ABSTRACT
In ribosomal translation, only 20 kinds of proteinogenic amino acids (pAAs), namely 19 l-amino acids and glycine, are exclusively incorporated into polypeptide chain. To overcome this limitation, various methods to introduce non-proteinogenic amino acids (npAAs) other than the 20 pAAs have been developed to date. However, the repertoire of amino acids that can be simultaneously introduced is still limited. Moreover, the efficiency of npAA incorporation is not always sufficient depending on their structures. Fidelity of translation is sometimes low due to misincorporation of competing pAAs and/or undesired translation termination. Here, we provide an overview of efforts to solve these issues, focusing on the engineering of tRNAs.
Introduction
In ribosomal translation system, 20 kinds of proteinogenic amino acids (pAAs), namely 19 l-amino acids and glycine, are exclusively used in polypeptide synthesis, and precisely introduced at the corresponding codons of mRNA. Of the 64 ( = 43) codons, 61 sense codons are used for designating the 20 pAAs, and the remaining 3 codons are used as stop codons. The correspondence between codons and amino acids is strictly defined by the genetic code, which is widely shared among all the 3 domains of life. However, various artificial methods to introduce non-proteinogenic amino acids (npAAs) beyond the 20 pAAs into polypeptides have been developed to date. If we wish to introduce npAAs, we need to break the rule of the genetic code and reassign the desired npAAs at some specific codons which are intrinsically occupied by particular pAAs or used as stop codons. As the key for the decoding is aminoacyl-tRNA (tRNA), use of “mischarged” npAA-tRNA enables incorporation of npAAs at the codon designated by the anticodon of the tRNA. Indeed, several methods to prepare artificial acyl-tRNAs are devised. For instance, ligation of aminoacyl-pdCpA dinucleotide with tRNA lacking the 3′-terminal CA dinucleotide is a widely adopted chemical method.Citation1 An aminoacylation ribozyme named flexizyme is also a powerful tool to enzymatically synthesize diverse acyl-tRNAs.Citation2,Citation3 By modifying natural aminoacyl-tRNA synthetases (ARSs), various artificial ARSs that can charge particular npAAs on specific tRNAs have also been developed.Citation4-Citation7
Such pre-charged npAAs can be introduced into peptides in combination with the following genetic code manipulation techniques. The genetic code reprogramming method and the stop codon method use sense codons and stop codons, respectively, for designating npAAs. In the genetic code reprogramming, pAAs designated at the codon to be reprogrammed are removed from the translation system to prepare vacant codons, and instead pre-charged npAA-tRNAs that designate the vacant codons are added to the translation system. The classical stop codon method utilizes a suppressor npAA-tRNA that designates a specific stop codon.Citation8 The competing release factor can be removed from the translation system.Citation9 The programmed frameshift method (using 4-base codon) and the non-standard base method can also create extra codons beyond the 64 canonical codons to assign npAAs. In the former method, the fourth nucleotide next to a rare codon, such as arginine AGG codon in Escherichia coli, can be recognized as a part of a 4-base codon (AGGU for instance) and used for designating additional npAA by an npAA-tRNA with a 4-base ACCU anticodon.Citation10 The non-standard base method introduces an artificial nucleotide pair in addition to the natural ones, i.e. A/U and G/C, to increase the number of available codons from 64 ( = 43) to 216 ( = 63).
However, there are several issues raised in incorporation of npAAs with these methods. First, the fidelity of translation is generally much lower than that of natural translation system due to misincorporation of undesired amino acids and/or premature translation termination. In the case of genetic code reprogramming, in which an npAA-tRNA designates a codon occupied by a particular pAA, the competing pAA-tRNA should be completely excluded from the translation system. Otherwise, the 2 different amino acids are possibly incorporated at the same codon in a competitive manner. Similarly, in the case of the stop codon suppression, competition of release factor against the suppressor npAA-tRNA causes undesired translation termination to yield a truncated protein. Thus, a mixture of full-length and truncated proteins is synthesized.
Second, the efficiency of npAA incorporation differs depending on their structures, and some npAAs cannot be efficiently introduced into peptides, which eventually induces misincorporation of competing amino acids. In an early report by Cornish and coworkers, diverse Ala and Phe derivatives, including N-methyl-amino, α-methyl-amino, β-amino, d-amino, and α-hydroxyl acid analogs were tested in fMet-npAA-Glu tripeptide synthesis.Citation11 They reported that N-methyl-amino, α-methyl-amino and α-hydroxyl acid could be introduced into peptides, whereas no incorporation of β- and d-amino acid was detected. Although several recent studies improved the translation conditions to accomplish the incorporation of β- and d-amino acid, the efficiencies were still quite low.Citation12-Citation15 Especially, consecutive incorporation of these amino acids was yet difficult to achieve.Citation12,Citation14
Third, the number of amino acids that are simultaneously available in ribosomal translation is limited. In the genetic code reprogramming, as all the 61 sense codons are already occupied by the 20 pAAs, the corresponding pAA designated at the codon to be reprogrammed to npAA should be removed from the translation system. Therefore, the number of available pAAs should be reduced to less than 20. Similarly in the stop codon suppression, as there are only 3 stop codons and at least one release factor must be used to terminate translation, only one stop codon can be used to introduce an extra amino acid without competition by the release factor.Citation8,Citation16
To solve these issues, researchers in this field have made a great deal of efforts for over the last few decades. In this article, we summarize several methodologies established by such efforts, especially focusing on tRNA engineering.
