ABSTRACT
This study aimed to establish a sustainable fabrication method for packaging paper using a Potassium-based delignification alkali; banana peel lye (BPL) to pulp banana stem fibers. This was an experimental study where Caustic soda was used as a positive control. The packaging paper was constructed using a handmade craft method described by TAPPI 2002. The packaging properties were tested according to the KEBS EAS 859:2017 standard for packaging, which outlines the minimum/maximum requirement for three properties; bursting strength, tearing resistance, and water absorbency. The results indicate that the pulping process using BPL yielded more fiber than the NaOH pulping process at 68% and 56%, respectively. The test results for the packaging paper revealed that BPL and NaOH packaging paper surpassed the minimum KEBS requirement for bursting strength and tearing resistance however it had a high water absorbency rate. The property tests statistical analysis showed that for all three tests, there was no significant difference between the packaging papers made with BPL and those made from NaOH indicating that BPL is a suitable replacement for NaOH. Further research is recommended to improve water absorption properties as well as to experiment with surface enhancement treatments such as dyeing.
摘要
本研究旨在建立一种使用钾基脱木素碱的可持续包装纸制造方法; 香蕉皮碱液(BPL)将香蕉茎纤维制浆. 这是一项实验研究,使用苛性钠作为阳性对照. 包装纸是使用TAPPI 2002描述的手工工艺方法构建的. 包装性能根据KEBS EAS 859:2017包装标准进行测试,该标准概述了三种性能的最低/最高要求; 爆裂强度、抗撕裂性和吸水性. 结果表明,BPL法制浆的纤维产量比NaOH法制浆高,分别为68%和56%. 包装纸的测试结果表明,BPL和NaOH包装纸在爆裂强度和抗撕裂性方面超过了KEBS的最低要求,但其吸水率很高. 性能测试统计分析表明,在所有三种测试中,用BPL制成的包装纸与用NaOH制成的包装纸张之间没有显著差异,这表明BPL是NaOH的合适替代品. 建议进一步研究以改善吸水性能,并试验表面增强处理,如染色.
Introduction
Packaging is a dynamic product that primarily serves the function of concealing other products. The typical global view of packaging has both bitter and sweet dimensions. It is seen as a necessary vice, as products can almost not exist without packaging yet the same packaging especially plastic packaging has caused major setbacks in environmental preservation (Vidal Citation2020). Similarly, the increase in demand for sustainable biodegradable packaging has fueled the rate of deforestation given that the most popular form of biodegradable packaging is made mainly from wood pulp (Poole Citation2021). However, different non-wood sources of fiber have been used as an alternative to create biodegradable paper. Jute, flax, bagasse, corn straw, bamboo, reed, grass, sisal, and banana stems are among these non-wood fiber sources which have a high cellulose content thus making them potentially great raw material for packaging paper (Liu, Wang, and Hui Citation2018; Subagyo and Chafidz Citation2018). Such an example is present in the work of Chollakup et al. (Citation2020) who established that the cellulosic pulp of rice cereal straw can make biodegradable paper. Non-wood fiber materials such as this are desirable because; they grow faster, they contain lesser lignin compared to wood, they require less energy in the form of heat and chemicals for pulping, and given that they are often discarded as agricultural waste they are readily available (Abd El-Sayed, El-Sakhawy, and El-Sakhawy Citation2020).
One of the major non-wood fiber sources produced in Kenya is banana stems. Kenya produces over 1 million tons of bananas annually (FAOSTAT Citation2019). Post-harvest, the banana waste is commonly used for making manure, livestock feed, craft items such as mats, bags, and even potentially; hair extensions (Dubey Citation2021; Koigi Citation2015; Padam et al. Citation2014). Banana pseudo stems have been determined as one of the major agricultural residues that have a high cellulose content of up to 69%, yet these stems are not fully exploited as a potential source of fiber for the production of products such as paper (Goswami, Kalita, and Rao Citation2008; Kamau Citation2016; Oyewo et al. Citation2023).
Banana stem fiber applications are extensive and have been utilized in industries such as food, textiles, and even construction (Subagyo and Chafidz Citation2018). Formerly, it was limited to making handcrafts or organic fertilizer for agriculture but the increase in popularity of eco-friendly materials has fueled its demand in multiple sectors (Temitayo et al. Citation2023). Banana stem fiber (BSF) is characterized as having great elasticity, tenacity, stiffness, moisture absorbency, and high cellulose content (Kumar Tripathi et al. Citation2019; Subagyo and Chafidz Citation2018). For this reason, they have been adapted into the production of textiles such as marine ropes given that they are resistant to seawater and they have great buoyancy (Subagyo and Chafidz Citation2018). BSF have also been utilized in creating hybrid composites, combining with fibers like coir, wheat gluten, jute, and carbon for various applications in the automotive industry, structural and interior applications, and electric cable insulation to list a few (Temitayo et al. Citation2023).
