ABSTRACT
Background: The explanation of uterine and vaginal embryogenesis in humans still poses many controversies, because it is difficult to assess early stages of an embryo. The literature review revealed many disagreements in Mullerian theory, inciting some authors to propose new embryological hypotheses. In the original Mullerian theory: the paramesonephral ducts form the Fallopian tubes, uterus and vagina; the mesonephral ducts regress in female embryos. Aims: The aim of this article is to investigate the development of Mullerian ducts in humans, using comparative analysis of fundamental embryological theory and various utero-vaginal anomalies. Material and methods: Between 1998 and 2015, 434 patients with various uterovaginal malformations had been operated on at the Scientific Centre of Obstetrics Gynaecology and Perynatology in Moscow. The anatomies of the uterovaginal malformations in these patients were diagnosed with ultrasound and MRI and then verified during surgical correction by laparoscopy. Results: A systematic comparison of uterovaginal malformations to those in the literature has allowed us to formulate a new theory of embryonic morphogenesis. The new theory is significantly different: ovary, ovarian ligamentum proprium, and ligamentum teres uteri derive from gonadal ridges; Fallopian tubes and vagina completely develop from mesonephral ducts. The uterus develops in the area of intersection between the mesonephral ducts with gonadal ridges by the fusion of the two. Conclusions: The new theory may to induce future embryological studies. The hypothetic possibility that the ovary and endometrium derive from the gonadal ridges could be the key to understanding the enigmatic aetiologies of extragenital and ovarian endometriosis.
INTRODUCTION
Congenital uterovaginal malformations are defined as deviations from normal anatomy resulting of embryological maldevelopment. The aim of this article is comparative analysis of fundamental embryological theory and various utero-vaginal anomalies. The literature review revealed many disagreements in Müllerian theory, inciting some authors to propose new hypotheses. The original theory we have proposed in this paper may be able to explain the process of normal uterovaginal embryogenesis and the abnormal development of the presented clinical cases.
According to the original Müllerian (1830) theory of uterovaginal embryogenesis, gonads appear as a pair of longitudinal ridges on the dorsolateral sides.Citation1,2 Initially two pairs of genital ducts, mesonephral (Wolffian) and paramesonephral (Müllerian), form in both sexes. On the medial part, immediately below of the gonadal ridges, the mesonephral ducts grow caudally. The Müllerian ducts develop autonomously and parallel to the paramedial parts of the Wolffian ducts. Two paramesonephral (Müllerian) ducts are conjoined in the midline in the caudo-cranial direction. The two ducts are initially separated by an intermediate septum. The intermediate uterovaginal septum decreases and fuses into a single canal, forming the unicavital (normal) uterus and vagina. The Fallopian tubes, uterus and vagina develop from pairs of paramesonephral (Müllerian) ducts. The vestibulum vaginae completely derive from the sinus urogenitalis. Although the mesonephros and mesonephral ducts form rete testis and seminiferous ducts in males, they reduce in females.Citation1,2
In most textbooks, the contemporary theories of uterovaginal development show some differences, considering the dual origin of vagina. The Fallopian tubes, uterus and proximal 1/3 of the vagina derive from the paramesonephral ducts. The vestibulum vaginae and distal 2/3 of the vagina derive from the urogenital sinus. For a review see refs.Citation3-9
Several authors had researched the origin of the vagina, they considered this issue has been insufficiently studied. For a review see refs.Citation10-21
MATERIAL AND METHODS
Between 1998 and 2015, 434 patients with various uterovaginal malformations had been operated on at the Scientific Center of Obstetrics Gynecology and Perynatology in Moscow (). The anatomies of the uterovaginal malformations in these patients were diagnosed with ultrasound and MRI and then verified during laparoscopy.
TABLE 1. The Types of Uterovaginal Malformation in 434 Patients and the Appropriate Surgical Treatment.
All cases were categorized using an embryological-clinical classification system of Müllerian malformation (i.e., the American Society for Reproductive Medicine [ASRM] classification). For a review see refs.Citation22-24
The ESHRE/ESGEFootnote (2013) Consensus has been accepted the ASRM classification, that provides a precise diagnosis and appropriate clinical management, even with complex or atypical malformations of the female genital tract due to congenital anomalies.Citation24
Surgical treatment performed according of the anomalies type (), the patient's symptoms and the concomitant gynecological pathology by laparoscopy and hysteroscopy, following histological investigation.
