 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Anticholinergic drugs are well-known to cause adverse effects, such as constipation, but their effects on baseline contractile activity in the gut driven by slow waves is not well established. In a video-based gastrointestinal motility monitoring (GIMM) system, a mouse's small intestine was placed in Krebs solution and recorded using a high definition camera. Untreated controls were recorded for each specimen, then treated with a therapeutic concentration of the drug, and finally, treated with a supratherapeutic dose of the drug. Next, the video clips showing gastrointestinal motility were processed, giving us the segmentation motions of the intestine, which were then converted via Fast Fourier Transform (FFT) into their respective frequency spectrums. These contraction quantifications were analyzed from the video recordings under standardised conditions to evaluate the effect of drugs. Six experimental trials were included with benztropine and promethazine treatments. Only the supratherapeutic dose of benztropine was shown to significantly decrease the amplitude of contractions; at therapeutic doses of both drugs, neither frequency nor amplitude was significantly affected. We have demonstrated that intestinal slow waves can be analyzed based on the colonic frequency or amplitude at a supratherapeutic dose of the anticholinergic medications. More research is required on the effects of anticholinergic drugs on these slow waves to ascertain the true role of ICC in neurologic control of gastrointestinal motility.
INTRODUCTION
Interstitial cells of Cajal (ICC) are responsible for electrical slow waves that drive motility in the gastrointestinal tract (GI), and are also closely linked with enteric neurons.Citation1 Currently under debate is the exact role and significance of ICC in enteric neurotransmission.Citation1-7 Enteric neurons utilize acetylcholine as their primary neurotransmitter,Citation8 and anticholinergic drugs are well known to block enteric neurotransmission and result in GI adverse effects, including constipation.Citation9,10 What is as yet unknown is the effect of anticholinergic drugs on the background rhythmic contractions driven by slow waves. By examining the effect of anticholinergic drugs, which are known inhibitors of neurotransmission, on the output of ICC, we inherently test the link between enteric neurons and ICC. This experiment aims to show the effects of anticholinergic drugs on ICC induced contractions, through blockade of cholinergic neurotransmission, as observed in vitro in an organ bath.
Gastrointestinal motility
The digestive system has a complex network of smooth muscle cells, neurons and interstitial cells, working in tandem to achieve propulsion of food and liquid through the GI tract. Smooth muscle cells are present in the muscularis mucosa, longitudinal smooth muscle and circular smooth muscle layers of the GI tract, and act as a syncytium in the presence of an action potential.Citation11
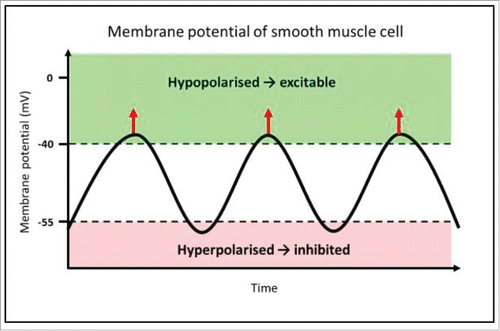
Two main electrical patterns of smooth muscle cells exist within the GI tract; these are slow waves and spike patterns.Citation8,12 Spike patterns represent an increase in the membrane potential such that an action potential is evoked and is transmitted through the syncytium of smooth muscle cells in the gut, resulting in active contraction. Slow waves are a continuous, undulating change in the membrane potential of smooth muscle cells, driven by ICC (). This cyclical rise and fall is transmitted from ICC to smooth muscle cells through gap junctions, causing undulation in smooth muscle cell membrane potential, and driving rhythmic contractions of smooth muscle.Citation13-16 It is these rhythmic contractions that we aim to observe in this experiment.
FIGURE 1. Mechanism of rhythmic contractions of smooth muscle, driven by pacemaker activity from ICC. This diagram represents the membrane potential of a smooth muscle cell, which is alternating between hyperpolarised and hypopolarised states; this pattern is referred to as slow waves. The function of slow waves is to change membrane potential from a state of low open probability (hyperpolarised) for voltage-dependent calcium ion (Ca2+) channels to open (−80 to −55 mV), to a hypopolarised state, where there is elevated probability of Ca2+ channel opening (−40 to −25 mV). Where the membrane potential is above the threshold for action potentials, voltage-gated Ca2+ channels open, and allow Ca2+ influx, indicated by the red arrows.Citation17 This transient Ca2+ influx then initiates smooth muscle contraction. This is the mechanism by which slow waves drive rhythmic contractions of smooth muscle.
Two main motor patterns in the small intestine are peristalsis and baseline rhythmic contractions driven by slow waves, as explained above.Citation18 Peristalsis occurs as a reflex to intraluminal distension, causing contraction oral to the stimulus and relaxation aboral to the stimulus, allowing forward propulsion of intraluminal contents.Citation19-21 Peristalsis differs from slow wave contractions in the following ways: slow waves occur continuously as background motility in the GI tract, while peristalsis only occurs in the presence of intraluminal distension. Furthermore, peristalsis is a coordinated motor pattern that moves longitudinally down the GI tract to achieve propulsion of substances through it. In contrast, rhythmic contractions driven by slow waves travel only short distances longitudinally, and do not have a propulsive mechanismCitation22; the function of the rhythmic contractions themselves is currently unclear.
As well as receiving input of slow wave activity from ICC, smooth muscle cells also receive neuronal input from the enteric nervous system. The enteric nervous system controls neural function for the entire GI tract, including blood flow, immune function, and hormonal and mucosal secretions.Citation11 It acts as a semi-independent component of the autonomic nervous system and contains reflex circuits that allow direct control of the GI system without commands from the brain or spinal cord.Citation23 The myenteric plexus, a component of the enteric nervous system, is largely responsible for neuronal control of smooth muscle, and lies between the longitudinal and circular muscle layers.Citation11
Excitatory circular muscle neurons provide motor output to circular smooth muscle cells, and utilize acetylcholine (ACh) as a neurotransmitter through nicotinic receptors.Citation11 These receive input from afferent neurons, which respond to chemical stimuli in the lumen and mechanical changes such as deformation, stretch and tension.Citation11
The enteric nervous system is also linked to the parasympathetic nervous system, where cholinergic preganglionic neurons exert effects on enteric neurons through both nicotinic and muscarinic receptorsCitation24 Varicose vagal efferents have been found to project to the myenteric plexus, and are believed to project to ‘command neurons’ in the myenteric plexus that then exert effects throughout the rest of the enteric nervous system.Citation24,25
Interstitial cells of Cajal and neurons
As well as being intimately connected with smooth muscle cells, neurons have also shown to be linked with ICC. Whether neuronal input to ICC is significant is a matter of debate,Citation1-7,17,26 however growing evidence suggests that ICC play a role in enteric neurotransmission. It has been shown that intramuscular ICC is closely associated with myenteric motor neurons, forming synapse-like junctions with nerve varicosities.Citation1,27-29 Immunofluorescence image of a transverse slice of mouse small intestine can allow us to examine the neurons network and ICC. Similarly, ICC in the deep muscular plexus (ICC-DMP) are closely linked to excitatory and inhibitory enteric neurons, as shown in the primate small intestine.Citation27 As well as this, it has been shown that mice with a genetic deficiency of ICC have deficient GI motility.Citation30 These studies provide positive evidence that ICC is involved in enteric neurotransmission.
Anticholinergic drugs
Anticholinergic drugs are one of the most widely used drug classes, especially in the elderly population; rates of anticholinergic use is estimated at 55% of residents of long-term care facilities,Citation31 and 27% of people aged over 65 y old.Citation32
There are 2 main types of anticholinergic drugs, acting on 2 different cholinergic receptors – muscarinic receptor antagonists and nicotinic receptor antagonists, or antimuscarinics and antinicotinics respectively. Muscarinic receptor antagonists compete with acetylcholine and antagonise muscarinic receptors within the parasympathetic nervous system.Citation33 Nicotinic receptor antagonists exert competitive inhibition at post-synaptic nicotinic receptors in the neuromuscular junction.Citation34 Nicotinic receptor antagonists are used mainly during anesthetic procedures to provide muscle relaxation,Citation35,36 while muscarinic antagonists treat a myriad of conditions including chronic pulmonary obstructive disease (COPD), Parkinson disease and urinary incontinence.Citation10
There are a multitude of adverse effects caused by anticholinergic drugs, including GI effects such as constipation, abdominal distension, nausea and vomiting, as well as effects related to the blockage of parasympathetic activation, including dry eyes, dry mouth, blurred vision and urinary retention.Citation10,37 Many of these adverse effects are mediated by muscarinic blockade of cholinergic neurotransmission within the parasympathetic nervous system, hence causing inhibition of parasympathetic functions (salivation, lacrimation, urination, digestion and defecation).Citation9,38 Due to the use of acetylcholine as a neurotransmitter in the enteric nervous systemCitation11 as well as the significant parasympathetic input involved in GI motility,Citation38 anticholinergic drugs hinder GI motility through blockade of neurotransmission.
