ABSTRACT
Solar ultraviolet (UV) radiation is the second most prevalent carcinogenic exposure in Canada and is similarly important in other countries with large Caucasian populations. The objective of this article was to estimate the economic burden associated with newly diagnosed non-melanoma skin cancers (NMSCs) attributable to occupational solar radiation exposure. Key cost categories considered were direct costs (healthcare costs, out-of-pocket costs (OOPCs), and informal caregiver costs); indirect costs (productivity/output costs and home production costs); and intangible costs (monetary value of the loss of health-related quality of life (HRQoL)). To generate the burden estimates, we used secondary data from multiple sources applied to computational methods developed from an extensive review of the literature. An estimated 2,846 (5.3%) of the 53,696 newly diagnosed cases of basal cell carcinoma (BCC) and 1,710 (9.2%) of the 18,549 newly diagnosed cases of squamous cell carcinoma (SCC) in 2011 in Canada were attributable to occupational solar radiation exposure. The combined total for direct and indirect costs of occupational NMSC cases is $28.9 million ($15.9 million for BCC and $13.0 million for SCC), and for intangible costs is $5.7 million ($0.6 million for BCC and $5.1 million for SCC). On a per-case basis, the total costs are $5,670 for BCC and $10,555 for SCC. The higher per-case cost for SCC is largely a result of a lower survival rate, and hence higher indirect and intangible costs. Our estimates can be used to raise awareness of occupational solar UV exposure as an important causal factor in NMSCs and can highlight the importance of occupational BCC and SCC among other occupational cancers.
Introduction
Skin carcinomas are the most common form of cancer in countries with large Caucasian populations and are one of the few malignancies with increasing incidence. In Canada, it is estimated that 34% of all cancers are skin related.[Citation1] The 3 most common forms of skin cancer are melanoma, basal cell carcinoma (BCC), and squamous cell carcinoma (SCC). The latter two are commonly referred to together as non-melanoma skin cancers (NMSCs). NMSCs are far more prevalent than melanoma, and BCC specifically is the most common form of any skin malignancy.[Citation2]
The risk of developing NMSCs is associated with several factors, however, it has long been accepted that ultraviolet (UV) radiation exposure is the main cause of this cancer.[Citation3,Citation4] CAREX Canada has identified solar UV radiation as the second most prominent carcinogenic exposure in Canada.[Citation5] Peters et al. reported over 1.5 million Canadian workers are exposed to solar UV at work, and approximately 900,000 of them spent more than 75% of their workdays outdoors (e.g., construction workers, farmers, and landscapers) and have a high level of exposure to solar UV (defined as 6 hr or more per workday spent outdoors).[Citation6] Exposure to solar UV radiation depends on a number of factors, including latitude, elevation, the presence of reflective surfaces, ozone concentration, cloud cover, and particulate matter in the atmosphere.[Citation7]
Estimating the number of cases and economic burden of NMSCs caused by occupational solar UV exposure can provide invaluable information for policy development and priority-setting for occupational cancer prevention purposes. Several studies have been undertaken that consider the economic burden of all skin cancers, or only occupational cases, in Canada and elsewhere. We present a subset of NMSCs economic burden studies from North America that provide both direct and indirect cost estimates and reported per-case cost in order to facilitate comparisons.
Krueger et al. reported on the cost of skin cancers in Canada for new cases in 2004.[Citation1] Based on their incidence numbers and total costs, we calculated average per-case costs for NMSCs of $1,162 in 2004 Canadian dollars ($1,429 in 2011 Canadian dollars). The average per-case direct costs for BCC and SCC were $436 and $660, respectively, in 2004 Canadian dollars. The authors considered 3 expenditure categories for direct costs: primary care-based treatment, day surgery in outpatient clinics, and inpatient/hospital stays. Based on the data presented in their report, indirect per-case costs for BCC and SCC were $362 and $1,945, respectively, in 2004 Canadian dollars. The SCC indirect costs are higher due to a higher mortality rate for this type of cancer. These costs include the value of work-loss days from mortality and morbidity.
In another Canadian study, Orenstein et al. estimated the economic burden of occupational cancers in Alberta for the year 2003.[Citation8] Their average cost estimate for NMSCs was $3,233 per-case in 2008 Canadian dollars ($3,533 in 2011 Canadian dollars). This study does not provide the cost for BCC and SCC separately. Since the authors only had healthcare costs information for lung cancer, they used the published scientific literature to estimate a proportion of the lung cancer costs that would be appropriate for NMSCs (a ratio of 0.19). The estimated direct and indirect costs per-case NMSCs are $2,484 and $749, respectively, in 2008 Canadian dollars. In their indirect costs, they included costs attributable to absenteeism, disability, and premature mortality.
Bickers et al. estimated the burden of NMSCs in the United States (US) for new cases in 2004.[Citation9] However, this study does not provide the costs for BCC and SCC separately. It also does not provide per-case cost. We estimated the average per-case cost for NMSCs to be $2,010 in 2004 US dollars ($2,472 in 2011 Canadian dollar). We also estimated their direct and indirect costs at $1,209 and $801, respectively, in 2004 US dollars. For direct costs, the authors considered hospital costs for inpatient, outpatient, and the emergency department. They also included physician office visits and prescription drugs in their direct cost calculations. For the indirect cost category, they considered time spent for healthcare consumption, lost or impaired ability to work or enjoy leisure activities as a result of morbidity, caregiver lost workdays and lost future earnings due to premature mortality. This study also reported an additional cost category, intangible costs associated with the impact of skin disease on quality of life. We estimated their per-case intangible cost at $801 in 2004 US dollars, as they did not provide per-case cost. These costs are based on the Dermatology Life Quality Index (DLQI) score used in conjunction with a willingness-to-pay approach to approximate the average amount a person is willing to pay to avoid this adverse health outcome.
