Abstract
Alternative pre-mRNA processing greatly increases the coding capacity of the human genome and regulatory factors involved in RNA processing play critical roles in tissue development and maintenance. Indeed, abnormal functions of RNA processing factors have been associated with a wide range of human diseases from cancer to neurodegenerative disorders. While many studies have emphasized the importance of alternative splicing (AS), recent high-throughput sequencing efforts have also allowed global surveys of alternative polyadenylation (APA). For the majority of pre-mRNAs, as well as some non-coding transcripts such as lncRNAs, APA selects different 3′-ends and thus modulates the availability of regulatory sites recognized by trans-acting regulatory effectors, including miRs and RNA binding proteins (RBPs). Here, we compare the available technologies for assessing global polyadenylation patterns, summarize the roles of auxiliary factors on APA, and discuss the impact of differential polyA site (pA) selection in the determination of cell fate, transformation and disease.
Abbreviations
| RBP | = | RNA binding protein |
| pA | = | Polyadenylation site |
| DM | = | Myotonic dystrophy |
| 3′ UTR | = | 3′-untranslated region |
| APA | = | Alternative polyadenylation |
| AS | = | Alternative splicing |
| HITS-CLIP | = | High-throughput sequencing coupled with crosslinking and immunoprecipitation |
| KD | = | Knockdown |
| KO | = | Knockout |
Alternative Polyadenylation Analysis: Sequencing and Validation
Approximately 70% of human gene transcripts undergo APA, which involves the selection of a specific 3′-endonucleolytic cleavage site followed by the addition of a polyA tail.Citation1,2 In addition to transcription and splicing, the tissue-specific mRNA landscape also depends on APA and the availability of miR and RBP accessible binding sites.Citation3 Indeed, several diseases highlight the importance of APA fidelity. Global 3′ UTR shortening leads to altered expression of cell growth control factors and is associated with cell transformation and cancer.Citation4 Enhanced selection of more proximal pA sites has also been reported in oculopharyngeal muscular dystrophy (OPMD), which is caused by expansions of a (GCG)n microsatellite in PABPN1, the major nuclear polyA tail binding protein.Citation5,6 Recently, we provided evidence that loss of muscleblind-like (MBNL) activity also results in APA defects in cell and mouse models of myotonic dystrophy type I (DM1) and in DM1 autopsied and biopsied skeletal muscles.Citation7
In the past, RNA-seq coverage of 3′ UTRs has been problematic due to several issues. First, technical factors complicate the use of RNA-seq to correctly determine 3′-ends, including 3′ UTR sequence composition, alternative 3′-end processing, and read depletion near 3′-ends in RNA fragmentation library generation protocols due to random priming and differential PCR amplification.Citation8-12 These problems lead to sequencing depth peaks and valleys resulting in inconsistent 3′ UTR quantitation. However, more recent RNA-seq approaches combined with enhanced de novo pA detection software and improved annotations have allowed more accurate APA assignments.Citation13,14 Second, RNA-seq provides sequence information for the entire transcriptome while PolyA-seq was developed specifically for quantitative estimation of pA selection in cells and tissues so it is less costly to obtain the high coverage required for statistically significant results.Citation15,2,7 Most of these 3′-end sequencing methodologies involve the isolation of polyA+ RNA, fragmentation, oligo(d)T and randomly primed cDNA synthesis followed by oligo(d)T primer-mediated sequencing. These techniques suffer from internal priming of templated A-rich stretches and polymerase slippage due to the repetitive polyA tail. Therefore, additional bioinformatic steps are required to filter out internally primed false pA peaks by either mapping the reads to a genome assembly annotated for polyadenylation sites or by defining a set of computational rules that exclude sites with less than a certain number of contiguous adenines. Variations of these techniques have also been developed that address such biases. For example, 3-P uses a splint ligation step to attach a biotinylated adapter to the 3′-end of the polyA tail to allow for improved purification and polyT misprimingCitation16 whereas 3′ Region Extraction And Deep Sequencing (3′ READS) uses a CU5T45 oligo primer to capture polyA+ RNA, followed by RNase H digestion of the polyA tail, 3′ and 5′ adapter ligation, reverse transcription and deep sequencing.Citation17 These PolyA-seq methods clearly delineate 3′-end cleavage sites, which often follows a CA dinucleotide.