The study had approved by institutional ethics committee.
RESULTS
Developmental uterovaginal anomalies (named after Mullerian) result from the influence of teratogenous lesions during critical periods of morphogenesis, each variant of them corresponds to persistent normal embryonic development stages.
The early stage of embryonal development () corresponds to uterovaginal aplasia, Mayer-Rokitansky-Kuster-Hauser (MRKH) syndrome ().
FIGURE 1. New Theory of Uterovaginal Embryogenesis. Schematic studies of the embryogenesis of the uterus (U) and vagina (V) from the gonadal ridges (GR) and mesonephral ducts (MD). Vestibulum vaginae (VV – yellow) from sinus urogenitalis (S).There are two pairs of ducts: on the left side - pairs of ducts in undifferentiated stages; and on the right side – corresponding uterine and vaginal derivatives.The gonadal ridges (GR-blue), as longitudinal pairs, grow caudally. The upper parts (before intersection with mesonephral ducts) form gonads (ovaries) and ligamentum ovary (LO); the lower part forms the ligamentum teres uteri (LT). Mesonephros (Ms) reduces in female embryo. The upper part of the mesonephral duct (MD- red) lies on the paramedial (lateral) sides of the GR and become the Fallopian tubes (F). The area of intersection between the mesonephral ducts with the gonadal ridge develops into the uterine fold (UF) with the endometrial (E) cavity inside. Below of the UF, both mesonephral ducts (MD) form the vagina. They grow caudally and conjoin together in the midline and pass into the sinus urogenitalis (S). The vestibulum vaginae (VV) derives from the sinus urogenitalis (S-yellow).
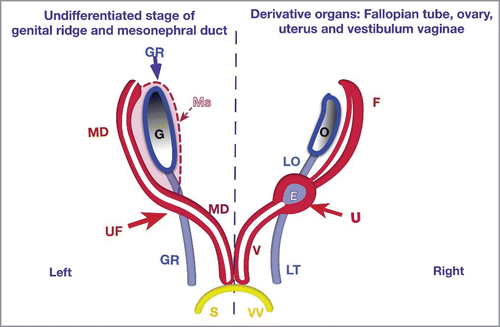
FIGURE 2. Uterovaginal Aplasia Corresponds to Early Stage of Embryogenesis. O – ovary, F – Fallopian tube, LO – ligamentum ovaricum proprium, LT – ligamentum teres uteri U – uterine rudiment, Laparoscopically there are two uterine rudiments (U) detected in the crossing area between the Fallopian tubes (F) with ligamentum teres uteri (LT) and ligamentum ovaricum proprium (LO).
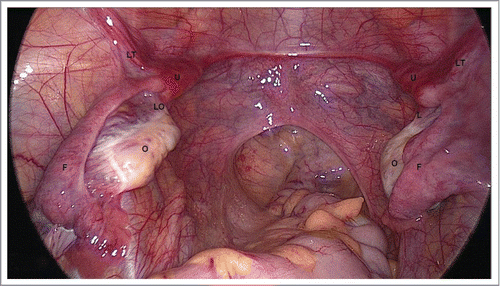
During laparoscopy, all (43) patients with uterovaginal aplasia (MRKH syndrome) had rudimentary uterine horns. The non-functional rudiments had 37 patients, approximately 20х12х15 mm in size (derivatives of Müllerian ducts) and normally shaped Fallopian tubes and ovaries. Both rudimentary horns were detected in the crossing area between the Fallopian tubes, with round ligaments and ovarian ligaments. The functional uterine rudiments with endometrial cavities had 6 patients, which removed laparoscopically.
Fusion defects of Müllerian ducts result in symmetric malformations: duplicate uterus and vagina (), bicornuate uterus, and septate uterus ().Citation1,2,6,8
FIGURE 3. Schematic Stage of Duplicated Uterus. The uterus (U) and vagina (V) are duplicated before fusion. The uterus is detected in the crossing area between the Fallopian tubes with ligamentum teres uteri and ligamentum ovaricum proprium.