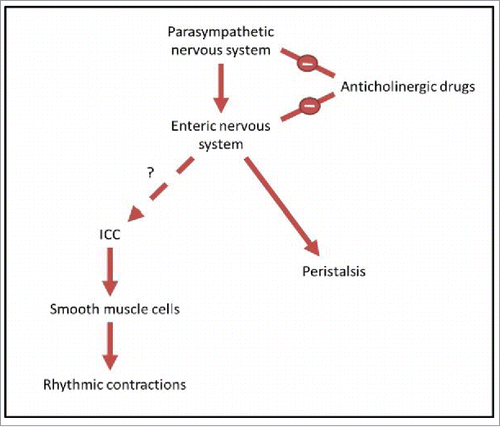
The anticholinergic drugs tested in this experiment were benztropine, an antimuscarinic anticholinergic agent used for symptomatic treatment of tremor in Parkinson diseaseCitation39,40 and promethazine, a first generation antihistamine with antimuscarinic actionCitation41,42 that can be used in the treatment of allergic rhinitis,Citation43 as well as being used as a sedative or sleep aid.Citation44,45 Both these drugs have strong anticholinergic activity, as rated on anticholinergic risk scales,Citation39,43-46 and are typical drugs that may be used long-term by patients. The relationship of anticholinergic drugs, the parasympathetic nervous system, and the enteric nervous system is shown in .
FIGURE 2. A diagrammatic representation of the relationship between neurons, ICC and motor patterns. The parasympathetic nervous system, a subgroup of the autonomic nervous system, provides input to the enteric nervous system, promoting digestion and defecation.Citation24,25 The enteric nervous system is known to control peristalsis.Citation11 However, the link between the enteric nervous system and ICC is debated, and this relationship is what we aim to test in this study. ICC transmit pacemaker activity to smooth muscle cells, causing slow wave changes in membrane potential.Citation13-16 This then drives rhythmic contractions of smooth muscle cells, and it is these contractions which we observe in the organ bath. Anticholinergic drugs are known to have negative input to both the parasympathetic nervous system and the enteric nervous system, by blocking cholinergic neurotransmission.Citation47,48 By treating the intestine with these drugs and measuring the rhythmic contractions driven by ICC, we examine whether there is a link between enteric neurons and ICC.
Aims and objectives
The specific objectives of this research are: 1) Setup of a gastrointestinal motility monitoring (GIMM) system organ bath, that is equipped with the necessary signal processing tools for quantitative analysis of frequency and amplitude of slow wave contractions; 2) Analyze rhythmic contractions generated by slow waves within murine small intestine; and 3) Investigate the effects of anticholinergic drugs (benztropine and promethazine) on frequency and amplitude of contractions driven by slow waves.
METHODS
Signal processing techniques used in gastrointestinal motility analysis
Smoothing of signal based on moving average filter
The moving average filter is mainly used to reduce signal noise, which we can express as:(1)
(1) where x(n) is the input signal, and y(n) is the output signal of the moving average. Here, the length of the inspection window is defined by N; and with the N increasing, the signal waveform becomes smoother. This allows us to perform the identification of amplitudes pertaining to the dominant frequencies more effectively based on changes in the inspection window. The aim is to eliminate the presence of non-relevant amplitudes and frequencies that belongs to noise, and so by smoothing the signal, the sharp peaks disappears, and the greater the smoothing of the signal, the clearer is the definition of the dominating pairs of amplitudes and frequencies. All we need is a smaller number of such amplitudes and frequencies for our examination of the intestinal motion.
Conversion of signals to frequency domain by Fast Fourier Transform
Assume that x(t) is a continuous signal in the time domain, and we assign x(t) = 0 for all t<0, then the equation for performing FFT can be written as:(2)
(2)
Here, , where
, and
and N are real and positive integers. We define a fixed time interval ΔT, such that it is sufficiently small, so that the change of x(t) from t = nΔT to t = (n+1)ΔT, which we define as Δx(t) = x((n+1)ΔT)-x(nΔT), is kept to a reasonable computation limit. As such, we can numerically define x(t)dt as x(nΔT), and the integral in Equation (Equation2
(2)
(2) ) can be expressed as:
(3)
(3)
If we set to be large enough, then for all 0≤n≤N-1, such that the change of x(nT) is sufficiently small, Equation (Equation3
(3)
(3) ) becomes:
(4)
(4)
If we define ω = 2πk / NΔT, then Equation (Equation4(4)
(4) ) becomes:
(5)
(5)
Here, the segment of the time domain signal x[n] = x(nΔT) from n = 0 to N-1 (comprising of N samples), which is processed by Discrete Fourier Transform (DFT) as shown in , to give X[k]. Defining, then Equation (Equation5
(5)
(5) ) becomes:
(6)
(6)
FIGURE 3. Fast Fourier Transform for conversion of signal from time to frequency domain. On the left is the signal in time domain of length N-1 samples, whereby the lower-case x[ ] represent signal value at every time point. Then, this signal is processed by forward DFT to give the frequency spectrum, whereby the amplitude X[ ] can be computed. Here, X[ ] has 2 components, whereby Re X[ ], being the real values, represents the amplitudes of the cosine wave, and Im X[ ], being the imaginary values, represents the amplitudes of sine wave, with each having a length of N/2+1. These are collectively referred to as X[ ], which sum up to a length of N-1.
![FIGURE 3. Fast Fourier Transform for conversion of signal from time to frequency domain. On the left is the signal in time domain of length N-1 samples, whereby the lower-case x[ ] represent signal value at every time point. Then, this signal is processed by forward DFT to give the frequency spectrum, whereby the amplitude X[ ] can be computed. Here, X[ ] has 2 components, whereby Re X[ ], being the real values, represents the amplitudes of the cosine wave, and Im X[ ], being the imaginary values, represents the amplitudes of sine wave, with each having a length of N/2+1. These are collectively referred to as X[ ], which sum up to a length of N-1.](/cms/asset/e96fa19d-46bb-4dff-967e-e4ae8c7f1f58/kogg_a_1295904_f0003_c.gif)
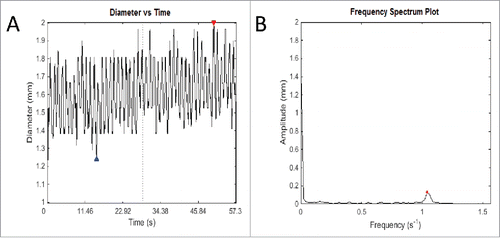
Now, based on FFT, we will be able to obtain. As such, we can convert the signal from the time domain to the frequency domain as shown in . It is worthwhile mentioning again that we are provided with the spatiotemporal plot in 3 dimensions—Diameter (mm) versus Time (s), and Diameter (mm) vs. Distance (mm). We also note that the same technique can be applied to convert the signal from distance domain to its respective frequency spectrum as well.
FIGURE 4. An example of the output of Fast Fourier Transform. (A) This graph records fluctuations in diameter of a transverse slice of intestine over time. The red and blue arrows represent the highest and lowest diameter respectively of that particular video analysis. Owing to the intrinsic width of the intestine, fluctuations cannot be about the zero mark, but rather around the length-dependent variable diameter of the intestine. (B) The frequency spectrum plot shows the most salient frequency – that is, the frequency with the highest amplitude, to choose the predominant frequency and amplitude. This allows elimination of other signals that contribute to ‘noise’ in the graph of diameter change over time, which ideally should be sinusoidal in shape. This method is used to eliminate human error in counting the number of contractions over time and using an average, which is used currently in the literature. As well as this, it allows more detailed information about the amplitude of contractions, which is difficult to measure, and as yet has not been studied in detail in gastrointestinal motility experiments in the literature.
Experimental design and method
Gastrointestinal motility monitoring hardware system
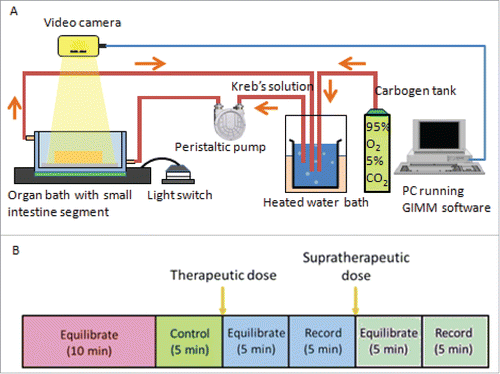
The GIMM hardware (Catamount Research and Development) is used to analyze colon's segmentation motion (see ). The system comprises the organ bath, video camera, circulating pumps, associated tubing and heating systems, and was set up in accordance to the recommended instruction manual.Citation49 The Krebs solution is heated to 37°C, and carbogen gas (95% oxygen and 5% carbon dioxide), is aerated through the solution which circulates to the organ.