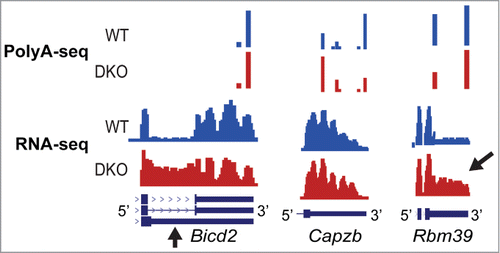
Although PolyA-seq methods may also show a bias, they allow for direct pA quantitation. We compared APA patterns using both RNA-seq and PolyA-seq datasets from Mbnl1−/−; Mbnl2−/− double knockout mouse embryonic fibroblasts (DKO MEFs). Whereas some overlap was noted for APA targets between the 2 data sets, a few differences were also detected. Of course PolyA-seq was not able to identify intron retention within the Bicd2 3 ′UTR whereas RNA-seq was successful (, left). This event is of significance because mutations in BICD2 cause autosomal dominant congenital spinal muscular atrophy and hereditary spastic paraplegia.Citation18-20 Conversely, a change in Capzb APA patterns was not clearly detected by RNA-seq due to uneven 3′ UTR read coverage but was successfully captured by PolyA-seq (, middle) whereas the Rbm39 APA pattern (, right) was captured by both techniques. On the horizon, long read single molecule methods are being developed that allow for whole transcript sequencing to estimate pA utilization and coordination of APA with other RNA processing events. Direct RNA sequencing has also been used for pA quantitation thereby removing the reverse transcription bias.Citation21 Additional validation methods include RNA blots and qRT-PCR for individual site quantitation and HITS-CLIP analysis combined with minigene reporters to study APA regulation by RBPs. These methodologies provide essential verifications that APA patterns reported by high-throughput sequencing technologies correctly ascertain pA selection in vivo.
Figure 1. Alternative Polyadenylation Site Identification by RNA-seq versus PolyA-seq. Comparison of PolyA-seq and RNA-seq wiggle plots of Bicd2 (left), Capzb (middle), and Rbm39 (right) in WT (blue) and Mbnl1;Mbnl2 DKO MEFs (red). RNA-seq detects internal 3′ UTR splicing in Bicd2 but fails to capture the pA shift in Capzb. Both techniques detect the pA shift in Rbm39.
Mechanisms and Auxiliary Regulatory Factors for Alternative Polyadenylation
Pre-mRNA 3′-end processing is regulated by RNA sequence elements that are recognized by several highly interactive protein complexes, which comprise the core polyadenylation machinery.Citation22,23 The AAUAAA polyadenylation sequence (PAS) is recognized by cleavage and polyadenylation specificity factor (CPSF), a U-/GU-rich element (DSE) downstream of the PAS is recognized by cleavage stimulation factor (CstF) and a U-rich/UGUA upstream element (USE) is recognized by cleavage factor Im (CFIm).Citation1,22,24,25 APA is often coupled to transcription and the core 3′-end processing machinery is known to interact with certain transcription factors for efficient loading of the polyadenylation machineryCitation26,27 and cis-elements and trans-acting factors may alter RNA polymerase II elongation dynamics to modulate pA site selection.Citation28,29 It is well established that the strength of the RNA sequence elements mentioned above, together with the local concentration of the core 3′-end processing machinery, defines APA patterns for many genes.
