Abstract
Reusable Powered Air Purifying Respirators (PAPRs) have been increasingly used as an alternative to disposable masks or respirators for healthcare workers needing protection from respiratory droplets containing respiratory viruses, but little information is available concerning how well PAPRs resist damage from repeat disinfection over their lifetime. This study tested parts from four PAPRs against four commercially available hydrogen peroxide and quaternary ammonium chloride disinfectants by immersion for 28 days to simulate prolonged exposure. Risk of surface damage was assessed through color change, mass change, and visual observation of damage. Minimal risk of damage was found for three of the disinfectants tested and for the fourth disinfectant, a risk of surface damage to a small number of parts. Exposure to tap water caused similar damage in many cases. The study demonstrated that risk of surface damage varied by part and disinfectant, indicating that some disinfectants are more likely to be compatible against the wide range of materials and parts in a commercial PAPR and other disinfectants may show varying compatibility, with more risk to certain materials or parts.
Introduction
Since early 2020, the COVID-19 pandemic has caused significant shortages of personal protective equipment (PPE), especially for healthcare workers (HCWs) in acute care facilities (Cohen and van der Meulen Rodgers Citation2020). The United States is both the country with the most reported cases and deaths from COVID-19 as of January 1, 2022, and the largest importer of personal protective equipment (PPE) used in healthcare facilities, including gloves, gowns, masks, and respirators (Cohen and van der Meulen Rodgers Citation2020). The United States Centers for Disease Control and Prevention (US-CDC) guidance for HCWs treating COVID-19 patients emphasizes the need for respiratory protection to protect the HCW from the risk of infection (CDC Citation2021). US-CDC recommends the use of a National Institute for Occupational Safety & Health (NIOSH)-approved disposable N95 filtering facepiece respirator, or other respirators providing a similar level of protection, or disposable face masks, during the delivery of care with the mask to be discarded and a new one donned after the patient care encounter (CDC Citation2021). A May 2020 survey in the United States found that 87% of nurses reported reusing single-use disposable masks or N95 respirators and 27% of nurses reported being exposed to confirmed COVID-19 patients without wearing appropriate PPE due to shortages of PPE supply (Cohen and van der Meulen Rodgers Citation2020). Before the COVID-19 pandemic, Chughtai et al. (Citation2019) tested used medical masks in hospitals in Beijing, China, and found 10.1% (15/148) were contaminated with respiratory viruses, with the probability of the mask being positive for a respiratory virus increasing with a longer time of wearing the mask. HCWs are at risk of touching their contaminated mask and potentially contaminating their hands as masks may be removed between patients, to better communicate with some patients, or to readjust a mask that has shifted on their face during patient care.
Reusable powered air-purifying respirators (PAPRs) are another type of respirator and are increasingly being considered as an alternative to disposable N95 respirators for several reasons. These include less waste generation, lower cost to use over time, increased comfort for the HCW, superior air filtration when compared to N95 respirators, improved communication with patients, and the ability to wear some models while having limited facial hair (CDC Citation2020). PAPRs may include a tight-fitting facepiece or loose-fitting hood or helmet, a tube for supplying filtered air, a power pack with a rechargeable battery, and replaceable filters. PAPRs may also protect from airborne hazardous drugs and splash protection to the eyes and face without needing goggles (CDC Citation2020). PAPRs can interfere with some HCWs’ visual field and prevent the use of stethoscopes, which may prevent PAPR use in all patient care situations (CDC Citation2020).
When used, PAPRs require a maintenance program to ensure worn components are replaced and that the PAPR is routinely cleaned and disinfected (CDC Citation2020). Because respiratory viruses and bacteria can survive on environmental surfaces for times varying from hours to weeks (Kramer et al. Citation2006), PAPRs must be routinely cleaned and disinfected to prevent fomite transmission. Chakladar et al. (Citation2021) found that traditional disinfection procedures for PAPRs were often not adequate to remove bacterial, fungal, and viral contamination and found contamination in 40% (10/25) of previously cleaned and disinfected PAPR units.