In another study in the U.S., Kyle et al. reported on an economic evaluation with different preventive program scenarios for the 1999 calendar year.[Citation10] Their average per-case cost for BCC and SCC are $1,785 and $4,523, respectively, in 1999 US dollars ($2,545 and $6,449 in 2011 Canadian dollars). Since they did not report the average cost per-case for NMSCs, nor the incident number of NMSCs, we are unable to estimate an NMSCs per-case cost for this study. The authors reported the direct cost of both BCC and SCC at $825, in 1999 US dollars. They drew on the healthcare costs from Chen et al. study,[Citation11] which was calculated based on Medicare current beneficiary survey data (1999–2000). They also reported the indirect costs at $959 and $3,698 for BCC and SCC, respectively, in 1999 US dollars. Their indirect costs estimation are based on a US Environmental Protection Agency analysis developed to support a regulatory impact analysis. Productivity loss costs per-case were estimated by multiplying the Environmental Protection Agency's estimates of the loss of work associated with illness from BCC and SCC, as well as caregiving performed by others, by the national mean annual wage for 1999. Work loss associated with morbidity and premature mortality were both included in the indirect cost estimation. The researchers also considered Quality-Adjusted Life-Years (QALYs) losses from an intervention program with different preventive scenarios, but did not report the per-case numbers in the study.
There have been several economic burden of skin cancer studies published in the scientific literature, but there are inconsistencies in the methods used, as well as a tendency to not include some key costs such as HRQoL costs. Some studies simply reported healthcare system costs as direct costs and ignore out-of-pocket costs (OOPCs) and informal caregiver costs. In terms of indirect costs, most studies considered productivity losses due to morbidity and premature mortality but differ in the details of the methods used. Some considered only productivity in paid-work, while others consider productivity in other social roles. Although some of these cost categories may present challenges for researchers to estimate, they may be worth including if at all possible, given that their values may contribute importantly to the overall burden magnitude.
Most studies on the economic burden of NMSCs do not focus on occupational exposure. In fact, we were unable to identify any peer-reviewed economic burden studies focused solely on occupational NMSCs arising from solar UV radiation. Thus, this study provides the first attempt to comprehensively estimate the economic burden of occupational NMSCs in Canada. Specifically, we used an incidence cost approach to estimate the economic burden for Canada of newly diagnosed NMSCs cases in the calendar year 2011, attributable to occupational solar radiation exposure.
Methods
This study draws on computation methods developed from an extensive review of the literature, particularly Tompa et al.[Citation12] and Leigh.[Citation13] Several studies have used similar approaches to estimate the societal burden of all occupational injuries and illnesses in Australia[Citation14] and Singapore.[Citation15] We used an incidence cost approach to estimate the lifetime cost of newly diagnosed NMSCs in the calendar year 2011. We consider the economic burden of NMSCs in three broad cost categories as indicated in , namely direct, indirect, and intangible costs. All monetary values were converted to 2011 Canadian dollars. Monetary flows in years after 2011 were discounted to the 2011 calendar year using a 3% discount/interest rate. Productivity growth was assumed to be 1%, based on data drawn from Canadian System of National Accounts (CSNA).[Citation16]
Table 1. Breakdown of items included in the calculation of the economic burden of non-melanoma skin cancer due to occupational solar radiation exposure.
Input data
To generate the estimates, we combined secondary data sources drawn from various sources with an estimation method described above. The principal data were NMSCs incidence rates, healthcare costs, Health Utilities Index (HUI) values for different health states, the probability of survival at different ages, consumer price indices, and employment rates. Data sources include the Occupational Cancer Research Centre (OCRC) based in Toronto (2011); Schedule facility fees and physician services under the health insurance act of Ontario, Canada (2013);[Citation17] Canadian Population Life Expectancy (2009–2011);[Citation18] Canadian Community Health Survey (CCHS) (2010);[Citation19] Labour Force Survey (LFS) (2011);[Citation20] Survey of Labour and Income Dynamics (SLID) (2010);[Citation20] CSNA (2011);[Citation16] General Social Survey (GSS) (2005);[Citation21] and Survey of Employment, Payrolls and Hours (SEPH) (2011).[Citation22] Other secondary data sources included data abstracted from the published literature for various estimates needed for the computations. Sources are identified in the description of these computations below.
The starting point for our burden estimates was the number of newly identified NMSCs in Canada in 2011 attributable to occupational solar radiation exposure, stratified by sex and age bracket, estimated by several members of our research team at OCRC and CAREX Canada. They identified the number of workers exposed to solar radiation based on an approach similar to the United Kingdom Burden of Occupational Cancer Study,[Citation23] but revised for the Canadian context.