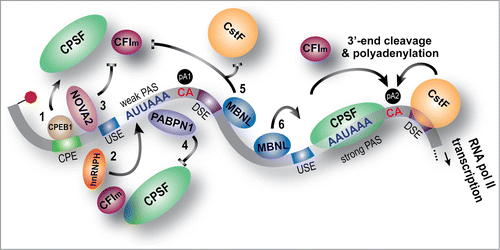
Previous studies have provided evidence for considerable variation of the canonical cis-elements in APA regulation probably due to the involvement of trans-acting RBP regulators that stabilize interactions between inherently weaker pA sites and the core machinery.Citation1,6,30,31 Interestingly, a number of these RBPs have multiple roles in RNA processing. For AS, enhancer and silencer elements in introns (ISE, ISS) and exons (ESE, ESS) are recognized by some RBPs to activate or suppress the splicing of alternative exons. Similarly, RBPs can act as auxiliary factors and activate or suppress polyadenylation at specific sites (). Incidentally, many APA regulators are well-known splicing factors. The neuron specific splicing factor, NOVA2, plays a crucial role in tissue-specific AS and APA regulation in the brain.Citation32 Both PTB and hnRNP H have also been reported to regulate both AS and APA.Citation33,34 High throughput sequencing-crosslinking immunoprecipitation (HITS-CLIP or CLIP-seq) demonstrates that hnRNP H binds to proximal (to the coding region termination codon) pA sites resulting in 3′ UTR shortening.Citation34 The cytoplasmic polyadenylation factor CPEB1, which shuttles between the nucleus and the cytoplasm, has multiple roles in AS, APA, and translation.Citation35 In the nucleus, CPEB1 binds to cytoplasmic polyadenylation elements (CPE) upstream of the CPSF binding site to activate weaker proximal pA sites. Therefore, any cellular processes or signaling events that induce shuttling of CPEB1 to the nucleus may lead to widespread 3′ UTR shortening.Citation25 Alternatively, ELAVL1/HuR is known to bind U-rich sequences near pA sites to sterically block activation.Citation36 A recent Drosophila study showed that RNA pol II-mediated ELAV recruitment to proximal pA sites suppresses selection at that site leading to long 3′ UTRs and the promoters of extended genes contained paused RNA pol II.Citation37 Replacement of these extension-associated ‘long promoters' with heterologous promoters shortened 3′ UTR lengths in the Drosophila nervous system whereas these promoters lengthened 3′ UTRs in other tissues.Citation37 In myotonic dystrophy, MBNL proteins either suppress or activate polyadenylation at specific sites.Citation7 HITS-CLIP analysis showed that MBNL binding sites occur in the vicinity of many pA sites and PolyA-seq analysis detected widespread changes in APA patterns in DKO MEFs, a polyCUG DM1 mouse model and in human DM1 muscle. Integration of sequencing and HITS-CLIP datasets into an RNA regulatory map indicated that MBNL suppresses polyadenylation at specific sites, preferably at, or downstream of, the cleavage site by blocking recruitment of some components of the core polyadenylation machinery. Consequently, CF Im68 (CPSF6) binding was reduced at the polyadenylation sites blocked by MBNL. MBNL proteins may also recruit the core polyadenylation machinery to activate some sites. Direct MBNL involvement in APA was confirmed by the loss of MBNL regulatory activity following mutation of MBNL binding sites in minigene polyadenylation reporters.Citation7
Figure 2. Mechanisms of RBP-mediated APA regulation. This illustration highlights the 3′ UTR (gray bar) downstream of the stop codon (red octagon) and the cis elements that bind the core 3′-end processing machinery. While the proximal site (pA1) is weaker (AUUAAA), the distal site (pA2) contains the canonical AAUAAA hexamer. Also shown are the U-rich upstream sequence element (USE) recognized by cleavage factor Im (CFIm), the U/GU-rich downstream sequence element (DSE) that binds cleavage stimulation factor (CstF) and the polyA signal (PAS) A(A/U)UAAA recognized by the cleavage and polyadenylation specificity factor (CPSF). Cleavage occurs 3′ of the CA dinucleotide (red). Also included in the figure are several examples of 3′-end processing modulated by RBP 3′ UTR binding: 1) In the nucleus, the cytoplasmic polyadenylation element binding protein 1 (CPEB1) recognizes the cytoplasmic polyadenylation element (CPE) upstream of the weaker proximal pA1 and recruits the CPSF complex to promote 3′ UTR shortening; 2) hnRNP H binds in the proximity of pA1 to recruit the core processing machinery to cause 3′ UTR shortening; 3) NOVA2 binds near pA1 to suppress proximal usage; 4) PolyA binding protein nuclear 1 (PABPN1) recognizes PASs and binds to the weaker pA1 thereby blocking CPSF binding (in OPMD, insufficient proximal pA1 suppression by PABPN1 leads to 3′ UTR shortening and PABPN1 cannot compete with CPSF for the strong canonical PAS); 5) MBNL proteins act by blocking the recruitment of the core machinery to intronic (not shown), proximal, and sometimes distal (not shown) sites when they bind within ± 50–100 nt of the pA site; 6) MBNL proteins also enhance pA selection by recruiting core polyadenylation factors.