Development of orthogonal tRNAs
To maintain the fidelity of npAA incorporation, it is necessary to completely remove the competitor aminoacyl-tRNA from the translation system. If there are 2 or more aminoacyl-tRNAs bearing the same anticodon charged with different kinds of amino acids, those amino acids can be competitively introduced at the same codon. To avoid this, the pair of ARS and tRNA used for the npAA acylation must be orthogonal to the other ARS/tRNA pairs (). When we use an artificial ARS to charge an npAA de novo in the translation system, the ARS should not cross-react with the other tRNAs existing in the system. Conversely, the tRNA used for the npAA incorporation should not be recognized by the other ARSs in the translation system.
Figure 1. Development of orthogonal tRNAs. (A) Conceptual scheme of orthogonal ARS/tRNA pairs. ARS1 specifically charges amino acid 1 (AA1) onto tRNA1, but not onto tRNA2. Conversely, tRNA2 is exclusively charged with amino acid 2 (AA2) by ARS2, but not with AA1 by ARS1. (B) Mutant TyrRS/tRNATyr CUA pair orthogonal to the E. coli wild-type (WT) TyrRS/tRNATyr pair. The mutant TyrRS was developed based on M. jannaschii TyrRS to charge O-methyltyrosine instead of tyrosine on M. jannaschii tRNATyr or mutRNATyr CUA. The mutRNATyr CUA has 5 nucleotide substitutions, C17A, U17aG, U20C, G37A, and U47G, to improve the orthogonality as well as the anticodon substitution from GUA to CUA to decode UAG codon. (C) Methanococcus maripaludis (Mm) SepRS/tRNASep CUA pair orthogonal to the E. coli wild-type (WT) CysRS/tRNACys pair. The tRNASep CUA was designed based on M. jannaschii tRNACys with 3 nucleotide changes (C20U, G34C, and C35U). (D) Secondary structure of tRNAAsnE2 GGA designed based on E. coli tRNAAsn. Nucleotide changes at the acceptor stem are introduced to give orthogonality to the other E. coli ARS/tRNA pairs. (E) Secondary structure of tRNAGluE2 GGA designed based on E. coli tRNAGlu.
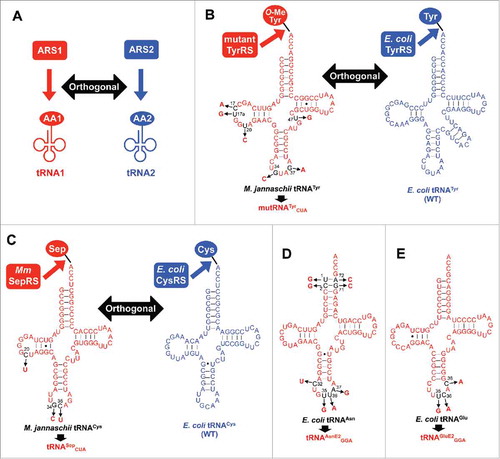
Schultz and coworkers developed various orthogonal ARS/tRNA pairs that can utilize npAAs. They introduced 5 point mutations around the active center of Methanocaldococcus jannaschii TyrRS to charge O-methyltyrosine instead of tyrosine on M. jannaschii tRNATyr, and expressed them in E. coli.Citation4 The mutant M. jannaschii TyrRS/tRNATyr pair and the wild-type E. coli TyrRS/tRNATyr pair are orthogonal to each other, and thus O-methyltyrosine can be exclusively charged on M. jannaschii tRNATyr, and tyrosine is charged on E. coli tRNATyr. To enhance the orthogonality, 5 nucleotide substitutions were introduced into the M. jannaschii tRNATyr (, mutRNATyr CAU, C17A, U17aG, U20C, G37A, and U47G). In addition, the anticodon sequence of M. jannaschii tRNATyr was changed from GUA to CUA to decode UAG stop codon, while the E. coli tRNATyr decodes the tyrosine codons (UAU and UAC). By using this system, they successfully demonstrated the incorporation of O-methyltyrosine at the UAG codon.