Calculating the Attribution Factor (AF) involved three major steps. The first was to select an appropriate relative risk from a high-quality, epidemiological study suitable for the Canadian context. The second step was to assess the prevalence of exposure to UV in the Canadian working population. The prevalence was based on exposure estimates previously developed by CAREX Canada. The final step was population modelling. Methods were used to model the working population in total and the working population ever exposed to UV included in the study. The number of workers ever exposed during the risk exposure period (in this case 1961–2000) was calculated by counting the number of all exposed workers in the first year of the risk exposure period (i.e., 1961) and the number of exposed new hires in each subsequent year (i.e., 1962–2000); the survival of all of these workers was then followed to the target year (i.e., 2011). More details about this method are described elsewhere.[Citation24]
Direct costs
We considered healthcare costs of NMSCs at three main phases in the cancer journey—diagnosis, treatment, and follow-up. We turned to several studies for the computation of healthcare costs,[Citation1,Citation8,Citation11,Citation17,Citation25] as well as the schedule facility fees and physician services under the health insurance act of Ontario, Canada.[Citation17,Citation26] Since healthcare is a provincial-level jurisdiction in Canada, treatment costs can vary to some degree from province to province. Canada-wide estimates by health condition and/or treatment paradigm are not available for BCC and SCC, hence the reason for the use of healthcare date from Canada's most populous province.
We assumed that if a worker survived 5 years after their initial diagnosis that they were cancer free, and at that point their risk of mortality returned to that of the general population. We used a survival rate of 99.98% and 99.30% for of BCC and SCC, respectively, based on Lucas et al.[Citation27] However, different survival rate scenarios were investigated in sensitivity analyses, ranging from 99% and 99.5% for BCC and SCC, respectively. To estimate the healthcare-related costs of recurrence, we approximated average recurrence rates of BCC and SCC based on the meta-analysis of Marcil et al.,[Citation28] at 44% and 18% for BCC and SCC, respectively. We also estimated the probability of conversion of BCC to SCC and vice versa at 44% and 6%, respectively, as indicated in .
Figure 1. Three-year cumulative risk of reoccurrence of non-melanoma skin cancer based on Marcil et al.[Citation28] Notes: BCC: Basal Cell Carcinoma; SCC: Squamous Cell Carcinoma.
![Figure 1. Three-year cumulative risk of reoccurrence of non-melanoma skin cancer based on Marcil et al.[Citation28] Notes: BCC: Basal Cell Carcinoma; SCC: Squamous Cell Carcinoma.](/cms/asset/b9d3be01-1031-430f-b9c4-f2553f3e4f56/uoeh_a_1447118_f0001_b.gif)
OOPCs include several costs such as travel to healthcare appointments, pharmaceuticals, vitamins, and supplements, purchased home healthcare services, and hotel accommodation costs. Since we could not identify a study specifically on OOPCs for NMSCs in Canada, we used OOPCs from Morries et al.[Citation29] which were £310.7 per case in 2002 British Pounds ($547 in 2011 Canadian dollars). Additionally, we undertook a sensitivity analysis using a range from $0–$2,300 per-case in 2011 Canadian dollars, based on a Longo et al.[Citation30]
To estimate the informal caregiving time, we divided the treatment of NMSCs in 2 phases: local and terminal phases. For informal caregiving cost during local disease treatment phase, we used Bickers et al., on 2006 study, which proposed a value of $26 per-case in 2011 Canadian dollars.[Citation9] For the terminal phase of the disease, which generally requires intensive care, we used 0.08 years of full-time caregiving, based on a World Health Organization (WHO) report.[Citation27] We also performed a sensitivity analysis over the range of 0 to 1 year at 6.8 hr per day, based on Yabroff et al.[Citation31]
Indirect costs
The most common approach to estimate output/productivity losses is the human capital approach, where it is assumed that losses are equivalent to the wage value of time off work. The human capital approach is appropriate for estimating macro-level output/productivity losses at the societal level, and is used in relevant work injury/illness burden studies.[Citation12,Citation14,Citation15] We used this approach for both paid-labor force output/productivity losses and lost home production.
We considered four indirect cost categories. The first is the time away from work seeking medical care, such as time spent in doctors’ office visits. Since we could not find a valid estimate of the number of hours spent per visit for NMSCs, we used the Chen et al. value at 0.9 days (7.2 hr per-case).[Citation11] We also performed a sensitivity analysis using a range from 5–10 working days as a rough estimate for all medical seeking purposes.
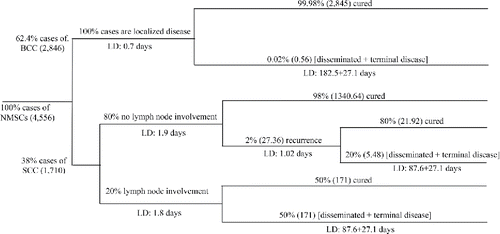
The second category is morbidity costs. NMSCs are generally caught early and treated efficiently, requiring very little time off work for most cases, and with little long-term consequences.[Citation1,Citation8] We estimated the reduced work output/productivity associated with absenteeism on the basis of a methodology by Kevin et al., in 2015.[Citation32] Specifically, to estimate the work days lost from BCC and SCC, the disability weight (DW) of each stage of the disease is multiplied by the stage length. We used DWs from the WHO report.[Citation27] For BCC the DWs were 0.05, 0.2, and 0.93 for localized, disseminated, and terminal stages, respectively. The duration of these stages were 0.04, 2.4, and 0.08 years, respectively.[Citation27] For SCC we considered two possibilities, no lymph node involvement, and lymph node involvement. For the former, the DW was 0.07 and duration 0.04 years. For lymph node involvement, the DW was 0.3 and duration 0.06 years. The DW for reoccurrence of SCC was 0.07 with a duration of 0.04 years. For the disseminated and terminal phases of SCC, the DWs were 0.2 and 0.93, and durations 1.2 and 0.08 years, respectively.[Citation27] illustrated the calculated lost working day for each stage of BCC and SCC. We also performed a sensitivity analysis using a range from 5 to 10 working days lost.