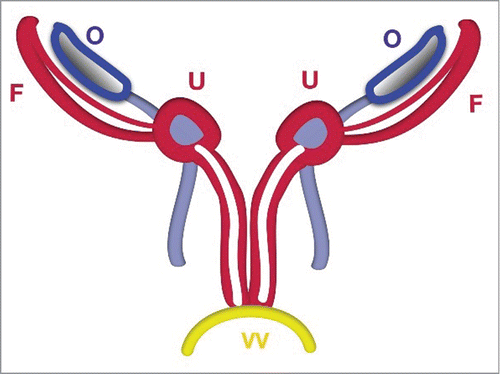
FIGURE 4. Schematic Stage of Two Utero-Vaginal Fusion. The 2 utero-vaginal ducts are initially separated by an intermediate septum. Fusion of the hemi-uterus and hemi-vagina forms a complete septate uterus with a duplicated vagina. The intermediate septum reduces and forms a normal uterus. The hymen (H-yellow) is a mucous membrane between the fused mesonephral ducts with the urogenital sinus.
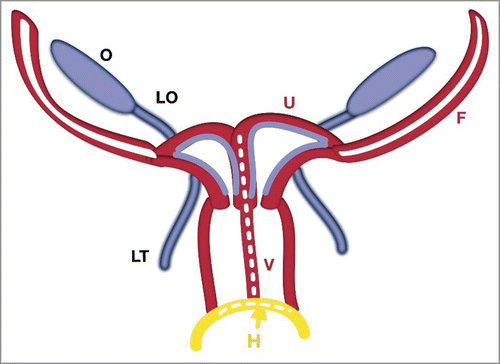
The clinical cases (in 56 patients) of completely duplicate uterus and vagina possibly arose during the developing stages of paired Müllerian ducts before their fusion.
These cases of fully duplicate vagina triggered controversy over contemporary views that describe the origin of the proximal 1/3 part of the vagina from paramesonephral (Müllerian) ducts and the distal 2/3 from the sinus urogenitalis. The urogenital sinus, as a part of the cloaca, is an unpaired structure.
Later, the pair of Müllerian ducts fuses together and the intermediate uterovaginal septum separates into two uterine cavities and cervical canals. Persistence of this stage corresponds to a completely septate uterus (45 patients).
Uterine malformation during subsequent stages results in a subseptate uterus (71 patients).
The uterovaginal septum reduces and finally forms the unicavital normal uterus.
The last stage of uterine development, after the reduction of the intermediate septum, is an arcuate uterus (42 patients). The normally shaped uterus becomes under the influence of high estrogen levels.
Distal aplasia of 1/3 or 2/3 of the vagina (39 patients) with obstruction of menstrual outflow are due to vaginal outgrowth defects of the cloaca.
Complete cervico-vaginal aplasia (11 patients) with functional uterus suppose the controversy of “unidirectional” caudo-cranial theory of Mullerian ducts fusion.
Hymen imperforatum (17) – is the last stage of vaginal connection onto the cloaca. Normally, the hymenal septum between vagina and vestibulum vaginae perforates due to physiological apoptosis (O'Rahilly, 1977).Citation5
The vestibulum vaginae had normal anatomy in all of the patients with uterovaginal aplasia (MRKH syndrome), partial or complete vaginal aplasia, or fully duplicated vagina. This demonstrated that the vestibulum vaginae derive from the sinus urogenitalis.Citation1,2
According to embryological data, the ureters and permanent kidneys develop from ureteric buds as outgrowths of the distal part of mesonephral ducts close to the entrance of the cloaca.Citation8
A high (12%) incidence of renal abnormalities was found in (52) patients with uterine malformations (from 434 patients). Clinical analysis estimates that unicornuate uterus (32 patients) is associated with ipsilateral renal aplasia in 64% of cases, whereas duplicated uterus and vagina (30 patients), with partial aplasia of the hemyvagina, is associated with ipsilateral renal aplasia in all (100%) cases.
Because all cases of unilateral renal agenesis are associated with ipsilateral blind vagina, it has been hypothesized that the vagina derives from the mesonephral (not the paramesonephral) ducts.
The 52% (225) patients with uterovaginal anomalies had concomitant gynecological diseases: leiomyoma (82), peritoneal adhesions (78), para-ovarian cysts (69), ovarian cysts (47), polycystic ovary (39) et al.
The extra-genital endometriosis removed in 46% patients with uterovaginal anomalies, deep infiltrative endometriosis in 23%, ovarian endometriosis in 6%, adenomyosis revealed in 8% females.
Presntation of Hypothesis
The ovary (O), ovarian ligamentum proprium (LO) and ligamentum teres uteri (LT) derive from the gonadal ridge (GR) ().