In East Africa, the present dominant use of BSF has been for simple domestic and craft applications. These include the fabrication of handcrafts such as doormats, bags, and ropes (Kamau Citation2016; Kamira et al. Citation2015). Different studies in this region as well as in other banana production regions outside Africa have highlighted the potential of banana stem fibers as a raw material for making paper and paper products. These products include tea bags, decorative paper, tissue paper, and even currency paper thus citing the potential for making packaging paper for various fields (Liu, Wang, and Hui Citation2018; Priyadarshana et al. Citation2022; Subagyo and Chafidz Citation2018). Nevertheless, limited sources document the properties of BSF paper for packaging.
Banana peels on the other hand are part of the undervalued banana waste by-products that have often been discarded in both domestic and industrial settings after the extraction of the fruit pulp (Gumisiriza et al. Citation2017). Value addition of these peels includes the generation of biogas for banana pulp extraction industries within rural areas (Gumisiriza et al. Citation2017). They have also been determined to produce a potentially suitable alkali solution for pulping useful in the paper-making process (Olabanji, Oluyemi, and Ajayi Citation2012). In various industries such as the soap making industry caustic soda (NaOH) has been replaced with organic alkalis such as banana peel lye (Waithaka and Muriuki Citation2019). This has been supported by other studies such as that by Olabanji et al. (Citation2012) where banana peel lye (BPL) was determined to be a fit replacement for caustic soda in soap making as it had a PH of 12.05 and 12.88, indicating it is a strong base just as caustic soda (NaOH) whose PH is 14. This indicates its potential to be used in pulping as an alkali delignification reagent to replace caustic soda (NaOH).
There is limited exploration of banana stems and peels as a resource for creating packaging paper. Similarly, the favored production method of banana fiber paper employs NaOH chemical as a delignification reagent. Eco-friendly alternatives for a delignification reagent are sparingly covered by previous literature. In an attempt to further add value to additional banana waste (banana peels) whose utility has been merited by various research, the main objective of this was to fabricate and establish some of the properties of banana stem fiber packaging paper made using banana peel lye (BPL) as a delignification reagent. Pulp yield of the pulping process, water absorptiveness, bursting strength, and tearing resistance of the NaOH and BPL pulped papers were examined. These findings will provide insight on the viability of banana peel lye as an organically derived delignification reagent and its significance in the banana stem fiber paper making process.
Materials and methods
Banana stems of the Kiganda variety (mainly used in matoke) were sourced from Kisii County, Kenya. Fifty stems were collected in total. The stems collected were those whose banana crop had been harvested after 12 months upon maturity of the crop. The collected stems were cut into pieces of 1 meter to ensure ease of transportation and handling of the stems during decortication.
Decorticating
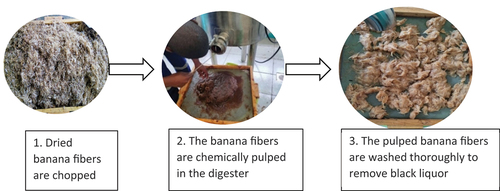
Before decortication, the sheaths of the stems were taken apart. From these sheaths, the outer and middle sheaths were selected, leaving out the inner sheaths which have weak fibers with very low cellulose content and more ash content (Jayaprabha, Brahmakumar, and Manilal Citation2011; Pereira et al. Citation2014). The Inner core sheaths of the banana stem were excluded since they contain weak fibers with lower cellulose content (Pereira et al. Citation2014). Mechanical decortication using a decorticating machine was used to isolate the fibers. Resultant fibers were washed, sundried, and stored under the following conditions; 65% ± 2% humidity and temperature of 20°C ±2°C. The dried fibers were then chopped into small pieces of between 2 and 2.5 cm in preparation for pulping.
Pulping
The alkaline pulping process was carried out under the standard conditions of 65% ± 2% humidity and a temperature of 20°C ±2°C. The time taken, the amount of fiber, and the concentration of the delignification reagents were kept constant. However, the type of delignification reagent was varied: one pulping process utilized [Banana peel lye (BPL)]. The other pulping process which was used as a control used Caustic soda (NaOH). The control, NaOH was used to assess the delignification ability of the BPL because it is the most common alkali used for alkaline pulping. For the pulping process, the materials included; 1000 g of dry banana stem fiber (Chopped), 480 g of BPL or NaOH, and 6 liters of distilled water. The ratio of fiber (g): alkali (g): distilled water (g) for 7% concentration is 1:0.48:6.
The cooking was done in a stainless steel bio-digester. To commence, the alkali was added to the distilled water. This mixture was stirred thoroughly to ensure all the alkali dissolved. One thousand grams of dry banana stem fibers were measured on a scale and put in the bio-digester. The alkali liquor was added to the fibers in the bio-digester and mixed with a stirrer. The temperature of the bio-digester was set at 105°C and the mixture was allowed to cook for 120 min. The resultant solution known as the cooking liquor was drained from the bio-digester and the fibers were thoroughly washed several times under running water to purify the pulp. The recovered fibers were dried and weighed to determine the fiber yield. The fibers were beaten into a smooth pulp using an electric blender to beat down the fibers into a smooth pulp. This process was repeated separately for the NaOH sample. The pulping process is presented in .