In methanogenic archaea, phosphoseryl-tRNA synthetase (SepRS) mischarges O-phosphoserine (Sep) on tRNACys, and then Sep-tRNA:Cys-tRNA synthase (SepCysS) converts the Sep into Cys by sulfhydrylation to give Cys-tRNACys. Söll and coworkers took advantage of Methanococcus maripaludis SepRS to express Sep-tRNASep in E. coli.Citation17 The tRNASep is an engineered tRNA based on M. jannaschii tRNACys (, tRNASep), which cannot be recognized by E. coli CysRS. Thus, Cys cannot be charged on tRNASep by E. coli CysRS. As E. coli does not have SepCysS gene, the mischarged Sep are not converted into Cys, and thus introduced into peptide as is. Similarly, E. coli tRNACys are not recognized by SepRS so that misacylation of Sep does not occur. Therefore, Sep is exclusively charged on the engineered tRNASep, and Cys is charged on wild-type tRNACys. By using this system, one or 2 Sep residues were introduced into human mitogen-activated ERK activating kinase 1 (MEK1).
Suga and coworkers developed a more versatile aminoacylation ribozyme named flexizyme, which catalyzes acylation of diverse tRNAs using pre-activated amino acids.Citation2,Citation3 Flexizyme recognizes the universally conserved 3′-terminal NCCA sequence of tRNA, and therefore virtually any tRNA species can be used in the flexizyme reaction, i.e., flexizyme is not orthogonal to any other ARS/tRNA pairs. Thus, pre-activated amino acids should be eliminated from the translation system to avoid the de novo flexizyme reaction. In addition, the tRNA used for the flexizyme acylation should be orthogonal to the other ARSs existing in the translation system. tRNAAsnE2 was developed as such tRNA having orthogonality against E. coli ARSs by introducing point mutations into the acceptor stem of E. coli tRNAAsn (, U1G, U2G, G71C, and A72C).Citation18-Citation20 Since then, tRNAAsnE2 has been used for incorporation of various npAAs including l-amino acids with non-natural side chains as well as N-alkyl-amino, β-amino, d-amino, α-hydroxyl acid, and so on.Citation12,Citation14,Citation15,Citation18,Citation21,Citation22
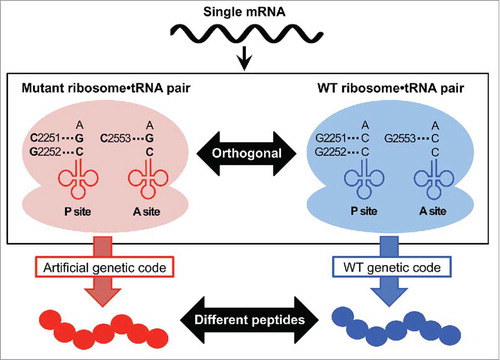
Suga's group also devised a new orthogonal ribosomal translation system in which the mutant ribosome exclusively utilizes the mutant tRNAs and does not recognize wild-type tRNAs ().Citation23 In the wild-type E. coli translation system, the Watson-Crick base-pairs between the 3′-end of tRNA (C74C75) and G2251G2252 at the P-site of 23S rRNA as well as with G2553 at the A-site play a critical role in the peptidyl transfer reaction. However, the compensatory mutant consisting of tRNAs with C75G and 23S rRNA with G2251C/G2553C mutations retains the translational activity with an orthogonality to the wild-type ribosome/tRNA pair. Therefore, this system can specifically use an orthogonal genetic code without cross-reading from the wild-type aminoacyl-tRNAs. Consequently, the wild-type and orthogonal machineries could function in parallel, producing 2 different peptides from a single mRNA template using the native and artificially reprogrammed genetic codes, respectively.
Figure 2. Overview of orthogonal ribosome/tRNA pairs. The mutant ribosome/tRNA pair consists of the mutant ribosome with G2251C/G2253C mutations in the 23S rRNA and the tRNA with C75G mutation. The mutant ribosome specifically recognizes the mutant tRNA, and is inert to the wild-type (WT) tRNA. Similarly, the WT ribosome exclusively utilizes the WT tRNA. These coexisting orthogonal machineries express 2 distinct peptides from a single mRNA template depending on the artificially reprogrammed genetic code and the WT genetic code.
Engineering of tRNA for improving efficiency of npAA incorporation
Efficiencies of npAA incorporation are generally much lower than that of pAAs. Especially, consecutive incorporation of d-amino and β-amino acids is extremely difficult. Even single incorporation of some d-amino and β-amino acids such as negatively charged ones are reported impossible. In the case of N-methyl amino acids, bulky N-methyl-amino acids such as N-methyl-Leu and negatively charged ones such as N-methyl-Asp are very poor substrates. One of the reason for the low efficiency of the incorporation of these amino acids would be slow accommodation rate of aminoacyl-tRNAs onto the A site of the ribosome. Although EF-Tu is responsible for the accommodation of elongator aminoacyl-tRNAs, affinity of npAAs to EF-Tu is considered weaker than that of pAAs because of their structural incompatibility, and thus EF-Tu cannot efficiently carries npAA-tRNAs. Therefore, enhancing tRNA•EF-Tu binding affinity would contribute to the incorporation of difficult npAAs by improving the accommodation rate.