Figure 2. Pathway for estimation of non-melanoma skin cancer working day loss. Notes:: NMSCs Non-Melanoma Skin Cancers; BCC: Basal Cell Carcinoma; SCC: Squamous Cell Carcinoma; LD: Lost days.
The third indirect cost category is the loss of future earnings due to premature mortality. To estimate the average present value of future earnings, we considered the number of fatalities from NMSC by age and sex, and compared them to the age and sex specific fatality rates of the general population. The present value in 2011 for labor-market earnings losses in years after 2011 was estimated for all paid labor-force time loss. For these estimates, we used the age and sex-specific average wage rates taken from the LFS,[Citation20] plus fringe benefits of 14%, consistent with other Canadian studies.[Citation33] All employment rates for the population were extracted from the 2011 LFS[Citation20] and the 2007 SLID.[Citation34] The LFS aggregates individuals above the age of 70, so the SLID public use file was used to get more granularity above age 70. A technical appendix summarizes the formulas for calculating output/productivity losses used for this study.
The fourth indirect cost category is home production losses associated with morbidity and premature mortality. In order to calculate this cost category, we identified the average time that men and women spend on various domestic related activities from the GSS.[Citation21] The monetary value of time in home production was estimated at the average hourly earnings for housekeepers and related occupations (occupation code 4412, $15.40 in 2011 Canadian dollars), based on SEPH.[Citation22] For morbidity, we estimated the potential home production losses based on the DWs and length of the disease stage. For terminal cases, home production was assumed to be zero, as cases usually lose their ability to perform daily domestic activities. For mortality, we estimated home production losses over the remaining standard life expectancy.
Intangible costs
Intangible costs (HRQoL costs) were captured through QALYs proxied with the HUI and then converted into monetary units using a value of $50,000 per QALY.[Citation35,Citation36] We identified HUI values of BCC and SCC for the local disease at 0.97 based on Chen et al.,[Citation37] and 0.67 for terminal cases based on Gaulin et al.[Citation38] Case QALYs were compared with population average QALYs, adjusted for age, sex, and population life expectancy. We also conducted a sensitivity analysis for this component using alternative monetary value of a QALY of, $100,000 and $150,000. The $50,000 rate has been used in Canada since the early 1990 s in the health technology assessment field. The $150,000 value is more reflective of willingness-to-pay values for a QALY identified in recent studies.[Citation39,Citation40] The $100,000 value is the midpoint between the two.
Results
There were 53,696 newly diagnosed cases of BCC and 18,549 newly diagnosed cases of SCC in Canada in 2011. As is illustrated in , based on OCRC estimates, 5.3% (2,846 cases) of BCC cases and 9.2% (1,710 cases) of SCC cases are attributable to occupational exposure to solar radiation.
Table 2. Incidence of basal cell carcinoma and squamous cell carcinoma for men and women according to the Occupational Cancer Research Center (2011).Footnotea
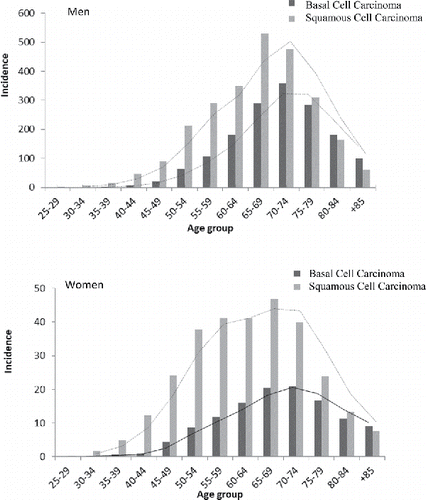
presents the distribution of the incidence rate of occupational NMSCs by age and sex group. For both sexes the age group of 65–69 has the highest rate of BCC and 70–74 has the highest rate of SCC. There were notable differences between men and women in the incidence of NMSCs attributable to occupational exposures. As expected, due to most outdoor workers being men, they have much higher occupational BCC incidence (9.0% of all cases) and occupational SCC (14.0% of all cases). For women, 1.3% of all NMSCs were attributable to occupational exposure (1.2% of occupational BCC, 1.7% of SCC), compared to 10.4% for men (see for details).
Figure 3. Distribution of occupational basal cell carcinoma and squamous cell carcinoma incidence by age and sex in 2011.