Preparation of the packaging paper
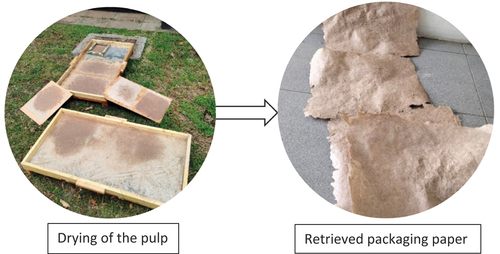
Packaging paper was constructed using a handmade craft method. The method employed was adapted from methods specified by Babcock (Citation2020) and Tappi (Citation2002). For this, the requirements included: a flat firm table, a 2 liter calibrated measuring jug, an A4 size mold and deckle, card boards (4 m by 1 m), a pair of scissors, a basin, and 3 cotton cloths (50 cm by 50 cm). The pulp retrieved from the pulping process was transferred to the mold and deckle to enable the fibers to matt into paper. The matted pulp was then transferred to a silk mesh frame and allowed to dry in the sun for 1 day (refer to ). Twenty A4 size papers of about 50 g/m2 were prepared. The dried paper samples were extracted from the frames, had rough edges trimmed, and stored in a dry place.
Testing of packaging paper
According to the Kenya Bureau of Standards – KEBS EAS 859:2017 standard minimum requirement for packaging paper; three tests are recorded as essential. These tests are Water absorptiveness, bursting strength, and tearing resistance. These tests were carried out for the packaging paper constructed with BPL as well as that constructed with NaOH. Before testing, the packaging papers were conditioned to 65% ± 2% humidity and temperature of 20°C ±2°C. Five A4-sized papers were allocated for each test. The statistical tests used for analysis of some of the parameters include; a two sample t-test used to evaluate if two independent groups differ from each otherin this case, BPL and NaOH delignification methods. A one sample t-test which were used to evaluate if a single group differs from a known value in this case an established result from a parameter test against a set minimum standard for good-quality paper packaging.
gives a summary of the tests done, the method number, the minimum requirement, and the equipment used.
Table 1. KEBS standards for packaging paper testing (Standard specification: KS EAS 859:2017 East African Standard for Paper Bag Packaging).
The surface morphology
The BPL packaging paper and the NaOH packaging paper samples were subjected to a scanning electron microscopy (SEM) analysis. The samples were independently scanned using a JEOL JCM-7000 Benchtop SEM machine. For the preparation, the samples were cut out and mounted on a double-sided adhesive carbon tape. The SEM machine was then vented to release the sample placement chamber. The samples were placed in the chamber and the machine was run to reach full vacuum, then the samples were scanned at an acceleration voltage of 15kV (Oyewo et al. Citation2023). The images from the scan were then acquired.
Thermo gravimetric analysis
The thermo gravimetric analysis was evaluated using a Shimadzu TGA 50 thermal analyzer. BPL and NaOH packaging paper samples of 3 g each were used for the analysis. The samples were heated from a temperature of 25°C to 600°C at a heating rate of 10°C/min and subsequent cooling at 25°C.
Fourier transform infrared spectroscopy (FTIR) characterization
The spectral data were acquired using an IR affinity – 1S FTIR spectrophotometer (Shhimadzu Corp., 03191) equipped with an ATR. The instrument was set up to perform a total of 20 scans with 4 cm -1 spectral resolution for both background and sample spectra, recorded rapidly at the range between 4000 and 400 cm -1.
Results and discussion
Pulping process
The resultant weight of the dry pulps from the two alkaline pulping processes was measured and compared to the original dry weight of the fiber before pulping to determine the yield (Briggs Citation1994).
The BPL alkaline pulping process had a higher yield by mass (680 g) compared to the NaOH alkaline pulping process which yielded (560 g) as shown on .
Table 2. Yield in grams produced by the BPL and NaOH pulping processes.
Alkali pulping is used as a surface treatment to eliminate non-cellulosic material from the fibers and thus improve their quality (Badanayak, Jose, and Bose Citation2023). The alkaline pulping process using NaOH is reported to yield about 50% of the original fiber weight (Goswami, Kalita, and Rao Citation2008). On the other hand, alkaline pulping processes using potassium-based alkalis give a yield that ranges from 47% to 53% (Kalyoncu Citation2022). The viability of BPL as a replacement alkali for various alkali-based processes has also been established by different studies that suggest that KOH solution derived from banana peels is a suitable replacement for NaOH in soap making due to similarity in pH as they are both strong alkalis with pH values of 11 and 13, respectively (Olabanji, Oluyemi, and Ajayi Citation2012; Waithaka and Muriuki Citation2019).
The results on yield show that the BPL pulping process surpassed the NaOH pulping process. The BPL pulping process yielded more fiber by mass compared to NaOH which retained 68% and 56%, respectively, in a mass of the original fiber before pulping thus indicating the viability of BPL as a pulping delignification reagent. This retention of more fibers by the BPL pulping process can be attributed to the increased retention of the cellulose and hemicellulose fiber layers during the pulping process, where only lignin is detached from the fiber walls. NaOH on the other hand has a slightly lower yield which could be attributed to increased breaking down and dissolution of more of the cellulose and hemicellulose fiber layers during the pulping process (Subagyo and Chafidz Citation2018).