Dale et al. have reported that the binding affinity of aminoacyl-tRNA to EF-Tu is determined by not only the amino acid structure but also the tRNA structure.Citation24,Citation25 For instance, ΔG0 values of l-Val-tRNAAsn and l-Val-tRNAGlu are −8.8 and −11.7 kcal/mol, respectively, indicating that l-Val-tRNAGlu has 110-fold higher affinity than l-Val-tRNAAsn. This result implies the possibility that use of tRNA with stronger affinity, such as tRNAGlu, improves the accommodation rate of difficult npAA-tRNAs. Suga's group devised a new tRNA named tRNAGluE2 based on E. coli tRNAGlu to have higher binding affinity to EF-Tu than the previously used tRNAAsnE2 ().Citation15,Citation23 Katoh et al.Citation15 demonstrated that tRNAGluE2 exhibited 3-fold higher translation yield of peptide containing 2 consecutive d-Ala compared with tRNAAsnE2. They also optimized the concentrations of EF-Tu, EF-G and IF2 in the translation system, and successfully introduced 10 consecutive d-Ser into a peptide. In addition, macrocyclic peptides with 4 or 5 consecutive d-amino acids consisting of d-Ser, d-Ala, d-Cys, and d-Phe closed by either a disulfide or thioether bond could also be synthesized. Similarly, Achenbach et al. also constructed an engineered tRNAGly with a high binding affinity for EF-Tu and demonstrated d-amino acid incorporation.Citation13
EF-Tu mutants that have higher binding affinity to specific npAA-tRNAs have been also developed. Doi et al. constructed a mutant EF-Tu that has a larger amino-acid binding pocket to accept bulky amino acids.Citation26 l-1-pyrenylalanine, l-2-pyrenylalanine and dl -2-anthraquinonylalanine, whose incorporation was hardly possible by wild-type EF-Tu, could be introduced into a protein by using the mutant EF-Tu.
Increasing amino acid repertoire by tRNA engineering
The genetic code reprogramming method involves (1) the preparation of a reconstituted in vitro translation systemCitation27 where certain pAAs and their cognate ARSs are omitted to create vacant codons, and (2) the addition of pre-charged npAA-tRNAs to encode the npAAs to the vacant codons. The shortcoming of this methodology is that some pAAs must be killed to make npAAs available.
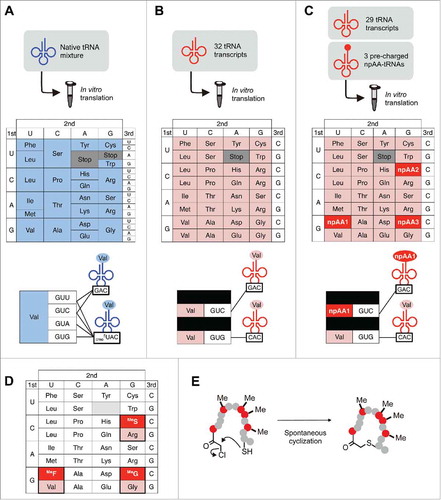
To overcome this limitation, Iwane et al. recently developed a method of “artificial division of codon boxes”,Citation28 which enables incorporation of more than 2 npAAs with all 20 pAAs retained in a reprogrammed genetic code (vide infra). Even though 61 codons are available for the polypeptide elongation in the native genetic code, only 20 pAAs are generally assigned to these codons. The connections between codons and amino acids are defined by fewer than 45 species of tRNAs in E. coli and other most organisms,Citation29 and therefore some tRNA species decode multiple cognate codons via ‘expanded’ wobble base pairing mechanisms.Citation30-Citation32 For example, the 4 codons constituting the valine codon box (GUN; N = U, C, A, or G) are decoded by 2 kinds of tRNAs possessing GAC and cmo5UAC (cmo5U: uridine 5-oxyacetic acid) anticodons, which are aminoacylated with Val by valyl-tRNA synthetase (). If we could manipulate the decoding system and reduce the redundancy, more npAAs could be used in addition to the 20 pAAs. To achieve this, an in vitro translation system was reconstituted with 32 in vitro transcribed tRNAs possessing SNN (S = G or C) anticodons. It has been demonstrated that these 32 tRNA transcripts can be charged with 20 pAAs by endogenous ARSs and orthogonally decode the corresponding 31 NNS elongation codons as well as the AUG initiation codon accurately ().Citation28 Among the 32 tRNAs, some redundant tRNAs can be replaced with pre-charged npAA-tRNAAsnE2s, which have a bioorthogonal body sequence and the same anticodons as the excluded tRNA transcripts. Accordingly, the corresponding codons can be reassigned to npAAs without the need to sacrifice any of the pAAs (). For example, when npAA-tRNAAsnE2 GAC and tRNAVal CAC are added, the corresponding GUC and GUG codons are translated to npAA and Val, respectively. In other words, the codon box is artificially divided and reprogrammed to accommodate 2 distinct amino acids. By this strategy, 3 codon boxes (valine GUN, arginine CGN, and glycine GGN) can be divided artificially, which expands the building block repertoire up to 23. Consequently, accurate ribosomal synthesis of 2 model peptides has been demonstrated28: (1) a 32-mer model peptide containing all the 20 pAAs and 3 npAAs (ϵ-N-acetyllysine, citrulline, and p-iodophenylalanine) and (2) a macrocyclic N-methyl-peptide “CM11–1” comprised of 14 amino acids (9 pAAs and 5 npAAs: N-chloroacetyl-d-tryptophan, N-methylserine, N-methylglycine, and 2 N-methylphenylalanines), which had been developed as an E6AP inhibitor (, ).Citation33 Notably, the reprogrammed codons were accurately decoded to the desired npAAs and the misincorporation of neither cognate or non-cognate pAAs occurred, which demonstrated the maintenance of translation accuracy. The proof-of-concept study on the artificial division of codon boxes opens a new opportunity for genetic code reprogramming,Citation34,Citation35 which, in principle, can create 11 vacant codons for npAAs with 20 pAAs retained.