FIGURE 5. Gastrointestinal Motility Monitor and organ bath setup. (A) The GIMM is a complete system required to measure GI non segmentation contractions in small animal models such as mice and guinea pigs. The organ bath setup is represented as follows: A pump circulates the warmed, aerated Krebs solution into the organ bath, where the dissected intestine sits. The intestine is pinned down in the organ bath, to allow a camera to record the organ motility. (B) For each trial, first the organ is allowed to equilibrate in the Krebs solution for 10 minutes. Next, a 5 minute video of the untreated organ is recorded and used as the internal control. After that, the drug was added at a therapeutic plasma concentration, allowed to equilibrate in the solution for 5 minutes, and then recorded for 5 minutes. A supratherapeutic concentration was then added, allowed to equilibrate for 5 minutes, and then recorded for 5 minutes.
Animal tissue
Animals used were provided by the Western Sydney University School of Medicine Animal Facility (Animal ethics approval A11220). The mice used were of the C57BL/6 strain, and were healthy adult mice between the ages of 6 months to 1 y old. These mice were euthanised using carbon dioxide asphyxiation and subsequent cervical dislocation. Following death, the GI tract from the stomach to the rectum was dissected, transported in 37°C Krebs solution, and placed in the organ bath, where further dissection isolated the duodenum and ileum for analysis.
Krebs buffer solution
The Krebs buffer solution serves the purpose of providing the oxygen, nutritional and electrolyte requirements to keep the organ functional after dissection. Its constituents are 121 mM NaCl, 5.9 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 25 mM NaHCO3, 1.2 mM NaH2PO4, and 8 mM glucose. This was prepared in accordance with recommendations for the GIMM system.Citation49
Anticholinergic drugs
The anticholinergic drugs benztropine and promethazine were chosen for this experiment. The drug concentration was titrated according to recommended therapeutic plasma concentrations; concentrations were 0.10µg/mL for benztropine (therapeutic range 0.01–0.18µg/mL) and 0.2 µg/mL for promethazine (therapeutic range 0.05–0.4µg/mL)Citation50,51 Drugs in these concentrations were added directly to the circulating Krebs solution, and 5 minutes was allowed for equilibration of the drug level throughout the solution. After this, 30 times the original dose was added, to reach a concentration above the therapeutic range, designated as the supratherapeutic experiment. For benztropine, this was 0.03mg, to reach a final concentration of 0.0301mg/L (toxic plasma concentration 0.0139mg/L).Citation50 For promethazine, this was 0.06mg, to reach a final concentration of 0.0602mg/L (toxic plasma concentration 0.002mg/L).Citation50
900 ml of milliQ water was measured out in a beaker. While stirring, 1 bottle of powdered Krebs – henseleit buffer, 0.373 g calcium chloride dihydrate 2.1 g of sodium bicarbonate and 100 ml of milliQ water were added to the beaker strictly in that order. The pH was checked and adjusted to 7.3 using a pH meter. The beaker was aerated with carbogen which is a mixture of 95% O2 and 5% CO2 and kept partially submerged in the water heater set to 38 ± 0.5 C. The preparation was equilibrated for at least 30 mins according to .
Organ isolation and organ transfer
20 ml of warmed and warmed carbogenated Krebs solution was stored in a tube. Nonfasted B6 mice of either sex, weighing 250–420 g were euthanised with carbon dioxide gas followed with cervical dislocation (protocol approved by Western Sydney University Animal Care and Ethics Committee). The mouse GI tract (not including the esophagus) was removed and placed carefully into the tube filled with warm, carbogenated Krebs solution. The contents of the tube were poured onto a petri dish and using fine tweezers the GI tract was transferred into the organ bath. In the organ bath, the mesentery, stomach and colon were removed quickly and carefully. The small intestine was separated into the duodenum and the ileum. The duodenum was pinned down first and then the ileum was pinned down in the illuminated organ bath so a video camera would record a 180 second video repeated 5 times.
Video data analysis
Each experiment consisted of a 5-minute video recording, of which 3 segments, each one minute in length, were analyzed. A spatiotemporal map measuring diameter, time and distance across the organ was used as an input to our in-house analysis program. After the video was recorded, the motor analysis function was used to generate a spatiotemporal map of a selected length of the mouse small intestine over a chosen time course within the video recording. Different shaded regions on the motor map represented different diameters of the small intestine. 3D graphs of diameter, distance and time were constructed from the spatiotemporal maps. One-way ANOVA tests with post-hoc Tukey tests (pairwise comparisons corrected for family-wise error rate) were utilised for data analysis to compare control results against therapeutic and supratherapeutic doses for both variables of frequency and amplitude.
RESULTS
Optimisation of organ bath and testing of our quantitative analytic tools
GIMM organ bath system
Mice were chosen because the majority of the literature on the role of ICC in neural control of the GI tract has been studied in mice; this is due to the fact that mice were the first animals to be able to be genetically manipulated to lack ICC.Citation1,51-53 Background motility driven by slow waves is a better measure of the function of ICC than peristalsis, and the effect of anticholinergic drugs on baseline contractility, rather than peristalsis, has not been studied before.
The organ was immersed in Krebs solution and recorded with a video camera. Instead of circulating the Krebs throughout the organ bath, they instead cannulated both ends of the organ and circulated the Krebs directly through the GI tract. They also recorded pressure measurements within the lumen of the GI tract.
Bubbling carbogen gas through the container used to transport the organ after dissection allowed greater dissolution of oxygen and carbon dioxide into the Krebs solution in the transport container. This allowed better preservation of the organ through increased delivery of carbon dioxide and oxygen during transport, and in turn, led to more consistent observations of regular contractions.
Furthermore, it was found that the greater the length of time required for dissection and transport of the organ, the weaker and more inconsistently the organ displayed motility. That is, if too long a time elapsed from mouse death to the organ being placed in the organ bath, contractions were either weak and irregular, or not able to be observed at all. To this end, all of the experimental organs had 16–20 minutes between mouse death and the beginning of recording, including dissection and equilibration time. Methods used to minimise this time included leaving clean-up of the dissection until after the experiment was finished, and practising dissection technique to improve speed. GIMM protocol(49) suggested organs could be kept on iced Krebs for up to 2 hours before experiments; however we found this was detrimental to motility.
Another area requiring optimisation was the camera settings. The software allowed manipulation of brightness, contrast and exposure settings (see ). It was suggested that for analysis, the settings were adjusted to high contrast between the organ and background.Citation49 This required heightening the contrast, and increasing the exposure and brightness. Any markings or debris within the camera viewfinder would create artifacts and noise in the spatiotemporal map. To avoid this, debris and distance markers were removed or covered up during recording.
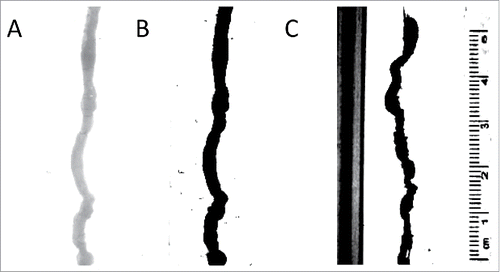
FIGURE 6. Analysis of video recordings. (A) An image of the organ with no contrast. The analysis program finds this difficult to analyze;(B) An image of the organ with full contrast; the black organ is easily measured by the analysis program; (C) An example of the image without covering up calibration markings and sides of the organ bath – the program in this case spuriously records calibration markers as part of the organ.
Program for quantitative analysis
An example of a time domain spectrum is shown in . It represents a typical complicated waveform extracted from the intestinal diameter fluctuation in the time domain. There exists a series of waveforms superimposed to form an irregular fluctuation. This wave may be presented as separate waves. A Fast Fourier Transformation (FFT) analysis of this time-based fluctuation was used to create a frequency plot shown in . Graphs displaying the time, distance and diameter scales constructed from the spatiotemporal map.
FIGURE 7. Spatiotemporal maps for data analysis of an untreated mouse small intestine. Using the motor analysis function, spatiotemporal motor maps of the mouse small intestine were generated and analyzed. (A) Two-dimensional spatiotemporal map: The software from the GIMM system includes the output of a spatiotemporal map recording distance, time and diameter measurements. This spatiotemporal map is used as the input for our analysis program. Distance is the x-axis, time is the y-axis and the diameter changes are recorded using shades from black to white. (B) Three-dimensional intensity map of the 3 variables, namely, distance, diameter and time, in the form of a 3D spatiotemporal plot. The area highlighted in red indicates the cross-section of the organ chosen, which we can see oscillates in diameter as contractions occur. This change in diameter over time is analyzed to measure frequency and amplitude of contractions. Diameters are represented in grayscale so that contractions were shown in black, relaxations are shown in white and the intermediate gray regions represent diameters from 0.7 mm to 5 mm as shown in the scale indicated by the red arrow.