These results imply that BPL presents a more economical and eco-friendly alternative for alkaline pulping. The process can even be fully established at a farm where the bananas are grown thus adding value to the banana stems and banana peels as banana farming by-products.
Packaging paper properties
a. Water absorbency
The water absorptiveness of BPL and NaOH packaging papers was tested. The results are presented in .
Table 3. Comparison of water absorptiveness of BPL packaging paper, NaOH packaging paper, and KEBS EAS 859:2017 standard minimum requirement.
The findings above in show that the Cobb value of the packaging paper fabricated using Lye (BPL) was higher than that fabricated by NaOH with values of 463 g/m2 and 205 g/m2 respectively. A statistical analysis of the results using a two-sample T-Test indicates that there is no significant difference in the water absorptiveness of the packaging paper fabricated using BPL and that fabricated using NaOH (p = .0452, p > .01).
Additionally, a one-sample t-test statistical analysis was done to determine whether the Cobb value of the packaging paper fabricated was higher, lower, or equal to the KEBS EAS 859:2017 standard maximum requirement Cobb value of 30 g/m2. The results showed that the Cobb value of the BPL packaging paper which was 463 g/m2 was significantly higher than the requirement of a maximum of 30 g/m2 (p = .9995, p > .01). This showed that it did not meet the requirement for water absorptiveness.
Paper made from non-wood fibers has been generally established to have poor water-filtering properties (Liu, Wang, and Hui Citation2018). Alkali-treated fibers will have a water absorption percentage of about 248% to 480% (Begum, Tanni, and Shahid Citation2021). The results of this study revealed that both BPL and NaOH packaging paper had a high water absorbency rate with no significant difference in the water absorptiveness of the packaging papers having water absorption percentages of 293% and 98%, respectively. This exhibition of high water absorbency rates is reflective of the inherent banana fiber absorbency qualities which are known to be highly hydrophilic (Liu, Wang, and Hui Citation2018; Motaleb, Mizan, and Milašius Citation2020). The high absorption rate is contributed by the de-lignification process which leaves the fibers bare free from the lignin, wax, and hemicellulose matrix which acts as a barrier to control the absorption of water by the fibers (Begum, Tanni, and Shahid Citation2021). Non-woven materials made from bananas have high water absorption rates and to counter this water repellent treatment should be included in the construction process.
b. Bursting strength
The bursting strength of the BPL and NaOH packaging paper was also determined under the KEBS EAS 859:2017 standard minimum requirement. The results are presented in .
Table 4. Comparison of bursting strength sample of BPL packaging paper, NaOH packaging paper, and KEBS EAS 859:2017 standard minimum requirement.
The results indicate that the packaging paper fabricated using NaOH had a higher bursting strength value of 293 kPa than that fabricated using BPL determined to be 98 kPa. A statistical analysis of the results using a two-sample T-Test indicates that there is no significant difference in the bursting strength of the packaging paper fabricated using BPL and that fabricated using NaOH (p = .0132, p > .01).
Additionally, a one-sample T-Test statistical analysis was done to determine whether the bursting strength of the packaging paper fabricated was higher, lower, or equal to the KEBS EAS 859:2017 standard minimum requirement bursting strength of 90 kPa. The results showed that the bursting strength of the BPL packaging paper which was 98 kPa was higher than the requirement of 90 kPa (p = .8397, p > .01). This shows that it met the requirement for bursting strength.
Bursting strength is the quality of paper that gives the ability of a packaging to hold its contents. It is described as a mechanical property of paper that measures its rupturing resistance (Adams Citation2021). The findings of this study show that the bursting strength of the packaging paper constructed using BPL and NaOH were 98 kPa and 293 kPa, respectively. These findings range close to those of Sakare et al. (Citation2020), which give the average bursting strength of alkali pulped banana stem paper as 157.8 kPa. Bursting strength is attributed to the long length of fibers and high cellulose content which in turn leads to higher fiber bonding (Nassar et al. Citation2021). Additionally, chemicals and the weight of paper are also factors that determine bursting strength.
Bursting strength gives a packaging material to capability to retain its contents. This therefore implies that the BPL paper packaging material surpassed the minimum requirement for packaging paper given by KEBS EAS 859:2017 indicating that it can retain its contents without bursting prematurely.
c. Tearing resistance
The results for the tear resistance test of BPL, NaOH packaging paper, and the KEBS EAS 859:2017 standard minimum requirement are presented in .
Table 5. Comparison of tear resistance sample of BPL packaging paper, NaOH packaging paper, and KEBS EAS 859:2017 standard minimum requirement.
The results of the tear resistance test showed that the BPL packaging paper has higher-tearing resistance compared to NaOH, having a tear resistance of 2252 mN and 1958 mN, respectively.
A statistical analysis of the results using a two-sample T-Test indicates that there is no significant difference in the tear resistance of the packaging paper fabricated using BPL and that fabricated using NaOH (p = .0570, p > .01).
Additionally, a one-sample T-Test statistical analysis was done to determine whether the tear resistance of the packaging paper fabricated was higher, lower, or equal to the KEBS EAS 859:2017 standard minimum requirement for tear resistance of 320mN. The results showed that the tear resistance of the BPL packaging paper which was 2252mN was higher than the requirement of 320mN (p = .9994, p > .01). This showed that it met the requirement for tear resistance.