Figure 3. Schematic representation of the artificial division of codon boxes. (A) The genetic code of a reconstituted translation system containing E. coli native tRNA mixture. The GUN codons in valine codon box are decoded by 2 kinds of native tRNAVals. (B) A reprogrammed genetic code where 32 in vitro transcribed tRNAs decodes 31 NNS (S = C or G) elongation codons along with the AUG initiation codon. (C) A reprogrammed genetic code containing 23 building blocks by means of artificial division of 3 codon boxes. The 3 nonproteinogenic amino acids (npAAs) are assigned to the black background codons by replacing the redundant tRNAVal GAC, tRNAArg GCG and tRNAGly GCC with the 3 bioorthogonal npAA-tRNAGNN's prepared by flexizyme-mediated aminoacylation.
Expansion of the codon table is a different approach to enable increase of the amino acid repertoire. The non-standard base method adds an artificial nucleotide pair in addition to the natural ones, namely A/U and G/C, in the mRNA codons and tRNA anticodons. The additional base pair made by artificial nucleotides increases the number of available codons from 64 ( = 43) to 216 ( = 63). As the codons consisting of the non-standard bases are intrinsically vacant, npAAs can be assigned without abolishing any pAAs. For instance, Benner's group developed the isoG-isoC pair as a new base pair to expand the codon table.Citation36 Unfortunately, the orthogonality of the non-standard pair to the natural bases is generally not sufficient,Citation37 and thus development of perfect artificial base pairs with full orthogonality in replication, transcription, and translation is anticipated.
Summary and outlook
To date, methods for synthesising npAA-tRNAs as well as genetic code manipulation techniques to assign the npAAs to specific codons have been established. Combination of those methods enables ribosomal synthesis of diverse polypeptides containing npAAs. However, the fidelity and efficiency of npAA incorporation are often insufficient to obtain enough quality and amount of desired translation products. In addition, the repertoire of amino acids that are available in translation is limited. In this review, methodologies to resolve these issues by engineering of tRNAs are introduced and discussed.
To maintain the fidelity, the ARS/tRNA pair used for npAA incorporation should be orthogonal to the other ARS/tRNA pairs existing in the translation system. Researchers in this field have made efforts to develop artificial ARS/tRNA pairs that have orthogonality. As the low efficiency of the desired npAA incorporation causes misincorporation of competitor amino acids, improvement of the efficiency is also important for maintenance of the fidelity. Here, we mentioned that the optimization of tRNA structure is effective for accelerating accommodation of npAA-tRNAs. However, the other steps in the ribosomal translation such as peptidyl transfer reaction and translocation should also be improved to obtain higher efficiency. For instance, Hecht's group introduced point mutations around the peptidyl transferase center of the ribosome to improve the incorporation of d- and β-amino acid. The E. coli ribosome with point mutations in 2 regions (2447–2450 and 2457–2462) of 23S rRNA showed higher efficiency in incorporation of d-Met and d-Phe into the DHFR protein.Citation38,Citation39 Similarly, they also developed mutant ribosomes with mutations in 2 regions (2057–2063 and 2496–2507) to improve β-amino acid incorporation.Citation40,Citation41
Peptidyl-tRNA drop-off from the ribosomal P site caused by mistranslocation would also be a possible reason for the low efficiency of npAA incorporation. As it has been previously reported that EF-G and several release factors are involved in such mistranslocation event,Citation42 development of a mutant EF-G with lower mistranslocation activity would be a possible approach for reducing undesired peptidyl-tRNA drop-off. Previously, it has been reported that lower EF-G concentration improves translation yield of peptides containing consecutive d-amino acids.Citation15 Development of new tRNAs that cause less drop-off would also be a good approach for this.
As the number of the amino acids available in natural translation system is limited to only 20, efforts to increase the repertoire of amino acids that can be simultaneously introduced into a peptide have been made. Here, we introduced the artificial division of codon boxes and the non-standard base method as examples of such approaches.
