
- Wang, L., C. Hu, and L. J. I. J. O. N. Shao. 2017. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. International Journal of Nanomedicine 12 (1227):s1227–1249. doi:10.2147/IJN.S121956.
- Wilson, N. H., M. Ragothaman, and T. J. Palanisamy. 2021. Bimetallic Copper–Iron Oxide Nanoparticle-coated leathers for lighting applications. Acs Applied Nano Materials 4 (4):4055–69. doi:10.1021/acsanm.1c00388.