Manufacturers of PAPRs recommend cleaning procedures in their instructions-for-use (IFU), but rarely test the PAPR components for compatibility with a wide range of common healthcare disinfectants. When disinfecting a PAPR, the US-CDC recommends using disinfectants on the US-EPA list N to ensure that the disinfectant is effective against SARS-CoV-2 and other potential respiratory viruses (CDC Citation2020). US-CDC also provides general cleaning and disinfection guidance for PAPRs on their website, and cautions that there have been some reports of specific disinfectants causing damage to certain PAPR components, and comments that disinfectant compatibility with PAPR components is not well understood (CDC Citation2020). The goal of this study was to test components of several commercially available PAPRs for damage from exposure to the effects of routine disinfection with commonly used hydrogen peroxide-based or quaternary ammonium-based disinfectants.
Materials and methods
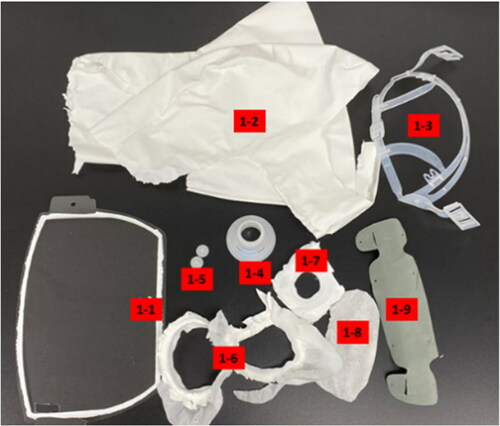
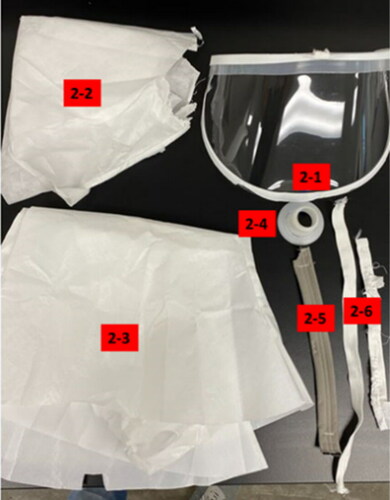
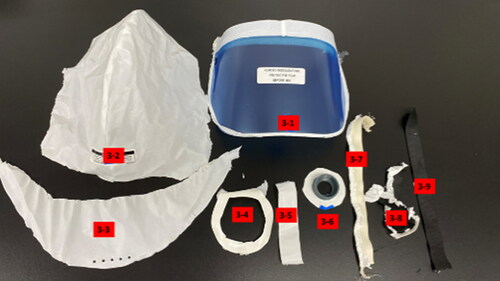
Four PAPRs were purchased from a commercial source. The PAPRs included in the testing included the 3M Versaflo S-553L, the 3M Versaflo S-403L, the 3M Versaflo S-133L-5 (St. Paul, MN), and the Sentinel XL PAPR (Frederica, DE). All four PAPRS were photographed, disassembled (or cut apart), parts labeled (), and photographed again, as shown below ().
Table 1. Grouping of materials tested with part number.
All components were analyzed by Fourier Transform Infrared Spectroscopy (FTIR) to determine the polymer materials used in construction. Parts made from foam and any absorbent fabrics were set aside as these materials cannot be disinfected with a liquid- or wipe-based disinfectant. Larger parts were cut into smaller pieces to facilitate testing and analysis. For the three PAPR models made by 3M, several components were identified as being the same size, shape, and material between models.
Four commercially available liquid disinfectants (Diversey, Fort Mill, SC) were used in the testing, as shown in . One of the quaternary ammonium chloride disinfectants (QA1) is labeled to be used at two different dilutions and was tested at both dilutions (QA1.1, QA1.2). Tap water was also tested and used as a control.
Table 2. Hydrogen peroxide and quaternary ammonium chloride disinfectants used in testing.
Face shield and plastic component samples were cleaned, weighed, and completely submerged in the test liquid in a method similar to that of Lawrence et al. (Citation2017). While most healthcare facilities will not routinely disinfect PAPRs by immersion in liquid disinfectants and PAPR manufacturers will commonly recommend disinfection by manually wiping the unit, using component immersion in liquid disinfectant is a common industry practice for material compatibility testing as it provides control of several important variables affecting the rate of surface damage and our internal testing has correlated results with those from manually wiping the surface.
The containers were sealed to prevent evaporation and stored at room temperature for 28 days. Samples were then removed, rinsed with water, and patted dry with a disposable paper towel. Once dry, the samples were weighed again and color change was measured by a Konica Minolta CM-2500D spectrophotometer (Ramsey, NJ), with color measurements taken in triplicate and averaged. Mass change and delta E (L*a*b*) data are reported below. Delta E is a widely used measure of color change that is the unitless result of the L*a*b calculation and is reported on a scale from 0 to 100 without any additional statistical analysis.