It is interesting, but not surprising, that many RBPs have dual roles in AS and APA. HITS-CLIP analyses indicate that other RBPs, such as TDP-43 and RBFOX, bind to the 3′ UTRs of their target RNAs.Citation38-41 With the advent of more refined sequencing techniques, RBPs are being increasingly profiled for their global binding signatures and roles in AS, APA and other RNA regulatory processes. The integration of this experimental information together with the predictive functions of novel machine learning software should further clarify the sequence codes and other rules underlying RNA processing events. Preliminary studies for revealing global RNA structure have been described for mapping the RBP-RNA interaction ‘structurome’, which will likely yield further insights into this type of RNA processing code.Citation42,43 Although global sequencing studies reveal snapshots of the dynamic transcriptome, its predictive value of the downstream functional proteome is still unclear mostly due to the difficulties and limitations associated with quantitative high-throughput proteome determination. Improvement in proteomic technologies should bridge the gap between meaningful transcriptome and proteome correlations and will give rise to highly predictive computer programs.
RNA 3′-end Modulation and Effects on Cell Function, Development and Disease
Dysregulation of 3′-end processing may alter RNA stability, localization, and translation and lead to deleterious effects on cellular homeostasis. As an example, BDNF mRNA localization is regulated by APA with short 3′ UTR isoforms present in the neuronal soma whereas long isoforms are localized in dendrites.Citation44 These isoforms exhibit different translational efficiencies, which has implications for activity-dependent synaptic function of the hippocampus.Citation45 MBNL proteins also regulate mRNA localization either directly or by regulating APA.Citation46 Furthermore, APA shifts either to upstream coding region exons or introns may cause the production of truncated and dominant-negative proteins. For instance, polyadenylation within an upstream exon of EPRS leads to the production of a truncated protein.Citation47 Full-length EPRS recruits the gamma interferon activated inhibitor of translation (GAIT) complex to the VEGFA 3′ UTR to shut down its interferon-gamma induced translation. However, the truncated protein produced by APA is unable to localize GAIT and hence it allows for a slow rate of translation.Citation47
Differential APA has also been linked to embryonic stem cell (ESC) differentiation and cancer. CPSF-associated factor Fip1 is essential for mouse ESC self-renewal and maintenance.Citation21 Fip1 knockdown (KD) results in the loss of stem cell morphology, decreased expression of stem cell markers and APA shifts to more distal sites. Time course experiments showed that APA dysregulation occurs long before ESC differentiation suggesting that these pA shifts are involved in differentiation. Additionally, somatic cell reprogramming was impaired in Fip1 KD cells and, based on Fip1 binding, APA data and similarity to prior studies,Citation22 high concentrations of the Fip1/CPSF complex may result in recognition of weaker proximal sites that are transcribed earlier than distal sites. Alternatively, when cleavage sites are in close proximity preventing a significant lag between their transcription, Fip1/CPSF binding between the 2 sites sterically blocks the proximal cleavage site. MBNL proteins also suppress ESC-specific splicing patterns by regulating FOXP1 exon 18b alternative splicing. MBNL overexpression induces splicing patterns associated with differentiation whereas MBNL KD results in ESC-like AS patterns and increased expression of pluripotency markers.Citation48 It will be interesting to investigate potential roles of MBNL-regulated APA in pluripotency and somatic reprogramming of induced pluripotent stem cells (iPSCs).
Changes in cell growth properties and differentiation is also associated with cancer.Citation4 RNA-seq of 7 tumor types (bladder urothelial carcinoma, head and neck squamous cell carcinoma, lung squamous cell carcinoma, lung adenocarcinoma, breast cancer, kidney renal clear cell carcinoma, uterine corpus endometrioid carcinoma) was performed recently for 358 patients and compared to matched normal tissues using the cancer genome atlas (TCGA, http://cancergenome.nih.gov/). A staggering 1,346 genes were identified with recurrent APA changes and the vast majority (91%) are proximal shifts leading to 3′ UTR shortening events. Furthermore, genes with shorter 3′ UTRs tend to be upregulated in tumors and increased expression of CSTF2/CstF-64 is observed.Citation14 Additional work has shown that elevation of CF Im25 levels enhances distal 3′ UTR selection in HeLa cells while KD causes 3′ UTR shortening, enhanced cell proliferation and increased expression of oncogenic factors, such as cyclin D1.Citation13 Moreover, CF Im25 is downregulated in glioblastoma (GBM) and its overexpression inhibits tumor growth in GBM cell lines. Future studies should focus on potential oncogenic and tumor suppressor RBPs that modulate APA in cancer.