The vagina (V), uterus (U) and Fallopian tubes (F) derive completely from fused mesonephral ducts.
The uterus (U) develops in the area of intersection between the mesonephral ducts with the gonadal ridges.
The normal (unicavital) uterus is created by the fusion of the uterine folds (UF).
The endometrium inside of the uterine fold derives from gonadal ridges (E).
The Fallopian tubes in females and the seminiferous ducts in males are analogs, and both develop from the mesonephral ducts (MD).
The vestibulum vaginae (VV) derives from the urogenital sinus (S).
The hymen is a mucous membrane between the fused mesonephral ducts with the urogenital sinus (part of the cloaca).
DISCUSSION
We found some very important evidence in the literature and in contemporary embryological investigations confirming our new theory.
Sanchez-Ferrer M. et al. (2006) immunohystochemically investigated the “experimental contributions to the study of the embryology of the vagina” in rats and found that the human vagina derives from the mesonephral ducts.Citation21
In accordance with contemporary embryology, the male prostatic utricle is a rudimentary analog of the female uterus. The prostatic utricle is believed to be a remnant of the fused caudal ends of the paramesonephral ducts.Citation3,6,7
Shapiro E et al (2004) used immunohystochemical probes and provided strong evidence that “the prostatic utricle is not a Müllerian duct remnant” but of a urogenital sinus origin.Citation25
JP Müller (1830) described two pairs of ducts: mesonephral ducts (Wolffian) located on the medial parts below the gonadal (genital) ridges and the paramesonephral ducts (so-called Mullerian) located on paramedial sides.Citation1
Recent embryological investigations with electronic microscopy have revealed some nuances: the mesonephral ducts are located on the external part of the mesonephros and the paramedial side of the gonadal ridges.
In the IX-th edition of Langman's Medical Embryology (Sadler, 2000; page 324, Fig. 14.3 C), there is an electron micrograph of a mouse female embryo showing the genital ridges (arrow) growing immediately below of the gonads. Mesonephral ducts (arrowheads) are located on the paramedial sides of the mesonephros.Citation3
According to Hill (2015) in the histological images of human female embryo on Carnegie stage 22 (approximately equal to week 8 of development) mesonephral ducts are located on the paramedial sides.Citation6
Hurst C et al (2002), investigated in utero exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) during the critical periods of female rats organogenesis. In support of our new theory, there is evidence of uterine development in the area of intersection between mesonephral ducts with gonadal ridges and fusion of both uterine folds. Figure 6 shows that there are two Müllerian ducts fusing with Wolffian ducts separately on the left and right sides in the early stage of gestation (GD 18 and 19) (Fig. 6 A, C). Then, on GD 21, both duct pairs fused and conjoined with one another on their medial parts (Fig. 6 A, C).Citation26
Hashimoto (2003) in a study of human embryos development by electron microscopy on Carnegie stages 18-23, detected the active cell-to-cell communications between the Mullerian and Wolffian cells.Citation27
Uterovaginal anomalies represent with a prevalence rate of 4–7% in females.Citation27
Using embryological origin for classification of these patients makes sense and enables effective diagnosis and treatment as well as a better understanding of their pathogenesis.Citation22,24
The prevalence of the extra-genital endometriosis in female population is 6–10%; the frequency increases to 35–60% in women with pain, infertility or uterovaginal malformations. For a review see refs.Citation28–30 Signorile et al (2010) demonstrated the presence of ectopic endometrium in a significant number (in 7.7% of cases) of human female fetuses analyzed by autopsy.Citation28
Some authors have reported endometriosis in patients with complete utero-vaginal aplasia.Citation31,32
The extra-genital endometriosis was the major concomitant pathology revealed in 46% patients with uterovaginal anomalies with obstruction of menstrual outflow (partial or complete vaginal aplasia, functional uterine horn) and in non-obstructive symmetric malformations (uterus duplex, septate uterus).
The most widely accepted theory for the pathogenesis of endometriosis is the retrograde menstruation and heterotopic implantation of endometrial glands and stroma at extra-uterine sites. Multiple hypotheses also have been postulated to explain the pathogenesis of endometriosis: lymphatic and vascular metastases, immunologic deficiency, and coelomic metaplasia. Knapp V (1999) has postulated that endometriosis is caused by small defects of embryogenesis. For a review see refs.Citation28-32
However, these theories fail to explain the presence of endometriosis under the peritoneum, especially in fetuses and in females with complete utero-vaginal aplasia.