Coated fabric specimens were tested according to ACT Test Method 1-2020: Assessing Resistance to Liquid Cleaners, Sanitizers, and Disinfectants, Part 2 (Association for Contract Textiles Citation2020). In this procedure, 2.5 mL of the liquid is applied to the coating side of the fabric and allowed to dry overnight. This was repeated five times, with any residue removed with a tap water rinse, and the sample wiped dry. The coating was then qualitatively evaluated for general damage, discoloration, gloss change, cracking, peeling, and bubbling and given a rating according to 2.6.1 of the ACT method () (Association for Contract Textiles Citation2020). Color measurements were made on the fabrics before and after the soaking procedure.
Table 3. Section 2.6.1 of ACT Test method 1-20207, qualitative damage ratings.
All treated samples were also evaluated by FTIR to investigate chemical changes in functional groups of the polymers. However, the analysis did not yield any useful results as the spectra did not change after treatment, even for samples with obvious damage (spectra/data not shown).
Data analysis methods: Changes in mass and color for each component were compared to the changes for the water-exposed components. Using the ACT Test Method (Association for Contract Textiles Citation2020), when the changes resulting from exposure to water were similar to the changes resulting from exposure to a disinfectant (no or slight changes), the disinfectant was qualitatively assessed as being compatible. When exposure to the disinfectant compared to water resulted in changes graded as moderate or severe, the disinfectant was qualitatively assessed as being incompatible. In this way, recommendations for disinfectant use can be made to facilities for the disinfectants and PAPRs assessed in this work.
Results
shows data for all face shield components ( Material groups 1, 5, 7, and 10). Most samples showed negligible mass gain and/or color change, consistent with soaking in water. Of the samples tested, 8.3% (2/24) showed obvious visual signs of damage, observed as changes in softening of the plastic and whitening and characterized by color change and mass change. Both damaged samples were a polyester material soaked in HP1, which caused significant surface damage, and would not be recommended for disinfecting the face shields of these PAPRs. Interestingly, sample group 7 was not damaged by HP1, even though it was also identified as being polyester material. This highlights that all materials in the same class of polymers (in this case polyester) may not have the same compatibility profile, even with the same disinfectant, and testing of the specific grade of polymer used in the component should be tested to ensure compatibility.
Table 4. Mass and color change data for face shield specimens soaked in test liquids.
shows the qualitative results of testing on coated fabrics (Material Groups 2, 6, 8, and 11). Of the tested components, only the polyurethane-coated fabrics (Material Group 2) showed any evidence of damage after soaking in the disinfectants. HP1 caused the coating to discolor slightly and begin to crack. QA2 and QA1.2 caused the coating to soften slightly, a degradation mode that is not listed in the ACT method, thus the ratings show only a slight change in general appearance. Testing of the other coated fabrics (Material Groups 6, 8, and 11) resulted in no apparent damage.
Table 5. Qualitative ratings of coated fabric materials tested per ACT Test Method 1-2020, part 2.
shows the color change data from the same test materials, and confirms that only the polyurethane-coated fabric soaked in HP’ had a significant color change (delta E > 1.0).
Table 6. Color change data (delta E) for coated fabrics.
shows the mass and color change data for the plastic components tested (Material Groups 3, 4, and 9). There were no obvious signs of damage to any of these parts after soaking in any of the disinfectants or the tap water control. Mass change, color change, and visual assessment showed minimal surface damage between all the tested disinfectants and the control (tap water). However, the PVC part (Group 9) soaked in HP1 did absorb 2.9% of its original mass, which is more than the other liquids tested. Although the mass change was higher in this case, it is still very low compared to the polyester materials, with obvious damage that gained >10% of their initial mass. Thus, HP1 would still be recommended for use on this part.
Table 7. Mass and color change data for plastic component specimens soaked in test liquids.