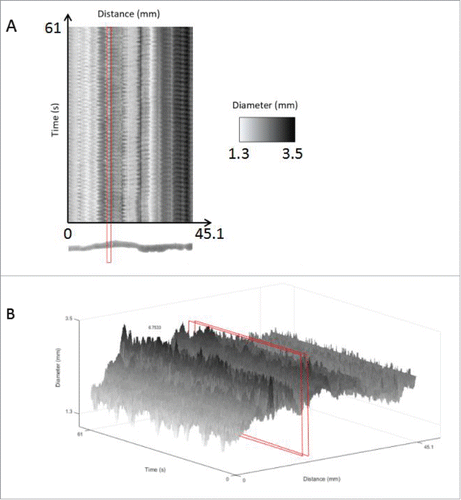
FIGURE 8. Signal processing of the time domain signals based on the following procedures: 1) Averaging of all signals in the Distance (mm) axis (represented by gray) into a single green signal (with its peaks and toughs highlighted by red and blue triangles respectively); 2) Perform moving average of the signal of interest to produce a smoothed signal that has its sharp peaks reduced; 3) Convert signal from time to frequency domain by applying Fast Fourier Transform (with dominant peaks highlighted by red dots); and 4) Deduce the dominant pairs of amplitude and frequency by identifying the dominating peaks of the frequency spectrum graph to give the stem plot (with the ends of every stem highlighted by a red dot). Note that A, B, and C denotes the increment of the inspection window in the moving average filter that leads to increased smoothing of the signal of interest. This corresponds to a reduction in the number of stems for the frequency spectrum stem plot.
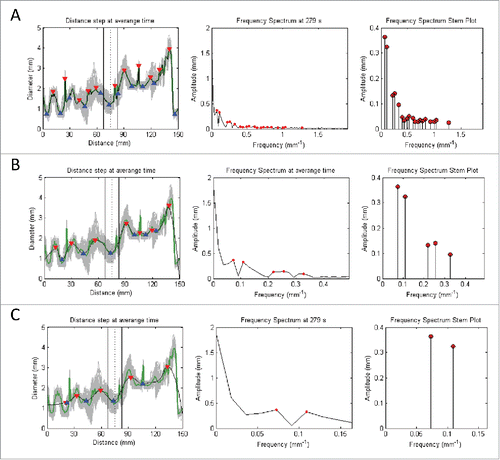
A cross-section of the organ was selected, chosen to be the most regular sinusoidal appearance of the graph of diameter vs. time. This exact same cross-section was then analyzed in the therapeutic and supratherapeutic trials, and compared against the control. The sensitivity function filters the various frequencies of diameter change over time, depending on the amplitude of the frequency. Therefore, at low sensitivity, only frequencies with very large amplitudes will be shown on the frequency spectrum stem plot. At a high sensitivity, many frequencies of varying amplitudes will be shown on the frequency spectrum stem plot. As such, the sensitivity is chosen to be low, and increased until the dominant frequency and amplitude pair with the highest amplitude arises, which was the frequency selected. From this, frequency and amplitude were recorded (in ).
Control tests
For this experiment, both an internal control and an external control were used. A mouse small intestine was recorded for 1 hour with no drug treatment, which is designated as the external control. In addition to this, each test organ was recorded pre-treatment of 5 mins, which is designated as the internal control. The external control determines the effect of prolonged in vitro conditions to motility. It is the internal control that is used for analysis, allowing for direct comparison of untreated and treated data as a pair. Pair-wise analysis was conducted based on the observation that there is considerable variability in the recorded frequency and amplitude of organ contractions between different organs. If experimental organs were averaged and compared against a control group, the observed change may have been obscured by the differences between individual organs. Another reason that pair-wise analysis was deemed valid is because there was minimal change in the frequency and amplitude in the untreated organ over 25 minutes (the complete length of a single trial including therapeutic and supratherapeutic doses). This ensures that the results are unlikely to be affected by the time elapsed during the experiment. Furthermore, with more experiments a control group and treatment group would have been more feasible, but with the limited number of trials due to time constraints, direct comparison within the same organ was deemed more appropriate. The final reason is that this method is used in other organ bath experiments involving a treatment following recorded control values.Citation54 We have performed signal processing based on the techniques outlined in Section 2.1 to provide the frequency spectrum pertaining to intestine treated with the different conditions, and extracted important quantifiable detailed from their respective frequency stem plots ().
FIGURE 9. Frequency and amplitude of gastrointestinal motility over 1 hour for organs that are: (A) Untreated baseline; (B) Treated with benztropine; and (C) Treated with promethazine. The 3 different conditions can be quantified based on their respective frequency spectrums.
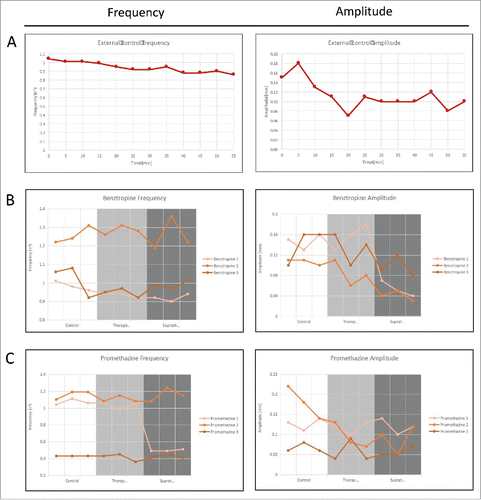
The results of the 1-hour external control are shown in . The frequency did not change significantly during the first 30 minutes, with values of between 0.92–1.04 contractions per second, and was at a low point of 0.86 contractions per second by the end of the hour. The amplitude however, varied greatly, from 0.07 mm to 0.18 mm.
All of the experimental trials followed the same timeline. At 0–5 min, the control was recorded. At 10–15 min, the experimental trial at therapeutic concentration was recorded. At 20–25 min, the experimental trial at supratherapeutic concentration was recorded. Thus, the control and the therapeutic trial would have had the same baseline level of contractility – however, results may have been affected by a loss of frequency in the supratherapeutic trials.
Experimental results
The results were analyzed with an in house frequency analysis program developed by the GI Motility Laboratory, Western Sydney University using MATLAB programming software. Video segments of 60 seconds were taken from the video, and analyzed by the GIMM motor analysis system, which produced a 2D visual diagram of diameter over time. This image was then analyzed using the inhouse MATLAB program, as described in .
When the means of the control, therapeutic and supratherapeutic results were analyzed only a significant decrease in amplitude was recorded at supratherapeutic drug concentrations of benztropine (p-value = 0.001), which is shown in . At therapeutic concentrations of both drugs, neither frequency nor amplitude was significantly affected.
When each experiment is analyzed individually, 3 out of 6 experiments showed a significant decrease in frequency or amplitude. The first experiment with benztropine showed significant decreases in frequency at therapeutic (p-value = 0.048) and supratherapeutic concentrations (p-value = 0.018), as well as amplitude at supratherapeutic concentration (p-value = 0.002). By comparison, the second benztropine experiment only showed significant decreases in amplitude at supratherapeutic concentration (p-value 0.005), and the third benztropine experiment showed no significant results ().
TABLE 1. Benztropine results.
For promethazine, the first experiment showed a decrease in frequency at supratherapeutic concentration (p value = 0.000), which is shown in , but the other 2 experiments showed no significant changes. These results are tabulated in . Next, we can compare the effect of 2 sets of drugs on frequency and amplitude in . Individual results table is shown in .
TABLE 2. Promethazine results.
TABLE 3. Individual results whereby significant results have been highlighted in bold.
TABLE 4. Summary of significant changes in individual experiments.
DISCUSSION
Rationale of using FFT for GI motility analysis
Anticholinergics and GI motility
Anticholinergic drugs are known to cause constipation as a common adverse effect.Citation9,10 Direct effects of anticholinergics – both muscarinic and nicotinic receptor antagonists – have been shown to impair peristalsis in both rat,5556 and guinea pig intestine.Citation57,58 Various effects were observed, including blockade of peristalsis, incomplete peristalsis, and increased intraluminal threshold pressure for peristalsis to occur. Past studies have focused on hexamethonium and atropine,Citation55-57 a nicotinic receptor antagonist and a muscarinic receptor antagonist respectively. These drugs are used mainly during anesthetic procedures, Citation35,59 and are not medications used by patients long-term. For our study we chose 2 anticholinergic drugs commonly prescribed over extended periods of time and are known to cause significant constipation. Benztropine is used in the treatment of Parkinson disease, and promethazine is used in allergic rhinitis or as a sleep aid. Past studies have focused on threshold pressure for peristalsis and the occurrence of electrically stimulated evoked potentials,Citation55-58 rather than baseline contractile activity, which is our primary goal in this study. However, the effects of anticholinergics on slow waves, rather than peristalsis, has not been thoroughly tested. By testing the effects of anticholinergic drugs on ICC-driven slow waves, we implicitly test the role of ICC in cholinergic neurotransmission.
ICC and enteric neurotransmission
Mounting evidence suggests that GI motility is mediated through neuronal input to ICC, rather than direct motor neuron input to smooth muscle cells alone. A study of ICC depletion in mice, achieved through genetic manipulation, showed inhibition of excitatory neurotransmission, in the form of reduced contraction in response to electrical stimulation.Citation30 Nerve varicosities have been shown to be in close association with ICC through immunohistochemistry techniques, with distances of around 20 nm between them.Citation1,27-29
Since ICC directly generate slow waves through an undulating rise and fall in membrane potential, and these slow waves drive the baseline contractions that were measured, this experiment is novel as it attempts to observe the in vitro effects of anticholinergic drugs on ICC, through inhibition of neuronal input. Findings would elucidate of the role of ICC in enteric neurotransmission. If rhythmic contractions driven by slow waves are dampened by anticholinergics, it suggests ICC are involved in neurotransmission; if they are unaffected by anticholinergics, it suggests ICC may not be involved in neurotransmission.