Developmental dysregulation of APA also occurs in myotonic dystrophy. DM is caused by either CTG microsatellite expansions in the DMPK 3′ UTR (DM1) or CCTG expansions in intron 1 of CNBP (DM2).Citation49 Transcription of these expansions yields C(C)UG expansion RNAs that sequester the MBNL proteins, thereby causing their loss-of-function and consequent disruption of developmental AS and APACitation7,50,51. Like AS, APA patterns change during development from a fetal to adult pattern allowing for tissue differentiation and to meet the ongoing functional requirements of adult tissues. Loss of MBNL in adult DM muscle leads to a reversion of APA to a more fetal pattern for target transcripts. Gene ontology and systems analysis reveals several different classes of genes misregulated in APA, including those involved in ubiquitination, IGF-1 signaling and the mTOR pathway, that are known to be play important roles in muscle hypertrophy and maintenance. Alterations in these pathways may explain the muscle atrophy phenotype associated with this disease.Citation7 It will not be surprising if APA defects are a recurrent theme in other microsatellite expansion and neurodegenerative diseases.
Coordinating RNA Processing
Since RBPs regulate multiple RNA processing steps, an intriguing question is how frequently these steps are coordinated at the molecular level. Such studies require the use of long read sequencing techniques, such as single molecule real time (SMRT) sequencing technologyCitation52 and direct RNA sequencing (DRS).Citation21,53 These methodologies allow RNA sequencing without the need for RNA fragmentation and amplification. Furthermore, comprehensive systems level analyses of APA along with gene expression patterns in disease should yield insights into altered regulatory pathways.
Disclosure of Potential Conflicts of Interest
R.B. is a 2015 Myotonic Dystrophy Foundation (MDF) postdoctoral fellow.
Funding
Our studies are funded by grants to M.S.S. from the National Institutes of Health (NS058901 and AR046799), the Muscular Dystrophy Association (MDA276063) and the Marigold Foundation.
References
- Shi Y. Alternative polyadenylation: new insights from global analyses. RNA 2012; 18:2105-17; PMID:23097429; http://dx.doi.org/10.1261/rna.035899.112
- Derti A, Garrett-Engele P, Macisaac KD, Stevens RC, Sriram S, Chen R, Rohl CA, Johnson JM, Babak T. A quantitative atlas of polyadenylation in five mammals. Genome Res 2012; 22:1173-83; PMID:22454233; http://dx.doi.org/10.1101/gr.132563.111
- Lianoglou S, Garg V, Yang JL, Leslie CS, Mayr C. Ubiquitously transcribed genes use alternative polyadenylation to achieve tissue-specific expression. Genes Dev 2013; 27:2380-96; PMID:24145798; http://dx.doi.org/10.1101/gad.229328.113
- Mayr C, Bartel DP. Widespread shortening of 3′UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 2009; 138:673-84; PMID:19703394; http://dx.doi.org/10.1016/j.cell.2009.06.016
- Jenal M, Elkon R, Loayza-Puch F, van Haaften G, Kuhn U, Menzies FM, Oude Vrielink JA, Bos AJ, Drost J, Rooijers K, et al. The poly(A)-binding protein nuclear 1 suppresses alternative cleavage and polyadenylation sites. Cell 2012; 149:538-53; PMID:22502866; http://dx.doi.org/10.1016/j.cell.2012.03.022
- de Klerk E, Venema A, Anvar SY, Goeman JJ, Hu O, Trollet C, Dickson G, den Dunnen JT, van der Maarel SM, Raz V, et al. Poly(A) binding protein nuclear 1 levels affect alternative polyadenylation. Nucl Acids Res 2012; 40:9089-101; PMID:22772983; http://dx.doi.org/10.1093/nar/gks655