Discussion
Commercially available Powered Air Purifying Respirators (PAPRs) are composed of a range of parts and polymeric (plastic) materials that need to withstand frequent disinfection to provide acceptable performance for HCW. This study is the first we are aware of to systematically test the various components of several commercially available PAPRs against a series of commercially available disinfectants to characterize the potential for surface damage from repeat exposure through routine disinfection. We found minimal risk of damage for three of the disinfectants tested and for the fourth disinfectant, a risk of surface damage to a small number of parts. We demonstrated that two disinfectants with similar levels of the active ingredient, in this case, two hydrogen peroxide disinfectants with the same activity level of peroxide, can have somewhat different surface compatibility risks, suggesting that facilities should be cautious in using disinfectants on PAPRs without compatibility testing or in extending compatibility data generated on one disinfectant to other similar disinfectants without additional testing.
Our study has several limitations. The method of preparing samples in our study may not reflect the real-world damage for a range of reasons including how the disinfectants are used, choice of disinfectant, and length of time the disinfectant is in contact with the surface. For HP1, while damage was observed to several parts, the exposure time used in the study would be approximately equivalent to over 40,000 applications (28 days of constant exposure = 40,320 min, which for a disinfectant with a 1-min contact time would be the equivalent of 40,000+ applications of the disinfectant if the disinfectant kept the surface wet only for the label contact time) and may not represent real-world conditions, potentially exceeding the expected life of the PAPR. Additionally, the disinfectants tested here may not be representative of all disinfectant chemistries on the market. Last, the manufacturers of PAPRs may change their components or materials used without notification to healthcare facilities, which may compromise the value of prior compatibility data.
Conclusions
This study showed that risk of surface damage varied by part and disinfectant, indicating that some disinfectants are more likely to be compatible against the wide range of materials and parts in a commercial PAPR and other disinfectants may show varying compatibility, with more risk to certain materials or parts. The data suggest that parts made of polyolefins, such as polyethylene and polypropylene, are less susceptible to damage by disinfectants. However, material alone does not necessarily predict the risk of surface damage as we found two different parts made from the same plastic were very different in their interaction with the disinfectants. This testing underscores the need for healthcare facilities to have compatibility data for their routinely used disinfectants with their PAPRs to ensure there is minimal risk of compromising the performance of the PAPR through damage to the components from routine disinfection.
Data availability statement
The data that support the findings of this study are available from the corresponding author, PT, upon reasonable request.
Additional information
Funding
References
- Association for Contract Textiles. 2020. ACT test method 1-2020: assessing resistance to liquid cleaners, sanitizers, and disinfectants; [accessed 2021 Jan 11]. https://contracttextiles.org/wp-content/uploads/2020/08/act_test_method_resistance_052720-FINAL.pdf.
- Centers for Disease Control and Prevention (CDC). 2020. Considerations for optimizing the supply of powered air-purifying respirators (PAPRs) for healthcare practitioners (HCP); [accessed 2022 Jan 6]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/powered-air-purifying-respirators-strategy.html.
- Centers for Disease Control and Prevention (CDC). 2021. Interim infection prevention and control recommendations for healthcare personnel during the Coronavirus Disease 2019 (COVID-19) pandemic; [accessed 2022 Jan 6]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html.
- Chakladar A, Jones CG, Siu J, Hassan-Ibrahim MO, Khan M. 2021. Microbial contamination of powered air purifying respirators (PAPR) used by healthcare staff during the COVID-19 pandemic: an in situ microbiological study. Am J Infect Control. 49(6):707–712. doi:10.1016/j.ajic.2021.02.006
- Chughtai AA, Stelzer-Braid S, Rawlinson W, Pontivivo G, Wang Q, Pan Y, Zhang D, Zhang Y, Li L, MacIntyre CR. 2019. Contamination by respiratory viruses on outer surface of medical masks used by hospital healthcare workers. BMC Infect Dis. 19(1):491. doi:10.1186/s12879-019-4109-x
- Cohen J, van der Meulen Rodgers Y. 2020. Contributing factors to personal protective equipment shortages during the COVID-19 pandemic. Prev Med. 141:106263. doi:10.1016/j.ypmed.2020.106263
- Kramer A, Schwebke I, Kampf G. 2006. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 6:130. doi:10.1186/1471-2334-6-130
- Lawrence C, Harnish DA, Sandoval-Powers M, Mills D, Bergman M, Heimbuch BK. 2017. Assessment of half-mask elastomeric respirator and powered air-purifying respirator reprocessing for an influenza pandemic . Am J Infect Control. 45(12):1324–1330. doi:10.1016/j.ajic.2017.06.034