The findings for tearing resistance indicate that BPL packaging paper had a higher tearing resistance of 2252 mN compared to packaging paper constructed using NaOH which was 1958 mN. It was also significantly higher than the KEBS EAS 859:2017 standard minimum requirement for tear resistance of 320mN. Tearing resistance or tearing strength is determined by the number of fibers per unit weight of material, this is often contributed by a high cellulose content (Nassar et al. Citation2021). This means that it is likely that the BPL packaging paper had a high number of fibers per unit weight of material, thus contributing to it having a greater tearing resistance.
Banana stem paper has been recorded to have good tear resistance. The tear resistance of banana paper has been determined to be an average of 1036mN (Khan et al., Citation2014). Additionally, the tear resistance of grease-proof banana paper was determined to be 980mN (Goswami, Kalita, and Rao Citation2008). These findings range closely to those of this study thus indicating that banana paper has generally good tear resistance.
The surface morphology
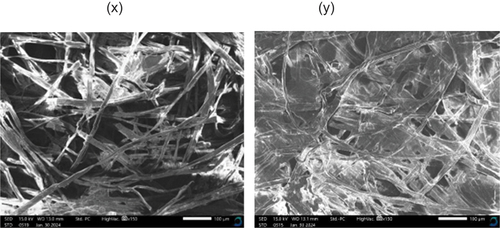
The images obtained from the SEM analysis are depicted in . The SEM images, as observed, revealed the bound intertwined non-woven network of fibers that appear like a mesh on both of the samples. The two samples distinctly exhibited voids within their structure with the BPL paper packaging sample appearing to have larger voids in between the intertwined fibers compared to the NaOH paper packaging sample. This justifies the results on the water absorbency rate of the samples, which revealed that the BPL paper packaging sample had a higher water absorption rate than that of the NaOH paper packaging sample. Yiga et al. (Citation2023) obtained similar morphological findings for the banana stem fiber paper they developed which aside from having a mesh-like appearance also had an inherent high moisture content. These inherent hydrophilic traits are attributed to the samples constituent banana stem fibers which have been established to have a great affinity to moisture (Jirukkakul Citation2019; Oyewo et al. Citation2023; Yiga et al. Citation2023).
Thermal gravimetric analysis (TGA)
Information on the stability of the material based on its subjection to varied temperatures was achieved through a thermal gravimetric analysis (TGA).
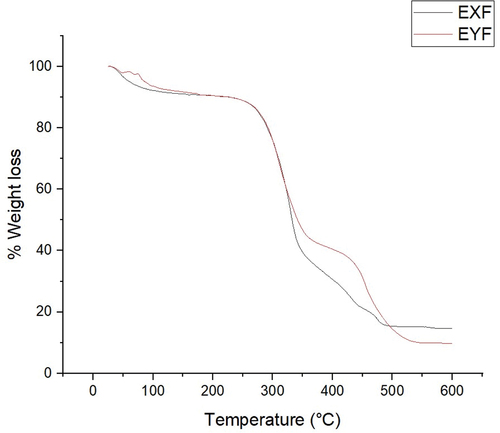
The TGA of the BPL and the NaOH paper packaging samples are shown in . Both the BPL and the NaOH recorded three distinct phases of weight decline over the temperature range of 25°C to 600°C. The estimated 7–8% initial loss of weight commences between 25°C to around 101°C for BPL and 25°C to 100.9°C for NaOH and this is related to moisture release by the samples (Oyewo et al. Citation2023).
The apparent commencement of decomposition took place at a temperature of approximately 273°C for BPL and 270°C for NaOH. Both packaging samples exhibit an estimated 15% weight loss at these temperatures. This phase marks the first stage of thermal degradation of the lignocellulosic components of the samples where hemicellulose is broken down (Oyewo et al. Citation2023; Yang et al. Citation2007). This was followed by a second phase which majorly marked the thermal decomposition of cellulose as well as the continuous degradation of any residual hemicellulose at temperatures of approximately 350°C for BPL and 359°C for NaOH which attributed to a weight loss of up to 61% and 56%, respectively (Joyline et al. Citation2023; Yang et al. Citation2007).
At a temperature of 450°C and 445°C for BPL and NaOH respectively the third and final distinct phase is witnessed giving a weight loss of up to 79% and 67%, respectively. Thermal Decomposition of the lignin took place mainly took place at this stage (Yang et al. Citation2007). Total thermal decomposition occurred beyond temperatures of 600°C for both BPL and y.
Joyline et al. (Citation2023) justify that the higher weight loss in phase 1 and 2 for BPL compared to NaOH can be attributed to their structural variances where BPL has an amorphous arrangement contrasting with NaOH that presents an oriented and compact knit structure. BPL having an amorphous structure is easier to thermally breakdown at a similar temperature range due to the weak interconnected fibers in contrast to NaOH with a stronger fiber interconnection (Joyline et al. Citation2023).