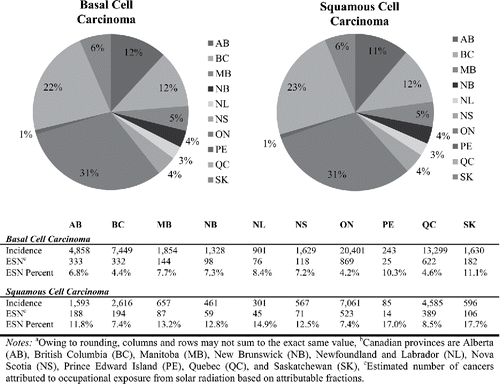
presents the distribution of occupational BCC and SCC cases by province. The estimated percentage of cancer cases attributable to occupational solar radiation exposure varies from the lowest percentages in Ontario to the highest in the province of Saskatchewan. However, data presented excludes the Yukon, Northwest Territories, and Nunavut, as there was not sufficient data to estimate cases for the territories. The provinces of Ontario (BCC: 868, SCC: 521) and Quebec (BCC: 622, SCC: 388) have the highest numbers of cases, which is due to their relatively large populations. The proportion of occupationally attributed NMSCs in Ontario and Quebec is 4.2% and 4.6% for BCC, and 7.4% and 8.5% for SCC, respectively. The highest proportion of occupationally attributed NMSCs is in Saskatchewan, where 11.1% of BCCs and 17.7% of SCCs are estimated to be caused by work-related sun exposure.
Figure 4. Distribution of occupational basal cell carcinoma and squamous cell carcinoma incidence and rates by province (2011).a,b
summarizes the direct, indirect and intangible costs of BCC and SCC. Direct costs are estimated at $14.4 million ($5,066 per-case) for BCC and $5.5 million ($3,198 per-case) for SCC. The total indirect costs are $1.1 million ($401 per-case) for BCC and $7.5 million ($4,379 per-case) for SCC. The estimated intangible costs for BCC and SCC are 0.6 million ($202 per-case) and $5.1 million ($2,978 per-case), respectively.
Table 3. Economic burden of occupational basal cell carcinoma and squamous cell carcinoma by age group (2011 Canadian dollars).Footnotea
As illustrated in , the sensitivity analyses indicated that the total BCC economic burden could be as low as $15.1 million and as high as $49.9 million, based on different assumptions for key parameters. For SCC, the range is $14.9 million to $29.3 million. Using different values for the monetary value of a QALY of $100,000 and $150,000 rather than $50,000 results in a change in total costs from 3.6–7.1% for BCC and 32.3–62.5% for SCC (see the Technical Appendix for details).
Table 4. Sensitivity analyses with different assumptions.Footnotea
To estimate a comparable burden for the U.S., we considered the incidence of NMSCs in the U.S. from the Guy et al. study (4.3 million in 2011).[Citation41] We assumed that 6.3% of all NMSC cases in the U.S. in 2011 (271,158 cases), were attributable to occupational sun exposure (similar to our study). We also assumed that the treatment methods and related costs are likely similar in both countries. Based on our conservative assumptions, we estimated the economic burden of occupational NMSCs in 2011 in the U.S. at $1.7 billion Canadian dollars. Since this calculation does not account for differences in the composition of the workforce or exposure differences associated with latitude (i.e., there are likely a higher number of cases per capita due to the lower latitude of the U.S.), it is likely that the estimate is low. It may also be low due to higher health care costs in the U.S. Thus, this example provides only a starting point for using our numbers to estimate burdens in other countries. For more precise country estimates, multiplier might be developed to adjust various cost components we have estimated for Canada.
Discussion
Our estimate of attributable fractions for occupational solar UV radiation exposure are 5.3% of newly diagnosed BCC cases and 9.2% of newly diagnosed SCC in 2011. Our estimate of the economic burden of newly diagnosed occupational NMSCs in Canada is $28.9 million for direct and indirect costs. Breakdown of our estimates between direct and indirect cost indicated that 70% of NMSC costs are associated with direct costs and 30% with indirect costs. Intangible costs are estimated at $5.7 million based on a value of $50,000 per QALY. We present direct and indirect costs separately from intangible costs due to possible overlap in these costs. Specifically, estimates of the value of a QALY may include output/productivity losses, since it is difficult to separate out HRQoL from other values derived from health.
This is the first study to exclusively consider occupational NMSCs, rather than all-cause NMSCs, therefore it is difficult to compare our estimated values with those found in the published literature. All of the quoted NMSC per-case costs from the literature are well below our estimate of $5,670 for BCC and $10,555 for SCC, respectively. For example, Krueger and colleagues estimated the direct costs for BCC and SCC at $536 and $812 in 2011 Canadian dollars,[Citation1] which is much lower than our direct costs. One of the reasons is that they only considered medical treatment costs, including primary care, hospital-based day surgery, and hospital inpatient care. Treatment costs associated with recurrences were not considered. They also did not consider informal caregiving and OOPCs in their direct costs calculation. They estimated the average indirect costs per-case of BCC and SCC at $445 and $2,392 in 2011 Canadian dollars, respectively. They used the algorithm developed by Lucas et al.,[Citation42] to determine the level of disability and the associated value of work-loss days following a diagnosis of BCC or SCC. They also attempted to address time costs such as unpaid work and leisure time to value what they describe as non-productive time. However, they did not include costs such as home production losses. Breakdown of their reported costs indicated that 41% of NMSC costs are attributed to direct costs and 59% attributed to indirect costs.