Factors affecting slow wave frequency and amplitude
The mechanism of generation of slow waves from ICC is still under debate. One of the predominant hypotheses for the mechanism is as follows: Ca2+ is released from intracellular Ca2+ stores, which in turn allows Cl− channels to open. These Cl− channels allow flow of Cl− ions, and this influx causes transient alterations in the membrane potential, creating the electrical slow wave pattern.Citation60-62
The frequency of slow waves is affected by a multitude of factors. Muscarinic agonists have been shown to have a positive chronotropic effect on slow wave frequency.Citation63,64 Similarly, it has also been shown that muscarinic antagonists decrease the frequency of slow waves.Citation64,65 Increased frequency of slow waves in the presence of muscarinic stimulation is mediated by muscarinic type 3 receptors (M3), which are linked to enhanced production of inositol-trisphosphate (IP3). It is well recognized increased levels of IP3 stimulate Ca2+ release from intracellular stores, which in turn initiates pacemaker activity.Citation17,66
Amplitude of the pacemaker activity of ICC has mostly been recorded through electrophysiological recordings in previous experiments, but what this experiment is recording is not the amplitude of the voltage change, but the amplitude of the contractions themselves. As such, it is hypothesized that changes in amplitude within our experiment are from smooth muscle factors, including the degree of neuronal excitation. It has been shown that acetylcholine increases the strength of contractions driven by slow waves in the intestinal smooth muscle,Citation67 while atropine (an antimuscarinic drug) decreases the amplitude of spontaneous smooth muscle contractions.Citation68 Thus, amplitude of contractions appears to be dependent on neuronal input. In addition to this, Ca2+-free solutions and calcium channel blocker drugs (nifedipine, verapamil) decreased the amplitude of contractions.Citation68 Smooth muscle contraction requires influx of Ca2+ ions to initiate contraction, and hence amplitude is also dependent on Ca2+ concentration and movement.
Proof of concept
Effect of anticholinergic drugs on slow wave motility
Reasons for discrepancies include lateral movement of the organ in the bath, which results in different cross sections being analyzed by the program as the organ moves. Studies recommend not pinning the organ until it is completely taut, as this inhibits contraction.Citation49,69 However, since the program analyses a cross-section and measures the change in diameter, if there is too much movement it could be analyzing instead the oscillation of adjacent cross-sections of the organ. A possible method to include this would be installing a tracking system where movement of the organ could be monitored, and negated during analysis.
In addition to this, another organ bath experiment exploring the effects of acetylcholine and atropine, an anticholinergic, showed seemingly paradoxical results where acetylcholine decreased contractile activity, whereas atropine reversed this effect and restored normal contractions.Citation70 This was reconciled when it was shown that acetylcholine caused shortening of the organ, which limited the variation in diameter and hence dampened the recorded contractions. Some degree of lengthening of the organ in the presence of anticholinergics may have negated the decrease in motility.
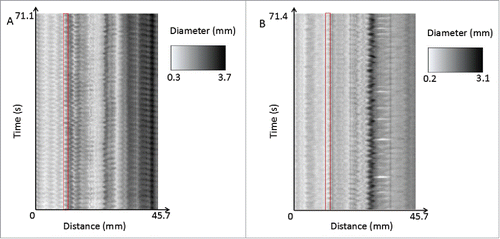
When analyzed individually, 3 out of 6 mice (benztropine n = 2, promethazine n = 1) showed significant changes in frequency or amplitude. Most of these changes were observed between the control and supratherapeutic doses, and included both frequency and amplitude. In addition to this, the spatiotemporal maps show that contractions become more irregular in frequency and amplitude with drug treatment. Dysrhythmia of slow waves with antimuscarinic treatment has been found in another study involving atropine.Citation65 However, this dysrhythmia was not always reflected in our quantitative measurements, as regularity is not actively measured in the program. A study on slow waves in gastroparesis has made a similar observationCitation71; it was found that measuring only frequency did not adequately reflect the dysfunctional irregularity of slow waves – in this case, it was found that abnormal initiation and conduction of slow waves occurred. If the software could be improved further to analyze regularity of contractions, this would add an extra dimension to quantitative analysis. An example of the importance of contractions can be given by the comparison of the spatiotemporal maps of one of the benztropine internal controls and its paired benztropine supratherapeutic trial as shown in .
FIGURE 10. A comparison of the spatiotemporal maps of one of the benztropine internal controls (A) and its paired benztropine supratherapeutic trial (B). The control shows more regular contractions throughout the entire length of the organ, whereas the supratherapeutic drug trial shows more irregular and less well-defined contractions. This can be seen for example in the areas highlighted in red, where in the control, the variation in diameter is regular and the amplitude is large, where in comparison the therapeutic trial shows more irregular, lower amplitude contractions.
There is evidence in the literature that there is a dampening effect on slow wave frequency and amplitude with anticholinergic treatment. Previous studies have shown that muscarinic antagonists decrease the frequency of slow waves.Citation64,65 In one study, the muscarinic antagonists studied were methoctramine, himbacine and 4-DAMP, which are selective muscarinic antagonists used mainly in laboratories for research purposes and not in the clinical setting; all these substances showed decrease in slow wave frequency.Citation64 This study involved electrophysiological studies of cultured murine gastric ICC. Another study showed that atropine (a muscarinic antagonist used to increase cardiac output and dry bodily secretions during anesthesia)Citation72 showed a decrease in slow wave frequency as well as dysrhythmia of slow waves in the stomach, but showed no effect in the small intestine.Citation65 The methodology in this study involved implanting electrodes in canine stomach and small intestine to observe slow waves in vivo. Additionally, it has been shown that acetylcholine and carbachol (a muscarinic agonist) increase frequency of slow waves both in the stomach and small intestine.Citation64,66,73
In terms of amplitude, it has been shown in a study of canine small intestine that ACh increases the amplitude of contractions driven by slow waves.Citation67 In addition to this, another study conducted with rabbit small intestine shows that atropine decreases the amplitude of contractions of ‘spontaneous rhythmic contractions’ – it is unclear whether these are related to slow waves or notCitation68
However, it is worth noting that some of these experiments have only shown results for gastric ICC, rather than ICC in the small intestine. There are some differences between ICC in the stomach and small intestine; ICC in the stomach are mainly situated within circular and longitudinal layers of muscle, while ICC in the small intestine occur both intramuscularly and in association with the deep muscular plexus.Citation74 It is the small intestinal ICC in association with the deep muscular plexus (a nerve plexus between the circular muscle and submucosal layers) that show close contacts with enteric neurons that suggest a link between ICC and the enteric nervous system.Citation75,76
Still, these previous studies showing muscarinic antagonism decreasing slow wave frequency and amplitude provides some evidence in support of our hypothesis that anticholinergic drugs will decrease frequency and amplitude of ICC induced contractions.
Observation of slow waves in murine small intestine
The frequency of slow waves in mice is estimated to be between 13 cycles/min in experiments conducted at room temperature,Citation15,77 to 40 cycles/min at 38°CCitation78 but have been observed up to 50 cycles/min.Citation77 Our experiments show contractions driven by slow waves at 25–75 per minute, with a mean of 59 per minute, recorded at 37–38°C, which is consistent with the literature.
This is also significant because the system is designed for guinea pig rather than mouse organs, and can provide the basis for further experiments on motility in mice. Mice are the predominant animal used for experiments studying ICC's role in enteric neurotransmission. This is because it is murine mutants that have been developed with genetic knockout disorders that lead to failure of formation of ICC, allowing observation of motility in ICC-depleted tissue.Citation1,51-53 Thus, optimising an organ bath for mouse intestine allows combination of these 2 methods of study investigating ICC's role in neurotransmission. The use of organ baths has a distinct advantage of allowing detailed analysis of motility through direct observation of the organ as a whole, and has been shown to have yield similar reflex reactions as in vivo experiments.Citation55
Quantitative frequency spectrum analysis program
This study also demonstrates a novel way of in depth analysis of GI motility. In the literature, measurement of effects of anticholinergic agents on GI motility has focused around evoked potentials in response to electrophysiological nerve stimulation, and peristalsis, including measurements of number of complete peristalsis, and the interruption and cessation of peristalsis.Citation55-58 Measurement of slow waves in the literature has been undertaken mainly through electrophysiological studies. These include dissection of the organ to produce intact smooth muscle, which is then electrically stimulated with electrodes, as well as using cultured ICC and measuring the membrane potential changes to assess slow waves, known as the patch-clamp technique.Citation64,79,80 While these methods allow quite accurate assessment of slow wave frequency, it does not assess the function of the organ as a whole. In this way, our method of an organ bath and video recording and analysis of contractions is a closer approximation of the organ's function as a whole. In the literature, spatiotemporal mapping using an organ bath has been used, however this has mainly been to visualize peristalsis,Citation81 and has not yet been used in the analysis of slow waves.