Fourier transform infrared spectroscopy (FTIR)
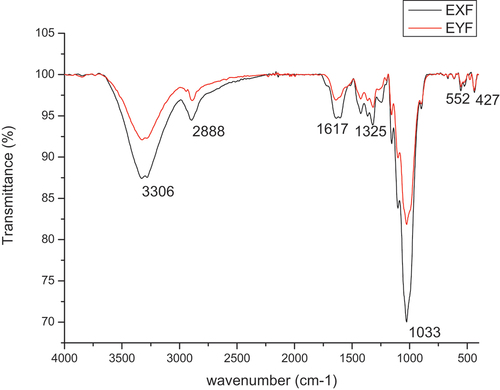
The FTIR band results are given in .
From the derived IR, BPL and NaOH generally mirrored each other indicating minor variance in the components.
The broad bands for (BPL, NaOH) at (3306,3306), (2888,2888), (1617,1637), (1325,1314), (1033,1028), (552 and 427) cm −1 respectively are associated with O-H stretching (H-bonded), C-H (stretching aromatic), C=C stretching or O-H bending or C=O stretching, O-H, in plane deformation, C-O (stretching) and C–C stretching functional groups (Oyewo et al. Citation2023; Pereira et al. Citation2014; Yang et al. Citation2007). gives a summary of the Transmittance bands of BPL and NaOH packaging paper alongside possible components.
Table 6. Transmittance bands of BPL and NaOH packaging paper alongside possible components.
The broad band at 3306 may be attributed to cellulose. The band at 2888 corresponded to the C-H stretching vibration, an indicator of either cellulose, lignin or carboxylic acids (Oyewo et al. Citation2023). A variance of transmittance was observed at (1617, 1637) for (BPL, NaOH), respectively. This would be attributed to a slightly higher hemicellulose content in NaOH. Additionally, the minor variance of transmittance was observed at (1325, 1314), (1033, 1028) cm −1 for (BPL, NaOH). The minor variance of transmittance observed at (1325,1314) attributed to the O-H, in plane deformation of cellulose present bands which is a strong indicator that the delignification process was extensive in both processes thus leading to the high cellulose component present (Oyewo et al. Citation2023; Pereira et al. Citation2014). Given that the variance is minimal it is possible to conclude that the delignification methods give similar paper quality justifying the possibility of using banana peel lye to replace NaOH as a delignification reagent. The possible lignin/hemicellulose presence in packaging samples of BPL slightly surpasses that in NaOH [(1033, 1028) cm −1] but given that the variance is minimal, both samples present similar delignification thresholds. The final minor bands at 552 and 427 for both samples would indicate the presence of aromatic hydrogen (Yang et al. Citation2007).
Conclusion
The assessment of BPL as a delignification reagent against NaOH as a control establishes its viability in paper making through various tests including the pulping process, paper tests as well as through material characterizations (SEM, TGA and FTIR). The pulping process using BPL gives a higher yield of fiber compared to the control NaOH which is the commonly used alkali. This indicates that BPL can easily replace NaOH in the pulping process. The findings show the BPL packaging paper as having good bursting strength and tearing resistance. This means that the BPL packaging paper had a stronger fiber-to-fiber bonding and a high number of fibers per unit weight of material. Similarly, banana stem packaging paper constructed using BPL and NaOH pulping processes has a high water absorption rate. Naturally, banana stem fibers are hydrophilic. This is maintained by both the BPL and NaOH banana stem packaging paper which was established to retain this hydrophilic quality. Therefore, water absorbency properties of banana stem packaging paper will need refinement. The SEM shows that the BPL paper packaging had more amorphous regions than the NaOH indicating less fiber to fiber bonds. Thermal analysis suggested similar stability of the samples across various temperatures with notable differences being on account of structural difference of the samples as it relates to orientation of fibers. FTIR results also showed that a close range of results for both NaOH and BPL paper packaging samples indicating similar extent of delignification in both samples. These results show that the paper packaging making process using BPL as a delignification reagent produces good-quality paper but if it is to be used enhancement of the qualities such as water resistance would be paramount. It is also recommended that more experiments such as dyeing ought to be done on the BPL paper packaging to establish a variety of finished products.
Highlights
Banana peel lye (BPL) is established to be a viable delignification reagent in the chemical pulping of banana stem fiber as it had a higher yield by mass than the control -NaOH.
The paper packaging produced from the BPL chemical pulping process was determined to have great tearing resistance and bursting strength.
The paper packaging produced from the BPL chemical pulping process was established to have a high-water absorption rate similar to the control packaging paper made from NaOH. It will need enhancement to improve this quality.
Material characterization of the paper packaging made using BPL further cements its similarity to that made using the control; NaOH establishing minor variances in property performance when assessed under the spectra of SEM, TGA and FTIR analysis.
Abbreviations
| BPL | = | Banana peel lye |
| NaOH | = | Sodium Hydroxide or caustic soda |
Acknowledgments
The author would like to thank; Kenyatta University and the National Research Fund (Kenya) who supported this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abd El-Sayed, E. S., M. El-Sakhawy, & M. A. M. El-Sakhawy. 2020. “Non-Wood Fibers As Raw Material for Pulp and Paper Industry.” Nordic Pulp and Paper Research Journal 35 (2): 215–13. https://doi.org/10.1515/npprj-2019-0064.