In the Orenstein et al. study in Alberta, Canada, researchers reported estimated per-case direct and indirect costs of NMSCs at $2,714 and $818 in 2011 Canadian dollars, respectively.[Citation8] These direct costs refer to healthcare expenditures by the government for the costs of treating these cancer patients, but they did not include OOPCs, nor informal caregiving costs in their direct costs calculation. In terms of indirect costs for calculating absenteeism, they only considered time seeking medical care (based on study of Chen et al.),[Citation43] however, this value is a part of total lost time, as the episodes of disease care includes other episodes such as illness episodes, disease episodes, health maintenance episodes, and care episodes.[Citation11] They also did not consider home production losses. Breakdown of their reported attributable costs indicated that 77% of total NMSC costs were direct costs and 23% were indirect costs.
Bickers et al. reported the direct and indirect costs of NMSCs in the US at $1,487 and $985 per-case in 2011 Canadian dollars, respectively.[Citation9] In terms of direct cost, although they considered different costs categories that relate to the hospital stays, doctors’ office visits, prescription drugs, and OOPCs in their calculations, they did not consider informal caregiving costs, but rather, put it under indirect costs. This may be one of the reasons that their indirect costs were higher than other studies. In their study, authors considered different indirect costs categories such as time spent seeking healthcare, lost or impaired ability to work, caregiver costs and premature mortality, but they did not consider home production loss costs. Breakdown of their reported costs indicated that 60% of total NMSC costs were attributed to direct and 40% attributed to indirect costs. The ratio of the intangible costs to the total direct and indirect costs was just 5%.
Kyle et al. reported direct costs of BCC and SCC at $2,545 and $6,449 per-case in 2011 Canadian dollars, respectively.[Citation10] They did not report the average cost per-cases for NMSCs. In terms of the direct costs, they only considered healthcare and did not include the OOPCs and caregiver costs. In terms of indirect costs, they considered the loss of productivity as a result of premature deaths, as well as caregiving performed by others, However, they did not include lost home production. Breakdown of BCC reported costs indicated that 46% of the costs were direct and 54% were indirect. The percentage of direct and indirect costs for SCC were 18% and 82%, respectively.
The magnitude and proportion of costs attributable to direct and indirect costs of NMSCs varies widely in the scientific literature, as indicated in previous studies such as Doran et al.[Citation44] Different studies have considered different cost elements in their direct and indirect cost components. In general, the main reason for our costs being higher than values from other studies is that we were able to include more cost elements. Our methodology includes many subcomponents of direct and indirect costs, based on previous frameworks and methodological approaches.[Citation13]
Lack of data for key inputs has often been cited as a limitation in occupational disease burden studies, and this is also the case with our study. Some assumptions (and sometimes compromises) were made to address data gaps. Our estimates are nonetheless underestimates, since not all resource implications of occupational NMSCs were included. This is because we lacked key data sources. For example, we did not include the effect of poor health on work performance, or presenteeism (working while in poor health).[Citation45] Presenteeism can decrease worker's productivity in the pre-diagnosis and after return to work periods. It is highly recommended that it be included as a factor in future studies to avoid underestimation of impacts on productivity. Additionally, we included in our calculations time off work for recovery, but we did not consider time off work for diagnostic procedures. Furthermore, the costs of lost work time can be substantially higher than the wage when perfect substitution of absent workers is not possible. This can also be a problem when there are team-based projects that require all workers to be present for full functioning, or where a penalty is associated with not meeting deadlines.[Citation46,Citation47]
Lack of data sources with which to develop our OOPCs estimates is another limitation. Since OOPCs can be markedly different for rural versus urban residents, we used an average value based on comparable studies and ran a sensitivity analysis for upper and lower limits of OOPCs. Lastly, we had data limitations in relation to return to work of cases with BCC and SCC.[Citation48]
To our knowledge, this is the first study in which the economic burden of occupational NMSCs attributable to solar radiation exposure has been estimated in Canada or elsewhere. We have attempted to be as comprehensive as possible in identifying the resource implications under three broad cost categories—direct, indirect, and intangible costs. Despite some uncertainties from required assumptions, the obtained results are as robust as possible, and the structure of the model gives insight into the total costs breakdown, pointing out the most relevant variables such as survival rate. The present study captured a significant portion of the economic burden of NMSCs that is sometimes described as the “hidden part of the iceberg of costs” in occupational health and safety.[Citation49] Future studies should attempt to account for a broader set of resource implications than those we included in the categories of direct, indirect, and intangible costs, in order to capture a larger part of the “hidden iceberg of costs.”
Conclusions
This study is part of a growing literature which attempts to put a monetary value on the costs of occupational carcinogen exposures. We anticipate that this study will provide insights for policy makers who make decisions about occupational cancer prevention resource allocations. Case-costing information from this study can also be used as an input for studies evaluating interventions to reduce occupational UV exposure in terms of their cost-benefit/cost-effectiveness. Thus, the results of this study can assist researchers interested in demonstrating the monetary impact of decreasing or eliminating occupational solar radiation exposures through various prevention measures.
Acknowledgments
The authors thank the Canadian Cancer Society, Occupational Cancer Research Centre (OCRC), and Institute for Work and Health (IWH) for their support.