The theory we have proposed in this paper may be able to explain the process of normal uterovaginal embryogenesis and the abnormal development of the presented clinical cases.
The key differences between existing and the proposed hypothesis are follows ():
TABLE 2. Comparative Data of the Original Müllerian Theory with Contemporary View and the New Theory.
in original Mullerian theory the mesonephral ducts regress in female embryos; paramesonephral ducts form the Fallopian tubes, uterus and vagina;
in new hypothesis () the mesonephral ducts in female embryos form Fallopian tubes, and vagina; the uterus derive in crossing area between mesonephral ducts with gonadal ridges. The paramesonephral ducts are absent.
The hypothetic possibility that the ovary and endometrium derive from the gonadal ridges could be the key to understanding the enigmatic aetiologies of extra-genital and ovarian endometriosis. At an early stage of development primordial germ cells appear among endoderm cells in the wall of the yolk sac close to the allantois. They migrate by amoeboid movement to the dorsolateral gonadal ridges along the mesentery of the hindgut.Citation3,6,8
Some of the polypotential germ cells fail to reach the ridges and stay in the peritoneal cavity, then may be transforming into endometrial heterotopy.
Future embryological studies with comparative clinical analysis are important for adjudicating between these theories.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Notes
1 ESHRE - European Society of Human Reproduction and Embryology ESGE ESGE ESGE ESGE – European Society of Gynecological Endocrinology
REFERENCES
- Müller JP. Bildungsgeschichte der Genitalien Düsseldorf: Arnz, 1830; 185-187
- Muller JP. Anatomie des Menschen Berlin: Miller, 1931; 272-275
- Sadler TW. “Langman's Medical Embryology.” IX-th edn Baltimore:Lippincott Williams&Wilkins, 2000; 324, Fig. 14.3-C.https://archive.org/stream/LangmansMedicalEmbryology#page/n342/mode/1up
- O'Rahilly R, Müller F. Embryologie und Teratologie des Menschen Huber Verlag 1999; 107-109.
- O'Rahilly R. The development of the vagina in the human. In: Blandau RJ, Bergsmall D (eds) Morphogenesis and Malformation of the Genital System. Birth Defects New York: Alan Liss 1977; 123-136.
- Hill MA. Embryology BGD Lecture - Sexual Differentiation. Retrieved December 27, 2015. http://php.med.unsw.edu.au/embryology/index.php?title=BGD_Lecture_-_Sexual_Differentiation.
- Jost AA. The new look at the mechanism controlling sex differentiation in mammals. Johns Hopkins Medical J 1972; 130:28-36.
- Sadler TW. “Langman's Medical Embryology.” XII-th edn Baltimore: Lippincott Williams&Wilkins, 2012; 232-259.
- Cunha GR. The dual origin of vaginal epithelium. Am J Anat 1975; 143:3:387-392; PMID:1155363; http://dx.doi.org/10.1002/aja.1001430309
- Spencer TE, Hayashi K, Hu J, Carpenter KD. Comparative developmental biology of the mammalian uterus. Curr Top Dev Biol 2005; 68:8-122.
- Yin Y, Ma L. Development of the mammalian female reproductive tract. J Biochem (Tokyo) 2005; 137:6:677-683; http://dx.doi.org/10.1093/jb/mvi087
- Acién P. Embryological observations on the female genital tract. Hum Reprod 1992; 7:437-445.
- Acién P, Susarte F, Romero J, Galán J, Mayol MJ, Quereda FJ, Sánchez-Ferrer M. Complex genital malformation: ectopic ureter ending in a supposed mesonephric duct in a woman with renal agenesis and ipsilateral blind hemivagina. Eur J Obstet Gynecol Reprod Biol 2004; Nov 10; 117(1):105-8
- Acien P, Arminana E, Garcia-Ontiveros E. Unilateral renal agenesis associated with ipsilateral blind vagina. Arch Gynecol 1987; 240:1-8; PMID:3827311; http://dx.doi.org/10.1007/BF02134057
- Acien P, Acien M, Sanchez-Ferrer M. Complex malformations of the female genital tract New types and revision of classification. Hum Reprod 2004 Nov 10; 117(1):105-8.