Although various npAAs are found in natural polypeptides, such npAAs are not directly introduced by ribosomal translation, but they are post-translationally converted from pAAs or de novo synthesized via non-ribosomal peptide synthesis independently of the genetic information on mRNA. The npAAs in the natural peptides include l-α-amino acids with non-natural side chains as well as d-amino acids, N-methyl amino acids and β-amino acids, and usually have biologically inportant roles in catalytic activity, peptidase resistance, structural rigidity, membrane permeability, and so on. Thus, peptides containing npAAs are now attracting much attention as a good drug-development platform. There is also an increasing demand for methods to ribosomally introduce such npAAs into peptides, as the ribosomal translation system can easily provide peptide libraries by randomizing the mRNA sequence, which can be applied for screening bioactive peptides with target binding affinities. Suga and coworkers developed an in-vitro selection method named RaPID (Random non-standard Peptides Integrated Discovery) system,Citation33,Citation43-Citation45 which is a combination of ribosomally synthesized peptide library having npAAs and the mRNA display method.Citation46,Citation47 For example, by using a macrocyclic peptide library containing N-methyl-Phe, Ser, Ala and Gly, inhibitors against the human oncoprotein E6AP were screened. The peptides exhibited high binding affinity with sub-nM KD values to E6AP, and inhibitory activity against the interaction between E6AP and the P53 tumor suppressor protein.
So far, the scope of amino acids available in ribosomal translation is still limited even with the aforementioned methods developed to date. For instance, negatively charged d-amino acids and N-methyl-amino acids, such as d-Asp and N-methyl-Asp, are still difficult to introduce. Consecutive incorporation of β-amino acids and even single incorporation of γ- and longer backbone amino acids have never been accomplished yet. Further engineering of the translation machinery is needed to overcome these limitations. Ribosomal synthesis of more diverse peptides with various npAAs will lead to construct of peptide libraries having wider chemical space, out of which peptide ligands with higher bioactivity against drug targets can be more efficiently screened.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Japan Science and Technology Agency (JST) PRESTO of Molecular Technology and Creation of New Functions (JPMJPR14K3); JST CREST Rising Star Award of Molecular Technology to T.K.; Grants-in-Aid for JSPS Fellows to Y.I. (26–9576); JST CREST of Molecular Technologies (JPMJCR12L2); the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (S) (26220204) to H.S.
References
- Robertson SA, Noren CJ, Anthony-Cahill SJ, Griffith MC, Schultz PG. The use of 5′-phospho-2 deoxyribocytidylylriboadenosine as a facile route to chemical aminoacylation of tRNA. Nucleic Acids Res 1989; 17:9649-60; PMID:2602139; https://doi.org/10.1093/nar/17.23.9649
- Murakami H, Ohta A, Ashigai H, Suga H. A highly flexible tRNA acylation method for non-natural polypeptide synthesis. Nat Methods 2006; 3:357-9; PMID:16628205; https://doi.org/10.1038/nmeth877
- Goto Y, Katoh T, Suga H. Flexizymes for genetic code reprogramming. Nat Protoc 2011; 6:779-90; PMID:21637198; https://doi.org/10.1038/nprot.2011.331
- Wang L, Brock A, Herberich B, Schultz PG. Expanding the genetic code of Escherichia coli. Science 2001; 292:498-500; PMID:11313494; https://doi.org/10.1126/science.1060077
- Ai HW. Biochemical analysis with the expanded genetic lexicon. Anal Bioanal Chem 2012; 403:2089-102; PMID:22322380; https://doi.org/10.1007/s00216-012-5784-2
- Chin JW, Cropp TA, Anderson JC, Mukherji M, Zhang Z, Schultz PG. An expanded eukaryotic genetic code. Science 2003; 301:964-7; PMID:12920298; https://doi.org/10.1126/science.1084772
- Liu CC, Schultz PG. Adding new chemistries to the genetic code. Annu Rev Biochem 2010; 79:413-44; PMID:20307192; https://doi.org/10.1146/annurev.biochem.052308.105824
- Noren C, Anthony-Cahill S, Griffith M, Schultz P. A general method for site-specific incorporation of unnatural amino acids into proteins. Science 1989; 244:182-8; PMID:2649980; https://doi.org/10.1126/science.2649980