Future directions
Future directions in the study of ICC and slow waves would include simultaneous recordings of membrane potential and video recordings of motility, to confirm that the contractions occurring are due to slow waves. This would also allow determination of the ratio of spike potentials to slow waves. In this experiment, it is assumed this ratio is 1:1 through correlation of the frequency of contractions in this experiment, and the measured frequency of slow waves in the literature.Citation15,78,79 At this point in time, studies of membrane potential are still done in clusters of cultured ICC, rather than an intact organ.Citation64,79,80,82 If the changes in membrane potential of ICC and smooth muscle were able to be recorded in a whole organ, this would give a greater idea of their behavior in a functional state.
Another expansion of the field would be reproducing murine experiments in humans. Currently, many of the experiments investigating ICC's role in enteric neurotransmission have focused on mice, due to the fact that murine mutants lacking ICC are available for study. These findings are yet to be extended to other species, and would hopefully one day be investigated in humans. Observation of slow waves in vitro have been studied in humans in a handful of studies,Citation83,84 however human experiments on the role of ICC in neurotransmission are lacking.
Within the pathophysiological realm, dysfunction of ICC has been implicated in a myriad of motility conditions, including achalasia,Citation85,86 gastroparesis,Citation71,87-89 intestinal pseudo-obstructionCitation90-93 and slow-transit constipation.Citation94-96 Lower numbers of ICC have been found in gastroparesis,Citation71 slow-transit constipation,Citation94,95 and intestinal pseudo-obstruction.Citation91 In achalasia, increased number of organelles (smooth endoplasmic reticulum and mitochondria) has been found in ICC located in the lower esophageal sphincter, possibly contributing to overactive contraction.Citation76 Gastroparesis also involves abnormal initiation and conduction of ICC pacemaker activity.Citation71
CONCLUSIONS
Anticholinergic drugs, while known to cause constipation and have dampening effects on peristalsis, have not been extensively studied in relation to ICC and slow waves. This experiment attempts to provide the framework for further investigation of the effect of commonly used therapeutic anticholinergic agents on background motility driven by slow waves produced by ICC. An organ bath system has been optimised to visualize contractions driven by slow waves in the murine small intestine. In addition to this, our quantitative frequency spectrum analysis program allows transformation of the video recording into measures of frequency and amplitude of contractions. By showing that blockade of neurotransmission with anticholinergic drugs affects ICC induced contractions, this would provide further evidence that ICC play an important role in neurotransmission.
ABBREVIATIONS
| Ach | = | Acetylcholine |
| Ca2+ | = | Calcium ion |
| CaCl2 | = | Calcium chloride |
| Cl− | = | Chloride ion |
| CO2 | = | Carbon dioxide |
| G | = | Gram |
| GI | = | Gastrointestinal |
| GIMM | = | Gastrointestinal motility monitoring system |
| ICC | = | Interstitial cells of Cajal |
| IP3 | = | Inositol-trisphosphate |
| KCl | = | Potassium chloride |
| MgCl2 | = | Magnesium chloride |
| mM | = | Millimoles |
| mV | = | Millivolts |
| NaCl | = | Sodium chloride |
| NaH2PO4 | = | Sodium dihydrogen phosphate |
| NaHCO3 | = | Sodium hydrogen carbonate |
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
AUTHORS' CONTRIBUTIONS
KKLW proposed the framework of this paper and gave the directions for all experiments. KKLW and LT designed and implemented the methods, as well as performed the experiments. JZ and KKLW analyzed and confirmed the validity of the experiments. JZ, KKLW, LT, and VH interpreted the results and drafted the manuscript. KKLW, LT, and VH reviewed the paper and gave reasonable comments for improvements. All authors have read and approved the manuscript.
ACKNOWLEDGMENTS
This project is completed in collaboration with Victoria University Sunshine Hospital laboratory, a laboratory with an established organ bath system in Melbourne, to aid optimisation of the organ bath system. Dr Kulmira Nurgali (Enteric Neuropathy Laboratory, Victoria University Sunshine Hospital laboratory) and her team have given the authors useful comments on the experimental setup.
REFERENCES
- Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neuroscience 2000; 20(4):1393-403.
- Goyal RK, Chaudhury A. Mounting evidence against the role of ICC in neurotransmission to smooth muscle in the gut. Am J Physiol Gastrointest Liver Physiol 2010; 298(1):G10-G3; http://dx.doi.org/10.1152/ajpgi.00426.2009
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterol 1996; 111(2):492-515; http://dx.doi.org/10.1053/gast.1996.v111.pm8690216
- Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol 2010; 588(23):4621-39; PMID:20921202; http://dx.doi.org/10.1113/jphysiol.2010.196030
- Sarna SK. Are interstitial cells of Cajal plurifunction cells in the gut? Am J Physiol Gastrointest Liver Physiol 2008; 294(2):G372-G90; PMID:17932226; http://dx.doi.org/10.1152/ajpgi.00344.2007
- Ward S, Sanders K. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol Motil 2004; 16(s1):112-7; PMID:15066015; http://dx.doi.org/10.1111/j.1743-3150.2004.00485.x
- Ördög T. Do we need to revise the role of interstitial cells of Cajal in gastrointestinal motility? Am J Physiol Gastrointest Liver Physiol 2008; 294(2):G368-G71; PMID:18270367; http://dx.doi.org/10.1152/ajpgi.00530.2007
- El-Sharkawy TY, Daniel EE. Electrical activity of small intestinal smooth muscle and its temperature dependence. Am J Physiol 1975; 229(5):1268-76; PMID:1200146
- Feinberg M. The problems of anticholinergic adverse effects in older patients. Drugs Aging 1993; 3(4):335-48; PMID:8369593; http://dx.doi.org/10.2165/00002512-199303040-00004
- Mintzer J, Burns A. Anticholinergic side-effects of drugs in elderly people. J R Soc Med 2000; 93(9):457; PMID:11089480
- Costa M, Brookes SJ, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut 2000; 47(suppl 4):iv15-iv9; PMID:11076898
- Bajaras-Lopez C, Huizinga JD. Different mechanisms of contraction generation in circular muscle of canine colon. Am J Physiol 1989; 256(3 pt 1):G570-G80; PMID:2923215
- Cousins H, Edwards F, Hickey H, Hill C, Hirst G. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol 2003; 550(3):829-44; PMID:12844505; http://dx.doi.org/10.1113/jphysiol.2003.042176
- Kelly KA, Force RCL. Role of the gastric pacesetter potential defined by electrical pacing. Can J Physiol Pharmacol 1972; 50(10):1017-9; PMID:4637178; http://dx.doi.org/10.1139/y72-147
- Lee JC, Thuneberg L, Berezin I, Huizinga JD. Generation of slow waves in membrane potential is an intrinsic property of interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 1999; 277(2):G409-G23.
- Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev 2014; 94(3):859-907; PMID:24987007; http://dx.doi.org/10.1152/physrev.00037.2013
- Sanders KM, Koh SD, M.Ward S. Interstitial cells of Cajal as pacemakers of the gastrointestinal tract. Annu Rev Physiol 2006; 68:307-43; PMID:16460275; http://dx.doi.org/10.1146/annurev.physiol.68.040504.094718
- Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 2012; 9(5):286-94; PMID:22392290; http://dx.doi.org/10.1038/nrgastro.2012.32
- Cannon WB. Peristalsis, segmentation, and the myenteric reflex. Am J Physiol 1912; 30(1):114-28.
- Husebye E. The patterns of small bowel motility: physiology and implications in organic disease and functional disorders. Neurogastroenterol Motil 1999; 11(3):141-61; PMID:10354340; http://dx.doi.org/10.1046/j.1365-2982.1999.00147.x
- Makhlouf G, Johnson L. Neuromuscular function of the small intestine. Physiology of the gastrointestinal tract. 3 ed. New York: Raven Press; 1994. p. 977-90.
- Lammers WJ, Stephen B, Slack JR. Similarities and differences in the propagation of slow waves and peristaltic waves. Am J Physiol Gastrointest Liver Physiol 2002; 283(3):G778-G86; PMID:12181194; http://dx.doi.org/10.1152/ajpgi.00390.2001
- Furness JB. The enteric nervous system. Carlton (AU): Blackwell Publishing; 2006.