- Batra R, Charizanis K, Manchanda M, Mohan A, Li M, Finn DJ, Goodwin M, Zhang C, Sobczak K, Thornton CA, et al. Loss of MBNL leads to disruption of developmentally regulated alternative polyadenylation in RNA-mediated disease. Mol Cell 2014; 56:311-22; PMID:25263597; http://dx.doi.org/10.1016/j.molcel.2014.08.027
- Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 2009; 10:57-63; PMID:19015660; http://dx.doi.org/10.1038/nrg2484
- Ramskold D, Wang ET, Burge CB, Sandberg R. An abundance of ubiquitously expressed genes revealed by tissue transcriptome sequence data. PLoS Comput Biol 2009; 5:e1000598; PMID:20011106
- Huang R, Jaritz M, Guenzl P, Vlatkovic I, Sommer A, Tamir IM, Marks H, Klampfl T, Kralovics R, Stunnenberg HG, et al. An RNA-Seq strategy to detect the complete coding and non-coding transcriptome including full-length imprinted macro ncRNAs. PLoS One 2011; 6:e27288
- Wang L, Yi R. 3′UTRs take a long shot in the brain. Bioessays 2014; 36:39-45; PMID:24115048; http://dx.doi.org/10.1002/bies.201300100
- Hansen KD, Brenner SE, Dudoit S. Biases in Illumina transcriptome sequencing caused by random hexamer priming. Nucl Acids Res 2010; 38:e131
- Masamha CP, Xia Z, Yang J, Albrecht TR, Li M, Shyu AB, Li W, Wagner EJ. CFIm25 links alternative polyadenylation to glioblastoma tumour suppression. Nature 2014; 510:412-6; PMID:24814343
- Xia Z, Donehower LA, Cooper TA, Neilson JR, Wheeler DA, Wagner EJ, Li W. Dynamic analyses of alternative polyadenylation from RNA-seq reveal a 3′-UTR landscape across seven tumour types. Nat Commun 2014; 5:5274; PMID:25409906; http://dx.doi.org/10.1038/ncomms6274
- Shepard PJ, Choi EA, Lu J, Flanagan LA, Hertel KJ, Shi Y. Complex and dynamic landscape of RNA polyadenylation revealed by PAS-Seq. RNA 2011; 17:761-72; PMID:21343387; http://dx.doi.org/10.1261/rna.2581711
- Jan CH, Friedman RC, Ruby JG, Bartel DP. Formation, regulation and evolution of Caenorhabditis elegans 3′UTRs. Nature 2011; 469:97-101; PMID:21085120; http://dx.doi.org/10.1038/nature09616
- Hoque M, Ji Z, Zheng D, Luo W, Li W, You B, Park JY, Yehia G, Tian B. Analysis of alternative cleavage and polyadenylation by 3′ region extraction and deep sequencing. Nat Methods 2013; 10:133-9; PMID:23241633; http://dx.doi.org/10.1038/nmeth.2288
- Oates EC, Rossor AM, Hafezparast M, Gonzalez M, Speziani F, MacArthur DG, Lek M, Cottenie E, Scoto M, Foley AR, et al. Mutations in BICD2 cause dominant congenital spinal muscular atrophy and hereditary spastic paraplegia. Am J Hum Genet 2013; 92:965-73; PMID:23664120; http://dx.doi.org/10.1016/j.ajhg.2013.04.018
- Peeters K, Litvinenko I, Asselbergh B, Almeida-Souza L, Chamova T, Geuens T, Ydens E, Zimon M, Irobi J, De Vriendt E, et al. Molecular defects in the motor adaptor BICD2 cause proximal spinal muscular atrophy with autosomal-dominant inheritance. Am J Hum Genet 2013; 92:955-64; PMID:23664119; http://dx.doi.org/10.1016/j.ajhg.2013.04.013
- Neveling K, Martinez-Carrera LA, Holker I, Heister A, Verrips A, Hosseini-Barkooie SM, Gilissen C, Vermeer S, Pennings M, Meijer R, et al. Mutations in BICD2, which encodes a golgin and important motor adaptor, cause congenital autosomal-dominant spinal muscular atrophy. Am J Hum Genet 2013; 92:946-54; PMID:23664116; http://dx.doi.org/10.1016/j.ajhg.2013.04.011
- Lackford B, Yao C, Charles GM, Weng L, Zheng X, Choi EA, Xie X, Wan J, Xing Y, Freudenberg JM, et al. Fip1 regulates mRNA alternative polyadenylation to promote stem cell self-renewal. EMBO J 2014; 33:878-89; PMID:24596251; http://dx.doi.org/10.1002/embj.201386537
- Tian B, Manley JL. Alternative cleavage and polyadenylation: the long and short of it. Trends Biochem Sci 2013; 38:312-20; PMID:23632313; http://dx.doi.org/10.1016/j.tibs.2013.03.005