- Acién P, Acién M. Unilateral renal agenesis and female genital tract pathologies. Acta Obstet Gynecol Scand 2010 Nov; 89(11):1424-31; http://dx.doi.org/10.3109/00016349.2010.512067
- Fritsch H, Richter E, Adam N. Molecular characteristics and alterations during early development of the human vagina. J Anatomy 2012; 220, Issue 4, pp. 363-71, April.
- Fatum M, Rojansky N, Shushan A. Septate uterus with cervical duplication: rethinking the development of Mullerian anomalies. Gynecol Obstet Invest 2003; 55:3:186-8; http://dx.doi.org/10.1159/000071535
- Martínez-Frías ML, Frías JL, Opitz JM. Errors of morphogenesis and developmental field theory. Am J Med Genet 1998; 76:291-6; http://dx.doi.org/10.1002/(SICI)1096-8628(19980401)76:4<291::AID-AJMG3>3.0.CO;2-T
- Giraldo JL, Habana A, Duleba AJ, Dokras A. Septate uterus associated with cervical duplication and vaginal septum. J Am Assoc Gynecol Laparosc 2000 May; 7(2):277-9; http://dx.doi.org/10.1016/S1074-3804(00)80057-2
- Sanchez-Ferrer M, Acien M, Sanchez Del Campo, Mayol-Belda M, Acién P. Experimental contributions to the study of the embryology of the vagina. Hum Reprod Embryol 2006; 21(6):1623-8.
- Acién P, Acién M. The presentation and management of complex female genital malformations. Hum Reprod Update 2016 Jan; 22(1):48-69 Epub 2015 Nov 3; http://dx.doi.org/10.1093/humupd/dmv048
- Buttram VC, Gibbons WE. The American Fertility Society classification of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, Mullerian anomalies and intrauterine adhesions. Fertil Steril 1988; Jun; 49(6):944-55; PMID:3371491
- Grimbizis G, Gordts S, Di Spiezio Sardo D, Brucker S, De Angelis C, Gergolet M, Tin-Chiu Li, Tanos V, BroЁlmann H, Gianaroli L, et al. The ESHRE/ESGE consensus on the classification of female genital tract congenital anomalies. Hum Reprod 2013 Aug; 28(8):2032-44; http://dx.doi.org/10.1093/humrep/det098
- Shapiro E, Huang H, McFadden D, Masch R, Eliza NG, Lepor H, Wu X.The prostatic utricle is not a Mullerian duct remnant: immunohistochemical evidence for a distinct urogenital sinus origin. J Urol 2004; 172:1753-6; PMID:15371806; http://dx.doi.org/10.1097/01.ju.0000140267.46772.7d
- Hurst CH, Abbott B, Schmid JE, Birnbaum LS. Feulgen staining of female rat reproductive tracts after GD 15 administration of 1.0 μg TCDD/kg showing width of interductal mesenchyme. Toxicol Sci Published online: Jan 2002; 65(1). Available from URL: http://toxsci.oxfordjournals.org/content/65/1/87/F6.expansion.html
- Hashimoto R. Development of the human Müllerian duct in the sexually undifferentiated stage. Anat Rec A Discov Mol Cell Evol Biol 2003 Jun; 272(2):514-9.
- Signorile PG, Baldi A. Endometriosis: New concepts in the pathogenesis. Int J Biochem Cell Biol 2010; 42:778-780; PMID:20230903; http://dx.doi.org/10.1016/j.biocel.2010.03.008
- Acién P. Endometriosis and genital anomalies: some histogenetic aspects of external endometriosis. Gynecol Obstet Invest 1986; 22(2):102-7; http://dx.doi.org/10.1159/000298899
- Knapp VJ. How old is endometriosis? Late 17th and 18th century European descriptions of the disease. Fertil Steril 1999; 72:10-14; PMID:10428141; http://dx.doi.org/10.1016/S0015-0282(99)00196-X
- Troncon JK, Zani AC, Vieira AD, Poli-Neto OB, Nogueira AA, Rosa-E-Silva JC. Endometriosis in a patient with mayer-rokitansky-küster-hauser syndrome. Case Rep Obstet Gynecol 2014; 2014:376231. Epub 2014 Dec 30; PMID:25610677
- Kawano Y, Hirakawa T, Nishida M, Yuge A, Yano M, Nasu K, Narahara H. Functioning endometrium and endometrioma in a patient with mayer-rokitanski-kuster-hauser syndrome. Jpn Clin Med 2014 Sep 5; 5:43-5. eCollection 2014.