Additional information
Funding
References
- Krueger, H., D. Williams, M. Chomiak, and L. Trenaman: “The Economic Burden of Skin Cancer in Canada: Current and Projected.” Available at http://krueger.ca/wp-content/uploads/2015/08/skincancer.pdf (accessed December 5, 2017)
- Bath-Hextall, F., J. Bong, W. Perkins, and H. Williams: Interventions for basal cell carcinoma of the skin: Systematic review. BMJ 329:705 (2004).
- De Gruijl, F.R.: Solar and ultraviolet radiation. Eur. J. Cancer. 35:2003–2009 (1999).
- Armstrong, B.K., and A. Kricker: The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. 63:8–18 (2001).
- Peters, C.E.: “Solar Ultraviolet Radiation and Outdoor Workers in Canada: A Program of Research on Exposure Assessment, Sun Protection Behaviours and Prostate Cancer Risk.” Available at https://open.library.ubc.ca/cIRcle/collections/24/items/1.0166296 ( accessed December 5, 2017).
- Peters, C.E, A.M. Nicol, and P.A. Demers: Prevalence of exposure to solar ultraviolet radiation (UVR) on the job in Canada. Can. J. Publ. Heal. 3:223–226 (2015).
- Fioletov, V., J.B. Kerr, and A. Fergusson: The UV index: Definition, distribution and factors affecting it. Can. J. Publ. Health. 101:5–9 (2010).
- Orenstein, M.R, T. Dall, P. Curley, et al.: “The Economic Burden of Occupational Cancers in Alberta.” Available at http://www.albertahealthservices.ca/poph/hi-poph-surv-phids-economic-burden-occup-cancer-2010.pdf ( accessed December 5, 2017).
- Bickers, D.R., H.W. Lim, D. Margolis, et al.: The burden of skin diseases: 2004: A joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J. Am. Acad. Dermatol. 55:490–500 (2006).
- Kyle, J.W., J.K. Hammitt, H.W. Lim, et al.: Economic evaluation of the US Environmental Protection Agency's SunWise program: Sun protection education for young children. Pediatrics 121:1074–1084 (2008).
- Chen, J.G., A.B. Fleischer, E.D. Smith, et al.: Cost of nonmelanoma skin cancer treatment in the United States. Dermatol. Surg. 27:1035–1038 (2011).
- Tompa, E., C. Kalcevich, C. McLeod, et al.: The economic burden of lung cancer and mesothelioma due to occupational and para-occupational asbestos exposure. Occup. Environ. Med. 74:816–822 (2017).
- Leigh, J.: Economic burden of occupational injury and illness in the United States. Milbank Quart. 89:728–772 (2011).
- Safe Work Australia: “The Cost of Work-related Injury and Illness for Australian Employers, Workers and the Community.” Available at https://www.safeworkaustralia.gov.au/system/files/documents/1702/cost-of-work-related-injury-and-disease-2012-13.docx.pdf ( accessed December 5, 2017).
- Yun, Y.L., J.W. Jin, K. Manikam, et al.: “WSH Institute Report: Economic Cost of Work-related Injuries and Ill-health in Singapore.” Workplace Safety and Health Institute. Available at https://www.wsh-institute.sg/files/wshi/upload/cms/file/Economic%20Cost%20of%20Work-related%20Injuries%20and%20Ill-health%20in%20Singapore.pdf ( accessed December 5, 2017).
- “Ontario Ministry of Healthcare. Schedule of Benefits.” Available at http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20151221.pdf ( accessed December 5, 2017).
- Statistics Canada: “Life Tables, Canada, Provinces and Territories 2010 to 2012.” Available at http://www.statcan.gc.ca/pub/84-537-x/84-537-x2016006-eng.htm ( accessed December 5, 2017).
- Canadian Community Health Survey (CCHS): “Canadian Health Utility Index (HUI).” Available at https://www.canada.ca/en/health-canada/services/food-nutrition/food-nutrition-surveillance/health-nutrition-surveys/canadian-community-health-survey-cchs.html ( accessed December 5, 2017).
- Statistic Canada: “Survey of Labour and Income Dynamics (SLID).” Available at http://statcan.gc.ca/pub/75f0011x/75f0011x2012001-eng.htm ( accessed December 5, 2017).
- Statistics Canada: “Sources of annual average growth in labour productivity in the total business sector, CANSIM table 383-0021.” Available at http://www.statcan.gc.ca/pub/15-206-x/2013030/t001-eng.htm (accessed December 5, 2017).
- Statistics Canada: “General Social Survey Cycle 24: Time-Stress and Well-Being Public Use Microdata File Documentation and User's Guide.” Available at http://gsg.uottawa.ca/data/teaching/eco/gssc24gid-ver4.pdf ( accessed 2011).
- Statistics Canada: “Survey of Employment, Payrolls and Hours (SEPH).” Available at http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=2818026&&pattern=&stByVal=1&p1=1&p2=31&tabMode=dataTable&csid= ( accessed December 5, 2017).
- Brown, T., A. Darnton, L. Fortunato, et al.: Occupational cancer in Britain. Respiratory cancer sites: Larynx, lung and mesothelioma. Br. J. Cancer 107:6–70 (2012).