- Adams, R. 2021. “Instrumentation in the Graphic Arts; Instruments and Tests for Paper. In Pressbooks. https://pressbooks.library.ryerson.ca/gainstrumentation/chapter/chapter-1/.
- Babcock, M. 2020. “How to Make Handmade Paper from Recycled Materials. Paperslurry. https://www.paperslurry.com/2014/05/19/how-to-make-handmade-paper-from-recycled-materials/.
- Badanayak, P., S. Jose, and G. Bose. 2023. “Banana Pseudostem Fiber: A Critical Review on Fiber Extraction, Characterization, and Surface Modification.” Journal of Natural Fibers 20 (1). https://doi.org/10.1080/15440478.2023.2168821.
- Begum, H. A., T. R. Tanni, and M. A. Shahid. 2021. “Analysis of Water Absorption of Different Natural Fibers.” Journal of Textile Science and Technology 7 (04): 152–160. https://doi.org/10.4236/jtst.2021.74013.
- Briggs, D. G. 1994. “Chapter 8: Pulp and Paper.” Forest Product Measurements and Conversion Factors: With Special Emphasis on the U.S. Pacific Northwest, 96–100.
- Chollakup, R., W. Kongtud, U. Sukatta, K. Piriyasatits, M. Premchookiat, and A. Jarerat. 2020. “Development of Rice Straw Paper Coated with Pomelo Peel Extract for Bio-Based and Antibacterial Packaging.” Key Engineering Materials 847 KEM (June), 141–146. https://doi.org/10.4028/www.scientific.net/KEM.847.141.
- Dubey, P. 2021. “An Overview of Fruit By-Products Valorization: A Step Towards Sustainable Utilization.” Indian Journal of Pure & Applied Biosciences 9 (1): 46–55. https://doi.org/10.18782/2582-2845.8565.
- FAOSTAT. 2019. “Production Quantities of Bananas Annually.” https://www.fao.org/faostat/en/#data/QC.
- Goswami, T., D. Kalita, and P. G. Rao. 2008. “Greaseproof Paper from Banana (Musa Paradisica L.) Pulp Fiber.” Indian Journal of Chemical Technology 15 (5): 457–461.
- Gumisiriza, R., J. F. Hawumba, M. Okure, and O. Hensel. 2017. “Biomass Waste-To-Energy Valorisation Technologies: A Review Case for Banana Processing in Uganda.” Biotechnology for Biofuels 10 (1): 1–29. https://doi.org/10.1186/s13068-016-0689-5.
- Jayaprabha, J. S., M. Brahmakumar, and V. B. Manilal. 2011. “Banana Pseudostem Characterization and Its Fiber Property Evaluation on Physical and Bioextraction.” Journal of Natural Fibers 8 (3): 149–160. https://doi.org/10.1080/15440478.2011.601614.
- Jirukkakul, N. 2019. “Physical Properties of Banana Stem and Leaf Papers Laminated with Banana Film.” Walailak Journal of Science and Technology 16 (10): 753–763. https://doi.org/10.48048/wjst.2019.3471.
- Joyline, G., P. Kareru, A. Gachanja, N. C. Nyambura, and E. S. Madivoli. 2023. “High Swelling Carboxymethyl Cellulose Synthesized from Coconut Fibers.” Journal of Natural Fibers 20 (2). https://doi.org/10.1080/15440478.2023.2283549.
- Kalyoncu, E. E. 2022. “Eco-Friendly Pulping of Banana Pseudo-Stem Wastes with Potassium-Based Processes.” Cellulose Chemistry and Technology 56 (1–2): 131–140. https://doi.org/10.35812/CelluloseChemTechnol.2022.56.12.
- Kamau, M. 2016. We Make Handbags, Sanitary Towels from Banana Fiber - the Standard. June 4. https://www.standardmedia.co.ke/smartharvest/article/2000204005/we-make-handbags-sanitary-towels-frombanana-fiber.
- Kamira, M., C. Sivirihauma, J. Ntamwira, O. W, K. Mg, J. Bigabwa, L. Vutseme, and G. Blomme. 2015. “DRC Banana Plant Application.Pdf.” Journal of Applied Biosciences 95 (1): 8915–8929. https://doi.org/10.4314/jab.v95i1.1.
- Khan, M. Z. H., M. A. R. Sarkar, F. I. Al Imam, M. Z. H. Khan, and R. O. Malinen. 2014. “Paper Making from Banana Pseudo-Stem: Characterization and Comparison.” Journal of Natural Fibers 11 (3): 199–211. https://doi.org/10.1080/15440478.2013.874962.
- Koigi, B. 2015. Banana Stalk Hair Extension. FairPlanet. https://www.fairplanet.org/story/banana-stalk-hair-extension/.
- Kumar Tripathi, S., N. Kant Bhardwaj, S. Chechi, and R. Varadhan. 2019. “Suitability of Banana Stem Pulp as Replacement of Softwood Pulp for Making Superior Grade Unbleached Paper from Agro Residue Pulp.” Peer Review Appita Journal Appita J 72 (3): 163. www.appita.com.