- Goyal RK, Hirano I. The enteric nervous system. N Engl J Med 1996; 334(17):1106-15; PMID:8598871; http://dx.doi.org/10.1056/NEJM199604253341707
- Kirchgessner A, Gershon M. Identification of vagal efferent fibers and putative target neurons in the enteric nervous system of the rat. J Comp Neurol 1989; 285(1):38-53; PMID:2568999; http://dx.doi.org/10.1002/cne.902850105
- Zhang RX, Wang XY, Chen D, Huizinga J. Role of interstitial cells of Cajal in the generation and modulation of motor activity induced by cholinergic neurotransmission in the stomach. Neurogastroenterol Motil 2011; 23(9):e356-e71; PMID:21781228; http://dx.doi.org/10.1111/j.1365-2982.2011.01753.x
- Blair PJ, Bayguinov Y, Sanders KM, Ward SM. Relationship between enteric neurons and interstitial cells in the primate gastrointestinal tract. Neurogastroenterol Motil 2012; 24:e437-e49; PMID:22805588; http://dx.doi.org/10.1111/j.1365-2982.2012.01975.x
- Horiguchi K, Sanders KM, Ward SM. Enteric motor neurons form synaptic-like junctions with interstitial cells of Cajal in the canine gastric antrum. Cell Tissue Res 2003; 311(3):299-313; PMID:12658438
- Mitsui R, Komuro T. Direct and indirect innervation of smooth muscle cells of rat stomach, with special reference to the interstitial cells of Cajal. Cell Tissue Res 2002; 309(2):219-27; PMID:12172781; http://dx.doi.org/10.1007/s00441-002-0592-1
- Klein S, Seidler B, Kettenberger A, Sibaev A, Rohn M, Feil R, Allescher HD, Vanderwinden JM, Hofmann F, Schemann M, et al. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat Commun 2013; 4:1630; PMID:23535651; http://dx.doi.org/10.1038/ncomms2626
- Kumpula EK, Bell JS, Soini H, Pitkälä KH. Anticholinergic drug use and mortality among residents of long-term care facilities: a prospective cohort study. J Clin Pharmacol 2011; 51(2):256-63; PMID:20489026; http://dx.doi.org/10.1177/0091270010368410
- Ness J, Hoth A, Barnett MJ, Shorr RI, Kaboli PJ. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother 2006; 4(1):42-51; PMID:16730620; http://dx.doi.org/10.1016/j.amjopharm.2006.03.008
- Caulfield MP. Muscarinic receptors - characterization, coupling and function. Pharmacol Ther 1993; 58(3):319-79; PMID:7504306; http://dx.doi.org/10.1016/0163-7258(93)90027-B
- Brenner GM, Stevens CW. Pharmacology. London: Elsevier Health Sciences; 2013. p. 63-8.
- Mirakhur R. Anticholinergic drugs. Br J Anaesth 1979; 51(7):671-9; PMID:399194; http://dx.doi.org/10.1093/bja/51.7.671
- Nair VP, Hunter JM. Anticholinesterases and anticholinergic drugs. Contin Educ Anaesth Crit Care Pain 2004; 4(5):164-8; http://dx.doi.org/10.1093/bjaceaccp/mkh045
- Peters NL. Snipping the thread of life: antimuscarinic side effects of medications in the elderly. Arch Inter Med 1989; 149(11):2414-20; PMID:2684071; http://dx.doi.org/10.1001/archinte.1989.00390110020006
- Hall JE. Guyton and Hall textbook of medical physiology. 13 ed. Philadelphia: Elsevier Health Sciences; 2015. p. 773-84.
- Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson's disease. Cochrane Database Syst Rev 2002; 3:CD003735
- Tourtellotte WW, Potvin AR, Syndulko K, Hirsch SB, Gilden ER, Potvin JH, Hansch EC. Parkinson's disease: Cogentin® with sinemet®, a better response. Prog Neuropsychopharmacol Biol Psychiatry 1982; 6(1):51-5; PMID:7202230; http://dx.doi.org/10.1016/S0364-7722(82)80107-0
- Pearlman DS. Antihistamines: pharmacology and clinical use. Drugs 1976; 12(4):258-73; PMID:9270; http://dx.doi.org/10.2165/00003495-197612040-00002
- Simons FER, Akdis CA. Histamine and H1 antihistamines. Middleton's allergy: principles and practice. 8 ed. Philadelphia: Saunders; 2014. p. 1503-33.
- Van Cauwenberge P, Bachert C, Passalacqua G, Bousquet J, Canonica G, Durham S, Fokkens WJ, Howarth PH, Lund V, Malling HJ, et al. Consensus statement on the treatment of allergic rhinitis. Allergy 2000; 55(2):116-34; PMID:10726726; http://dx.doi.org/10.1034/j.1398-9995.2000.00526.x
- Morin AK, Jarvis CI, Lynch AM. Therapeutic options for sleep-maintenance and sleep-onset insomnia. Pharmacother 2007; 27(1):89-110; http://dx.doi.org/10.1592/phco.27.1.89
- Viukari M, Miettinen P. Diazepam, promethazine and propiomazine as hypnotics in elderly inpatients. Neuropsychobiology 1984; 12(2-3):134-7; PMID:6152029; http://dx.doi.org/10.1159/000118126
- Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatrics 2015; 15(1):1; PMID:25559550; http://dx.doi.org/10.1186/s12877-015-0029-9
- Zlotos DP, Bender W, Holzgrabe U. Muscarinic receptor agonists and antagonists. Expert Opin Ther Pat 1999; 9(8):1029-53; http://dx.doi.org/10.1517/13543776.9.8.1029
- Galligan J, North R. Pharmacology and function of nicotinic acetylcholine and P2X receptors in the enteric nervous system. Neurogastroenterol Motil 2004; 16(s1):64-70; PMID:15066008; http://dx.doi.org/10.1111/j.1743-3150.2004.00478.x
- Hoffman JM, Brooks EM, Mawe GM. Gastrointestinal Motility Monitor (GIMM). J Vis Exp 2010(46):e2435.
- Regenthal R, Krueger M, Koeppel C, Preiss R. Drug levels: therapeutic and toxic serum/plasma concentrations of common drugs. J Clin Monit Comput 1999; 15(7):529-44; PMID:12578052; http://dx.doi.org/10.1023/A:1009935116877
- Sanders KM, Ordög T, Ward SM. Physiology and pathophysiology of the interstitial cells of Cajal: from bench to bedside. IV. Genetic and animal models of GI motility disorders caused by loss of interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 2002; 282(5):G747-56; PMID:11960771; http://dx.doi.org/10.1152/ajpgi.00362.2001
- Burns AJ, Lomax A, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Science 1996; 93(21):12008-13; http://dx.doi.org/10.1073/pnas.93.21.12008
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol 1994; 480(Pt 1):91-7; PMID:7853230; http://dx.doi.org/10.1113/jphysiol.1994.sp020343
- Wood MJ, Hyman NH, Mawe GM. The effects of daikenchuto (DKT) on propulsive motility in the colon. J Surg Res 2010; 164(1):84-90; PMID:19631346; http://dx.doi.org/10.1016/j.jss.2009.03.068
- Bogeski G, Shafton A, Kitchener P, Ferens D, Furness J. A quantitative approach to recording peristaltic activity from segments of rat small intestine in vivo. Neurogastroenterol Motil 2005; 17(2):262-72; PMID:15787946; http://dx.doi.org/10.1111/j.1365-2982.2004.00605.x
- Hata F, Kataoka T, Takeuchi T, Yagasaki O, Yamano N. Differences in control of descending inhibition in the proximal and distal regions of rat colon. Br J Pharmacol 1990; 101(4):1011-5; PMID:2085703; http://dx.doi.org/10.1111/j.1476-5381.1990.tb14198.x
- Bartho L, Holzer P, Donnerer J, Lembeck F. Effects of substance P, cholecystokinin octapeptide, bombesin, and neurotensin on the peristaltic reflex of the guinea-pig ileum in the absence and in the presence of atropine. Naunyn Schmiedeberg Arch Pharmacol 1982; 321(4):321-8; http://dx.doi.org/10.1007/BF00498521
- Tonini M, Frigo G, Lecchini S, D'Angelo L, Crema A. Hyoscine-resistant peristalsis in guinea-pig ileum. Eur J Pharmacol 1981; 71(4):375-81; PMID:7250196; http://dx.doi.org/10.1016/0014-2999(81)90181-3
- Adams A. Techniques of vascular control for deliberate hypotension during anaesthesia. Br J Anaesth 1975; 47(7):777-92; PMID:240372; http://dx.doi.org/10.1093/bja/47.7.777
- Hirst G, Bramich N, Teramoto N, Suzuki H, Edwards F. Regenerative component of slow waves in the guinea-pig gastric antrum involves a delayed increase in Ca2+ and Cl− channels. J Physiol 2002; 540(3):907-19; PMID:11986379; http://dx.doi.org/10.1113/jphysiol.2001.014803
- Kito Y, Fukuta H, Suzuki H. Components of pacemaker potentials recorded from the guinea pig stomach antrum. Pflügers Archiv 2002; 445(2):202-17; http://dx.doi.org/10.1007/s00424-002-0884-z
- Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca2+‐activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol 2009; 587(20):4905-18; http://dx.doi.org/10.1113/jphysiol.2009.176206
- El-Sharkawy T, Szurszewski JH. Modulation of canine antral circular smooth muscle by acetylcholine, noradrenaline and pentagastrin. J Physiol 1978; 279:309; PMID:671353; http://dx.doi.org/10.1113/jphysiol.1978.sp012346
- Kim TW, Koh SD, Ördög T, Ward SM, Sanders KM. Muscarinic regulation of pacemaker frequency in murine gastric interstitial cells of Cajal. J Physiol 2003; 546(2):415-25; PMID:12527728; http://dx.doi.org/10.1113/jphysiol.2002.028977
- Liu S, Xu J, Chen JD. Roles of putative neurotransmitters in the regulation of gastric and intestinal slow waves in conscious dogs. J Gastroenterol Hepatol 2007; 22(7):1044-50; PMID:17608850; http://dx.doi.org/10.1111/j.1440-1746.2007.04916.x
- Malysz J, Donnelly G, Huizinga JD. Regulation of slow wave frequency by IP3-sensitive calcium release in the murine small intestine. Am J Physiol Gastrointest Liver Physiol 2001; 280(3):G439-G48; PMID:11171626
- Hara Y, Szurszewski JH. Effect of potassium and acetylcholine on canine intestinal smooth muscle. J Physiol 1986; 372(1):521-37; PMID:3723417; http://dx.doi.org/10.1113/jphysiol.1986.sp016023
- Grasa L, Rebollar E, Arruebo M, Plaza M, Murillo M. The role of Ca2+ in the contractility of rabbit small intestine in vitro. J Physiol Pharmacol 2004; 55(3):639-50; PMID:15381833
- Dickson EJ, Spencer NJ, Hennig GW, Bayguinov PO, Ren J, Heredia DJ, Smith TK. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterol 2007; 132(5):1912-24; PMID:17484884; http://dx.doi.org/10.1053/j.gastro.2007.02.047
- Benard T, Bouchoucha M, Dupres M, Cugnenc P-H. In vitro analysis of rat intestinal wall movements at rest and during propagated contraction: a new method. Am J Physiol Gastrointest Liver Physiol 1997; 273(4):G776-G84.