- Di Giammartino DC, Nishida K, Manley JL. Mechanisms and consequences of alternative polyadenylation. Mol Cell 2011; 43:853-66; PMID:21925375; http://dx.doi.org/10.1016/j.molcel.2011.08.017
- Xiang K, Tong L, Manley JL. Delineating the structural blueprint of the pre-mRNA 3′-end processing machinery. Mol Cell Biol 2014; 34:1894-910; PMID:24591651; http://dx.doi.org/10.1128/MCB.00084-14
- de Klerk E, t Hoen PA. Alternative mRNA transcription, processing, and translation: insights from RNA sequencing. Trends Genet 2015; PMID:25648499
- Martincic K, Alkan SA, Cheatle A, Borghesi L, Milcarek C. Transcription elongation factor ELL2 directs immunoglobulin secretion in plasma cells by stimulating altered RNA processing. Nat Immunol 2009; 10:1102-9; PMID:19749764; http://dx.doi.org/10.1038/ni.1786
- Ji Z, Luo W, Li W, Hoque M, Pan Z, Zhao Y, Tian B. Transcriptional activity regulates alternative cleavage and polyadenylation. Mol Syst Biol 2011; 7:534; PMID:21952137; http://dx.doi.org/10.1038/msb.2011.69
- Shukla S, Kavak E, Gregory M, Imashimizu M, Shutinoski B, Kashlev M, Oberdoerffer P, Sandberg R, Oberdoerffer S. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 2011; 479:74-9; PMID:21964334; http://dx.doi.org/10.1038/nature10442
- Pinto PA, Henriques T, Freitas MO, Martins T, Domingues RG, Wyrzykowska PS, Coelho PA, Carmo AM, Sunkel CE, Proudfoot NJ, et al. RNA polymerase II kinetics in polo polyadenylation signal selection. EMBO J 2011; 30:2431-44; PMID:21602789; http://dx.doi.org/10.1038/emboj.2011.156
- Kamasawa M, Horiuchi J. Identification and characterization of polyadenylation signal (PAS) variants in human genomic sequences based on modified EST clustering. In Silico Biol 2008; 8:347-61; PMID:19032167
- Ji Z, Lee JY, Pan Z, Jiang B, Tian B. Progressive lengthening of 3′ untranslated regions of mRNAs by alternative polyadenylation during mouse embryonic development. Proc Natl Acad Sci U S A 2009; 106:7028-33; PMID:19372383; http://dx.doi.org/10.1073/pnas.0900028106
- Licatalosi DD, Mele A, Fak JJ, Ule J, Kayikci M, Chi SW, Clark TA, Schweitzer AC, Blume JE, Wang X, et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature 2008; 456:464-9; PMID:18978773; http://dx.doi.org/10.1038/nature07488
- Castelo-Branco P, Furger A, Wollerton M, Smith C, Moreira A, Proudfoot N. Polypyrimidine tract binding protein modulates efficiency of polyadenylation. Mol Cell Biol 2004; 24:4174-83; PMID:15121839; http://dx.doi.org/10.1128/MCB.24.10.4174-4183.2004
- Katz Y, Wang ET, Airoldi EM, Burge CB. Analysis and design of RNA sequencing experiments for identifying isoform regulation. Nat Methods 2010; 7:1009-15; PMID:21057496; http://dx.doi.org/10.1038/nmeth.1528
- Bava FA, Eliscovich C, Ferreira PG, Minana B, Ben-Dov C, Guigo R, Valcarcel J, Mendez R. CPEB1 coordinates alternative 3′-UTR formation with translational regulation. Nature 2013; 495:121-5; PMID:23434754; http://dx.doi.org/10.1038/nature11901
- Zhu H, Zhou HL, Hasman RA, Lou H. Hu proteins regulate polyadenylation by blocking sites containing U-rich sequences. J Biol Chem 2007; 282:2203-10; PMID:17127772; http://dx.doi.org/10.1074/jbc.M609349200
- Oktaba K, Zhang W, Lotz TS, Jun DJ, Lemke SB, Ng SP, Esposito E, Levine M, Hilgers V. ELAV Links paused pol II to alternative polyadenylation in the drosophila nervous system. Mol Cell 2015; 57:341-8; PMID:25544561; http://dx.doi.org/10.1016/j.molcel.2014.11.024
- Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, Kayikci M, Konig J, Hortobagyi T, Nishimura AL, Zupunski V, et al. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci 2011; 14:452-8; PMID:21358640; http://dx.doi.org/10.1038/nn.2778
- Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling SC, Sun E, Wancewicz E, Mazur C, et al. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci 2011; 14:459-68; PMID:21358643; http://dx.doi.org/10.1038/nn.2779
- Weyn-Vanhentenryck SM, Mele A, Yan Q, Sun S, Farny N, Zhang Z, Xue C, Herre M, Silver PA, Zhang MQ, et al. HITS-CLIP and integrative modeling define the Rbfox splicing-regulatory network linked to brain development and autism. Cell Rep 2014; 6:1139-52; PMID:24613350; http://dx.doi.org/10.1016/j.celrep.2014.02.005
- Lovci MT, Ghanem D, Marr H, Arnold J, Gee S, Parra M, Liang TY, Stark TJ, Gehman LT, Hoon S, et al. Rbfox proteins regulate alternative mRNA splicing through evolutionarily conserved RNA bridges. Nat Struct Mol Biol 2013; 20:1434-42; PMID:24213538; http://dx.doi.org/10.1038/nsmb.2699
- Kertesz M, Wan Y, Mazor E, Rinn JL, Nutter RC, Chang HY, Segal E. Genome-wide measurement of RNA secondary structure in yeast. Nature 2010; 467:103-7; PMID:20811459; http://dx.doi.org/10.1038/nature09322
- Spitale RC, Crisalli P, Flynn RA, Torre EA, Kool ET, Chang HY. RNA SHAPE analysis in living cells. Nat Chem Biol 2013; 9:18-20; PMID:23178934; http://dx.doi.org/10.1038/nchembio.1131
- An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, et al. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 2008; 134:175-87; PMID:18614020; http://dx.doi.org/10.1016/j.cell.2008.05.045
- Orefice LL, Waterhouse EG, Partridge JG, Lalchandani RR, Vicini S, Xu B. Distinct roles for somatically and dendritically synthesized brain-derived neurotrophic factor in morphogenesis of dendritic spines. J Neurosci 2013; 33:11618-32; PMID:23843530; http://dx.doi.org/10.1523/JNEUROSCI.0012-13.2013
- Wang ET, Cody NA, Jog S, Biancolella M, Wang TT, Treacy DJ, Luo S, Schroth GP, Housman DE, Reddy S, et al. Transcriptome-wide regulation of pre-mRNA splicing and mRNA localization by muscleblind proteins. Cell 2012; 150:710-24; PMID:22901804; http://dx.doi.org/10.1016/j.cell.2012.06.041
- Yao P, Potdar AA, Arif A, Ray PS, Mukhopadhyay R, Willard B, Xu Y, Yan J, Saidel GM, Fox PL. Coding region polyadenylation generates a truncated tRNA synthetase that counters translation repression. Cell 2012; 149:88-100; PMID:22386318; http://dx.doi.org/10.1016/j.cell.2012.02.018
- Han H, Irimia M, Ross PJ, Sung HK, Alipanahi B, David L, Golipour A, Gabut M, Michael IP, Nachman EN, et al. MBNL proteins repress ES-cell-specific alternative splicing and reprogramming. Nature 2013; 498:241-5; PMID:23739326; http://dx.doi.org/10.1038/nature12270
- Poulos MG, Batra R, Charizanis K, Swanson MS. Developments in RNA splicing and disease. Cold Spring Harb Perspect Biol 2011; 3:a000778; PMID:21084389
- Kanadia RN, Shin J, Yuan Y, Beattie SG, Wheeler TM, Thornton CA, Swanson MS. Reversal of RNA missplicing and myotonia after muscleblind overexpression in a mouse poly(CUG) model for myotonic dystrophy. Proc Natl Acad Sci U S A 2006; 103:11748-53; PMID:16864772; http://dx.doi.org/10.1073/pnas.0604970103
- Charizanis K, Lee KY, Batra R, Goodwin M, Zhang C, Yuan Y, Shiue L, Cline M, Scotti MM, Xia G, et al. Muscleblind-like 2-mediated alternative splicing in the developing brain and dysregulation in myotonic dystrophy. Neuron 2012; 75:437-50; PMID:22884328; http://dx.doi.org/10.1016/j.neuron.2012.05.029
- Sharon D, Tilgner H, Grubert F, Snyder M. A single-molecule long-read survey of the human transcriptome. Nat Biotechnol 2013; 31:1009-14; PMID:24108091; http://dx.doi.org/10.1038/nbt.2705
- Ozsolak F, Platt AR, Jones DR, Reifenberger JG, Sass LE, McInerney P, Thompson JF, Bowers J, Jarosz M, Milos PM. Direct RNA sequencing. Nature 2009; 461:814-8; PMID:19776739; http://dx.doi.org/10.1038/nature08390