- Johnson DB, Xu J, Shen Z, Takimoto JK, Schultz MD, Schmitz RJ, Xiang Z, Ecker JR, Briggs SP, Wang L. RF1 knockout allows ribosomal incorporation of unnatural amino acids at multiple sites. Nat Chem Biol 2011; 7:779-86; PMID:21926996; https://doi.org/10.1038/nchembio.657
- Hohsaka T, Ashizuka Y, Murakami H, Sisido M. Incorporation of nonnatural amino acids into streptavidin through in vitro frame-shift suppression. J Am Chem Soc 1996; 118:9778-79; https://doi.org/10.1021/ja9614225
- Tan Z, Forster A, Blacklow S, Cornish V. Amino acid backbone specificity of the Escherichia coli translation machinery. J Am Chem Soc 2004; 126:12752-3; PMID:15469251; https://doi.org/10.1021/ja0472174
- Fujino T, Goto Y, Suga H, Murakami H. Reevaluation of the D-amino acid compatibility with the elongation event in translation. J Am Chem Soc 2013; 135:1830-7; PMID:23301668; https://doi.org/10.1021/ja309570x
- Achenbach J, Jahnz M, Bethge L, Paal K, Jung M, Schuster M, Albrecht R, Jarosch F, Nierhaus KH, Klussmann S. Outwitting EF-Tu and the ribosome: translation with D-amino acids. Nucleic Acids Res 2015; 43:5687-98; PMID:26026160; https://doi.org/10.1093/nar/gkv566
- Fujino T, Goto Y, Suga H, Murakami H. Ribosomal synthesis of peptides with multiple β-amino acids. J Am Chem Soc 2016; 138:1962-9; PMID:26807980; https://doi.org/10.1021/jacs.5b12482
- Katoh T, Tajima K, Suga H. Consecutive Elongation of D-Amino Acids in Translation. Cell Chem Biol 2017; 24:46-54; PMID:28042044; https://doi.org/10.1016/j.chembiol.2016.11.012
- Xie J, Schultz PG. An expanding genetic code. Methods 2005; 36:227-38; PMID:16076448; https://doi.org/10.1016/j.ymeth.2005.04.010
- Park HS, Hohn MJ, Umehara T, Guo LT, Osborne EM, Benner J, Noren CJ, Rinehart J, Soll D. Expanding the genetic code of Escherichia coli with phosphoserine. Science 2011; 333:1151-4; PMID:21868676; https://doi.org/10.1126/science.1207203
- Ohta A, Murakami H, Higashimura E, Suga H. Synthesis of polyester by means of genetic code reprogramming. Chem Biol 2007; 14:1315-22; PMID:18096500; https://doi.org/10.1016/j.chembiol.2007.10.015
- Murakami H, Kourouklis D, Suga H. Using a solid-phase ribozyme aminoacylation system to reprogram the genetic code. Chem Biol 2003; 10:1077-84; PMID:14652075; https://doi.org/10.1016/j.chembiol.2003.10.010
- Cload ST, Liu DR, Froland WA, Schultz PG. Development of improved tRNAs for in vitro biosynthesis of proteins containing unnatural amino acids. Chem Biol 1996; 3:1033-8; PMID:9000011; https://doi.org/10.1016/S1074-5521(96)90169-6
- Kawakami T, Murakami H, Suga H. Ribosomal synthesis of polypeptoids and peptoid-peptide hybrids. J Am Chem Soc 2008; 130:16861-3; PMID:19053417; https://doi.org/10.1021/ja806998v
- Kawakami T, Ishizawa T, Murakami H. Extensive reprogramming of the genetic code for genetically encoded synthesis of highly N-alkylated polycyclic peptidomimetics. J Am Chem Soc 2013; 135:12297-304; PMID:23899321; https://doi.org/10.1021/ja405044k
- Terasaka N, Hayashi G, Katoh T, Suga H. An orthogonal ribosome-tRNA pair via engineering of the peptidyl transferase center. Nat Chem Biol 2014; 10:555-7; PMID:24907900; https://doi.org/10.1038/nchembio.1549
- Dale T, Sanderson LE, Uhlenbeck OC. The affinity of elongation factor Tu for an aminoacyl-tRNA is modulated by the esterified amino acid. Biochemistry 2004; 43:6159-66; PMID:15147200; https://doi.org/10.1021/bi036290o
- Dale T, Uhlenbeck OC. Amino acid specificity in translation. Trends Biochem Sci 2005; 30:659-65; PMID:16260144; https://doi.org/10.1016/j.tibs.2005.10.006
- Doi Y, Ohtsuki T, Shimizu Y, Ueda T, Sisido M. Elongation factor Tu mutants expand amino acid tolerance of protein biosynthesis system. J Am Chem Soc 2007; 129:14458-62; PMID:17958427; https://doi.org/10.1021/ja075557u
- Shimizu Y, Inoue A, Tomari Y, Suzuki T, Yokogawa T, Nishikawa K, Ueda T. Cell-free translation reconstituted with purified components. Nat Biotechnol 2001; 19:751-5; PMID:11479568; https://doi.org/10.1038/90802
- Iwane Y, Hitomi A, Murakami H, Katoh T, Goto Y, Suga H. Expanding the amino acid repertoire of ribosomal polypeptide synthesis via the artificial division of codon boxes. Nat Chem 2016; 8:317-25; PMID:27001726; https://doi.org/10.1038/nchem.2446