- O'Grady G, Angeli TR, Du P, Lahr C, Lammers WJ, Windsor JA, Abell TL, Farrugia G, Pullan AJ, Cheng LK. Abnormal initiation and conduction of slow-wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterol 2012; 143(3):589-98. e3; PMID:22643349; http://dx.doi.org/10.1053/j.gastro.2012.05.036
- Shutt L, Bowes J. Atropine and hyoscine. Anaesthesia 1979; 34(5):476-90; PMID:382907; http://dx.doi.org/10.1111/j.1365-2044.1979.tb06327.x
- Forrest AS, Ördög T, Sanders KM. Neural regulation of slow-wave frequency in the murine gastric antrum. Am J Physiol Gastrointest Liver Physiol 2006; 290(3):G486-G95; PMID:16166340; http://dx.doi.org/10.1152/ajpgi.00349.2005
- Streutker C, Huizinga J, Driman D, Riddell R. Interstitial cells of Cajal in health and disease. Part I: normal ICC structure and function with associated motility disorders. Histopathology 2007; 50(2):176-89; PMID:17222246; http://dx.doi.org/10.1111/j.1365-2559.2006.02493.x
- Rumessen JJ, Thuneberg L, Mikkelsen HB. Plexus muscularis profundus and associated interstitial cells. II. Ultrastructural studies of mouse small intestine. Anat Rec 1982; 203(1):129-46; PMID:7103120; http://dx.doi.org/10.1002/ar.1092030112
- Wang XY, Patterson C, Huizinga JD. Cholinergic and nitrergic innervation of ICC-DMP and ICC-IM in the human small intestine. Neurogastroenterol Motil 2003; 15:531-43; PMID:14507353; http://dx.doi.org/10.1046/j.1365-2982.2003.00429.x
- Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nature Med 1998; 4(7):848–851.
- Der-Silaphet T, Malysz J, Hagel S, Arsenault AL, Huizinga JD. Interstitial cells of Cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterol 1998; 114(4):724-36; http://dx.doi.org/10.1016/S0016-5085(98)70586-4
- Wu MJ, Kee KH, Na J, Kim SW, Bae Y, Shin DH, Choi S, Jun JY, Jeong HS, Park JS. Pituitary adenylate cyclase-activating polypeptide inhibits pacemaker activity of colonic interstitial cells of cajal. Kor J Physiol Pharmacol 2015; 19(5):435-40; http://dx.doi.org/10.4196/kjpp.2015.19.5.435
- Yoon PJ, Parajuli SP, Zuo DC, Shahi PK, Oh HJ, Shin HR, Lee MJ, Yeum CH, Choi S, Jun JY. Interplay of hydrogen sulfide and nitric oxide on the pacemaker activity of interstitial cells of cajal from mouse small intestine. Chonnam Med J 2011; 47(2):72-9; PMID:22111064; http://dx.doi.org/10.4068/cmj.2011.47.2.72
- Kendig DM, Hurst NR, Grider JR. Spatiotemporal mapping of motility in Ex vivo preparations of the intestines. J Vis Exp 2016; 107:53263; http://dx.doi.org/10.3791/53263
- Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol 1998; 513(1):203-13; PMID:9782170; http://dx.doi.org/10.1111/j.1469-7793.1998.203by.x
- Rhee PL, Lee JY, Son HJ, Kim JJ, Rhee JC, Kim S, Koh SD, Hwang SJ, Sanders KM, Ward SM. Analysis of pacemaker activity in the human stomach. J Physiol 2011; 589(24):6105-18; PMID:22005683; http://dx.doi.org/10.1113/jphysiol.2011.217497
- Ryoo S-B, Oh H-K, Moon SH, Choe EK, Yu SA, Park S-H, Park KJ. Electrophysiological and mechanical characteristics in human ileal motility: Recordings of slow waves conductions and contractions, in vitro. Kor J Physiol Pharmacol 2015; 19(6):533-42; http://dx.doi.org/10.4196/kjpp.2015.19.6.533
- Faussone-Pellegrini M, Cortesini C. The muscle coat of the lower esophageal sphincter in patients with achalasia and hypertensive sphincter. An electron microscopic study. J Submicrosc Cytol 1985; 17(4):673-85.
- Khelif K, De Laet M-H, Chaouachi B, Segers V, Vanderwinden J-M. Achalasia of the cardia in Allgrove's (triple A) syndrome: histopathologic study of 10 cases. Am J Surg Pathol 2003; 27(5):667-72; PMID:12717251; http://dx.doi.org/10.1097/00000478-200305000-00010
- Faussone-Pellegrini MS, Grover M, Pasricha PJ, Bernard CE, Lurken MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, et al. Ultrastructural differences between diabetic and idiopathic gastroparesis. J Cell Mol Med 2012; 16(7):1573-81; PMID:21914127; http://dx.doi.org/10.1111/j.1582-4934.2011.01451.x
- Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone-Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterol 2011; 140(5):1575-85. e8; http://dx.doi.org/10.1053/j.gastro.2011.01.046
- Ordög T. Interstitial cells of Cajal in diabetic gastroenteropathy. Neurogastroenterol Motil 2008; 20(1):8-18; http://dx.doi.org/10.1111/j.1365-2982.2007.01056.x
- Feldstein AE, Miller SM, El-Youssef M, Rodeberg D, Lindor NM, Burgart LJ, Szurszewski JH, Farrugia G. Chronic intestinal pseudoobstruction associated with altered interstitial cells of cajal networks. J Pediatr Gastroenterol Nutr 2003; 36(4):492-7; PMID:12658043; http://dx.doi.org/10.1097/00005176-200304000-00016
- Isozaki K, Hirota S, Miyagawa J-I, Taniguchi M, Shinomura Y, Matsuzawa Y. Deficiency of c-kit+ cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. Am J Gastroenterol 1997; 92(2).
- Kenny S, Vanderwinden J-M, Rintala R, Connell M, Lloyd D, Vanderhaegen J, De Laet MH. Delayed maturation of the interstitial cells of Cajal: a new diagnosis for transient neonatal pseudoobstruction. Report of two cases. J Pediatr Surg 1998; 33(1):94-8; PMID:9473109
- Yamataka A, Ohshiro K, Kobayashi H, Lane GJ, Yamataka T, Fujiwara T, Sunagawa M, Miyano T. Abnormal distribution of intestinal pacemaker (c-kit-positive) cells in an infant with chronic idiopathic intestinal pseudoobstruction. J Pediatr Surg 1998; 33(6):859-62; PMID:9660215; http://dx.doi.org/10.1016/S0022-3468(98)90660-1
- He CL, Burgart L, Wang L, Pemberton J, Young-Fadok T, Szurszewski J, Farrugia G. Decreased interstitial cell of Cajal volume in patients with slow-transit constipation. Gastroenterol 2000; 118(1):14-21; http://dx.doi.org/10.1016/S0016-5085(00)70409-4
- Lyford G, He C, Soffer E, Hull T, Strong S, Senagore A, Burgart LJ, Young-Fadok T, Szurszewski JH, Farrugia G. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut 2002; 51(4):496-501; PMID:12235070; http://dx.doi.org/10.1136/gut.51.4.496
- Wedel T, Spiegler J, Soellner S, Roblick UJ, Schiedeck TH, Bruch H-P, Krammer HJ. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterol 2002; 123(5):1459-67; PMID:12404220; http://dx.doi.org/10.1053/gast.2002.36600