- Liu, Z., H. Wang, and L. Hui. 2018. “Pulping and Papermaking of Non-Wood Fibers.” Pulp and Paper Processing. https://doi.org/10.5772/INTECHOPEN.79017.
- Motaleb, K. Z. M. A., R. A. Mizan, and R. Milašius. 2020. “Development and Characterization of Eco-Sustainable Banana Fiber Nonwoven Material: Surface Treatment, Water Absorbency and Mechanical Properties.” Cellulose 27 (14): 7889–7900. https://doi.org/10.1007/s10570-020-03343-y.
- Nassar, M. M., I. I. Ibrahim, H. M. Elsherif, and M. Abdallah. 2021. “Optimization of Banana Stem Pulp to Substitute Softwood Pulp for High Quality Paper.” Egyptian Journal of Chemistry 64 (3): 1461–1469. https://doi.org/10.21608/EJCHEM.2020.46294.2954.
- Olabanji, I. O., E. A. Oluyemi, and O. S. Ajayi. 2012. “Metal Analyses of Ash Derived Alkalis from Banana and Plantain Peels (Musa Spp.) in Soap Making.” 11 (August 2014): 16512–16518. https://doi.org/10.5897/AJB12.2255.
- Oyewo, A. T., O. O. Oluwole, O. O. Ajide, T. E. Omoniyi, P. Akhter, M. H. Hamayun, B. S. Kang, Y. K. Park, and M. Hussain. 2023. “Physico-Chemical, Thermal and Micro-Structural Characterization of Four Common Banana Pseudo-Stem Fiber Cultivars in Nigeria.” Journal of Natural Fibers 20 (1). https://doi.org/10.1080/15440478.2023.2167031.
- Padam, B. S., H. S. Tin, F. Y. Chye, and M. I. Abdullah. 2014. “Banana By-Products: An Under-Utilized Renewable Food Biomass with Great Potential.” Journal of Food Science and Technology 51 (12): 3527–3545. Springer. https://doi.org/10.1007/s13197-012-0861-2.
- Pereira, A. L. S., D. M. Do Nascimento, M. D. S. M. Souza, A. R. Cassales, J. P. Saraiva Morais, R. C. M. de Paula, M. D. F. Rosa, and J. P. A. Feitosa. 2014. “Banana (Musa sp. Cv. Pacovan) Pseudostem Fibers Are Composed of Varying Lignocellulosic Composition Throughout the Diameter.” Bio Resources 9 (4): 7749–7763. https://doi.org/10.15376/biores.9.4.7749-7763.
- Poole, J. 2021. European Commission Proposes New Deforestation Rules but Carton Industry Insists Forests Are Not Just Carbon Sinks. https://www.packaginginsights.com/news/european-commission-proposes-new-deforestation-rules-but-carton-industry-insists-forests-are-not-just-carbon-sinks.html.
- Priyadarshana, R. W. I. B., P. E. Kaliyadasa, S. R. W. M. C. J. K. Ranawana, and K. G. C. Senarathna. 2022. “Biowaste Management: Banana Fiber Utilization for Product Development.” Journal of Natural Fibers 19 (4): 1461–1471. https://doi.org/10.1080/15440478.2020.1776665.
- Sakare, P., A. K. Bharimalla, J. Dhakane-Lad, and P. G. Patil. 2020. “Development of Greaseproof Paper from Banana Pseudostem Fiber for Packaging of Butter.” Journal of Natural Fibers (00): 1–9. https://doi.org/10.1080/15440478.2019.1710652.
- Subagyo, A., and A. Chafidz. 2018. “Banana Pseudo-Stem Fiber: Preparation, Characteristics, and Applications.” Banana Nutrition - Function and Processing Kinetics. https://doi.org/10.5772/INTECHOPEN.82204.
- Tappi. 2002. “Forming Handsheets for Physical Tests of Pulp. Test Method TAPPI/ANSI T 205 Sp-18.” Tappi 1–9. https://www.tappi.org/content/sarg/t205.pdf.
- Temitayo, A., O. Olugbemiga, O. Olufemi, T. Emmanuel, and M. Hussain. 2023. “Banana Pseudo Stem Fiber, Hybrid Composites and Applications: A Review.” Hybrid Advances 4 (September): 100101. https://doi.org/10.1016/j.hybadv.2023.100101.
- Vidal, A. 2020. Relocating Rubbish. When Southeast Asia Is Overflowing with Western Waste - Aude Vidal - Visionscarto. May 21. https://visionscarto.net/relocating-rubbish.
- Waithaka, P. N., and J. N. Muriuki. 2019. “Preparation of Soap Using Banana Peel and Olive Tree Ashes.” Animal Science Journal 10 (1): 22–29.
- Yang, H., R. Yan, H. Chen, D. H. Lee, and C. Zheng. 2007. “Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis.” Fuel 86 (12–13): 1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013.
- Yiga, V. A., M. Lubwama, J. Opio, E. Menya, D. Nono, and H. Nalubega Lubwama. 2023. “Production and Characterization of Paper from Banana Stem Fiber: Optimization Using Box-Behnken Design (BBD).” Journal of Natural Fibers 20 (1). https://doi.org/10.1080/15440478.2023.2192019.