- Cancer Care Ontario: “Burden of Occupational Cancer in Ontario: Major Workplace Carcinogens and Prevention of Exposure.” Available at http://www.occupational-cancer.ca/wp-content/uploads/2017/09/Burden-of-Occupational-Cancer-in-Ontario.pdf ( accessed December 5, 2017).
- Guy, G.P., and D.U. Ekwueme: Years of potential life lost and indirect costs of melanoma and non-melanoma skin cancer. Pharmacoeconomics 29:863–74 (2012).
- De Oliveira, C., K.E. Bremner, R. Pataky, et al.: Understanding the costs of cancer care before and after diagnosis for the 21 most common cancers in Ontario: A population-based descriptive study. C Open. 1:1–8 (2013).
- Lucas, R., T. McMichael, W. Smith, and B. Armstrong: “Solar Ultraviolet Radiation Global Burden of Disease from Solar Ultraviolet Radiation.” Available at http://www.who.int/uv/publications/solaradgbd/en/ ( accessed December 5, 2017).
- Marcil, I., and R.S. Stern: Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer. Arch. Dermatol. 136:1524–1530 (2000).
- Morris, S., B. Cox, and N. Bosanquet: Cost of skin cancer in England. Eur. J. Heal. Econ. 10:267–73 (2009).
- Longo, C.J., and B.G. Bereza: A comparative analysis of monthly out-of-pocket costs for patients with breast cancer as compared with other common cancers in Ontario, Canada. Curr. Oncol. 18:1–8 (2011).
- Yabroff, K.R., and Y. Kim: Time costs associated with informal caregiving for cancer survivors. Cancer. 115:4362–4373 (2009).
- Frick, K.D., S.M. Joy, D.A. Wilson, K.S. Naidoo, and B.A. Holden: The global burden of potential productivity loss from uncorrected presbyopia. Ophthalmology 122:1706–1710 (2015).
- Statistic Canada: “Economic Burden of Illness in Canada, 1998.” Available at https://www.canada.ca/content/dam/phac-aspc/migration/phac-aspc/publicat/ebic-femc/2005-2008/assets/pdf/ebic-femc-2005-2008-eng.pdf ( accessed 2002).
- Statistic Canada: “Survey of Labour and Income Dynamics (SLID).” Available at http://www.statcan.gc.ca/pub/75f0002m/75f0002m2012003-eng.pdf ( accessed December 5, 2017).
- Sander, B., J.C. Kwong, C.T. Bauch, et al.: Economic appraisal of Ontario's universal influenza immunization program: A cost-utility analysis. PLoS Med. 7:e1000256 (2010).
- Oliveira, C., H.V. Nguyen, H.C. Wijeysundera, et al.: Estimating the payoffs from cardiovascular disease research in Canada: An economic analysis. C Open 1:E83–90 (2013).
- Chen, S.C., A.M. Bayoumi, S.L. Soon, and A. Kent: A catalog of dermatology utilities: A measure of the burden of skin diseases. J. Investig. Dermatol. Symp. Pro.c 9:160–168 (2004).
- Gaulin, C., D.F. Sebaratnam, and P. Fernández-Peñas: Quality of life in non-melanoma skin cancer. Australas. J. Dermatol. 56:70–76 (2015).
- Neumann, P.J., J.T. Cohen, and M.C. Weinstein: Updating cost-effectiveness—The curious resilience of the $50,000-per-QALY. Threshold N. Engl. J. Med. 371:796–797 (2014).
- Laupacis, A., D. Feeny, A.S. Detsky, and P.X. Tugweli: How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 146:473–481 (1992).
- Guy, G.P., S.R. Machlin, D.U. Ekwueme, and K.R. Yabroff: Prevalence and costs of skin cancer treatment in the U.S., 2002−2006 and 2007−2011. Am. J. Prev. Med. 48:183–187 (2015).
- Lucas, R.M., A.J. McMichael, B.K. Armstrong, and W.T. Smith: Estimating the global disease burden due to ultraviolet radiation exposure. Int. J. Epidemiol. 37:654–567 (2008).
- John Chen, G., C.B. Yelverton, S.S. Polisetty, et al.: Treatment patterns and cost of nonmelanoma skin cancer management. Dermatol. Surg. 32:1266–1271 (2006).
- Doran, C.M., R. Ling, J. Byrnes, et al.: Estimating the economic costs of skin cancer in New South Wales, Australia. BMC Publ. Health 15:952 (2015).
- Roy, J.S., J.C. MacDermid, B.C. Amick III, et al.: Validity and responsiveness of presenteeism scales in chronic work-related upper-extremity disorders. Phys. Ther. 91:254–266 (2011).
- Pauly, M.V., S. Nicholson, J. Xu, et al.: A general model of the impact of absenteeism on employers and employees. Health Econ. 11:221–231 (2002).
- Nicholson, S., M.V. Pauly, D. Polsky, et al.: Measuring the effects of work loss on productivity with team production. Health Econ. 15:111–123 (2006).
- Vuong, K., K. McGeechan, B. K., Armstrong, and A.E. Cust: Occupational sun exposure and risk of melanoma according to anatomical site. Int. J. Cancer. 134:2735–2741 (2014).
- Sun, L., O. Paez, D. Lee, S. Salem, and N. Daraiseh: Estimating the uninsured costs of work-related accidents, part I: A systematic review. Theoret. Iss. Ergon. Sci. 7:227–245 (2006).