- Komine Y, Adachi T, Inokuchi H, Ozeki H. Genomic organization and physical mapping of the transfer RNA genes in Escherichia coli K12. J Mol Biol 1990; 212:579-98; PMID:PMID:2184240; https://doi.org/10.1016/0022-2836(90)90224-A
- Murphy FVt, Ramakrishnan V. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat Struct Mol Biol 2004; 11:1251-2; PMID:15558050; https://doi.org/10.1038/nsmb866
- Gustilo EM, Vendeix FA, Agris PF. tRNA's modifications bring order to gene expression. Curr Opin Microbiol 2008; 11:134-40; PMID:18378185; https://doi.org/10.1016/j.mib.2008.02.003
- Crick FH. Codon—anticodon pairing: the wobble hypothesis. J Mol Biol 1966; 19:548-55; PMID:5969078; https://doi.org/10.1016/S0022-2836(66)80022-0
- Yamagishi Y, Shoji I, Miyagawa S, Kawakami T, Katoh T, Goto Y, Suga H. Natural product-like macrocyclic N-methyl-peptide inhibitors against a ubiquitin ligase uncovered from a ribosome-expressed de novo library. Chem Biol 2011; 18:1562-70; PMID:22195558; https://doi.org/10.1016/j.chembiol.2011.09.013
- Forster AC, Tan Z, Nalam MN, Lin H, Qu H, Cornish VW, Blacklow SC. Programming peptidomimetic syntheses by translating genetic codes designed de novo. Proc Natl Acad Sci USA 2003; 100:6353-7; PMID:12754376; https://doi.org/10.1073/pnas.1132122100
- Josephson K, Hartman MC, Szostak JW. Ribosomal synthesis of unnatural peptides. J Am Chem Soc 2005; 127:11727-35; PMID:16104750; https://doi.org/10.1021/ja0515809
- Sismour AM, Benner SA. The use of thymidine analogs to improve the replication of an extra DNA base pair: a synthetic biological system. Nucleic Acids Res 2005; 33:5640-6; PMID:16192575; https://doi.org/10.1093/nar/gki873
- Switzer C, Moroney SE, Benner SA. Enzymatic incorporation of a new base pair into DNA and RNA. J Am Chem Soc 1989; 111:8322-23; https://doi.org/10.1021/ja00203a067
- Dedkova LM, Fahmi NE, Golovine SY, Hecht SM. Enhanced D-amino acid incorporation into protein by modified ribosomes. J Am Chem Soc 2003; 125:6616-17; PMID:12769555; https://doi.org/10.1021/ja035141q
- Dedkova LM, Fahmi NE, Golovine SY, Hecht SM. Construction of modified ribosomes for incorporation of D-amino acids into proteins. Biochemistry 2006; 45:15541-51; PMID:17176075; https://doi.org/10.1021/bi060986a
- Dedkova LM, Fahmi NE, Paul R, del Rosario M, Zhang L, Chen S, Feder G, Hecht SM. β-Puromycin selection of modified ribosomes for in vitro incorporation of β-amino acids. Biochemistry 2012; 51:401-15; PMID:22145951; https://doi.org/10.1021/bi2016124
- Maini R, Nguyen DT, Chen S, Dedkova LM, Chowdhury SR, Alcala-Torano R, Hecht SM. Incorporation of β-amino acids into dihydrofolate reductase by ribosomes having modifications in the peptidyltransferase center. Bioorg Med Chem 2013; 21:1088-96; PMID:23375097; https://doi.org/10.1016/j.bmc.2013.01.002
- Rao AR, Varshney U. Specific interaction between the ribosome recycling factor and the elongation factor G from Mycobacterium tuberculosis mediates peptidyl-tRNA release and ribosome recycling in Escherichia coli. EMBO J 2001; 20:2977-86; PMID:11387230; https://doi.org/10.1093/emboj/20.11.2977
- Hayashi Y, Morimoto J, Suga H. In vitro selection of anti-Akt2 thioether-macrocyclic peptides leading to isoform-selective inhibitors. ACS Chem Biol 2012; 7:607-13; PMID:22273180; https://doi.org/10.1021/cb200388k
- Morimoto J, Hayashi Y, Suga H. Discovery of macrocyclic peptides armed with a mechanism-based warhead: isoform-selective inhibition of human deacetylase SIRT2. Angew Chem Int Ed Engl 2012; 51:3423-7; PMID:PMID:22374802; https://doi.org/10.1002/anie.201108118
- Tanaka Y, et al. Structural basis for the drug extrusion mechanism by a MATE multidrug transporter. Nature 2013; 496:247-51; PMID:23535598; https://doi.org/10.1038/nature12014
- Nemoto N, Miyamoto-Sato E, Husimi Y, Yanagawa H. In vitro virus: bonding of mRNA bearing puromycin at the 3′-terminal end to the C-terminal end of its encoded protein on the ribosome in vitro. FEBS Lett 1997; 414:405-8; PMID:9315729; https://doi.org/10.1016/S0014-5793(97)01026-0
- Roberts RW, Szostak JW. RNA-peptide fusions for the in vitro selection of peptides and proteins. Proc Natl Acad Sci USA 1997; 94:12297-302; PMID:9356443
