ABSTRACT
Osteoblastic bone formation and osteoclastic bone resorption dynamically maintain the bone homeostasis; in the present study, we attempt to investigate the mechanism of the excessive activation of osteoclasts inducing the deregulation of bone homeostasis from the perspective of non-coding RNA regulation. Differentially expressed patterns of circRNAs were examined in non-treated and RANKL + CSF1-treated bone marrow monocyte/macrophage (BMM) cells and differentially-expressed miRNAs during osteoclast differentiation were analyzed and identified. We found that circRNA_28313 was significantly induced by RANKL + CSF1 treatment. circRNA_28313 knockdown significantly inhibited RANKL + CSF1-induced differentiation of osteoclasts within BMM cells in vitro, while suppressed ovariectomized (OVX)-induced bone resorption in mice in vivo. Via bioinformatics analyses, it has been demonstrated that miR-195a might bind to circRNA_28313 and CSF1 and together form a circRNA-miRNA-mRNA network. circRNA_28313 relieves miR-195a-mediated suppression on CSF1 via acting as a ceRNA, therefore modulating the osteoclast differentiation in BMM cells. In conclusion, circRNA_28313, miR-195a, and CSF1 form a ceRNA network to function in RANKL + CSF1-induced osteoclast differentiation, thus affecting OVX-induced bone absorption in mice.
Introduction
Osteoblastic bone formation and osteoclastic bone resorption dynamically maintain the bone homeostasis [Citation1]. The excessive activation of osteoclasts, the only cell type that can resorb bone, may result in osteolytic diseases, including osteoporosis [Citation2].
Osteoclasts are multinuclear cells of monocyte/macrophage hematopoietic progenitor cells [Citation3]. The critical cytokines, such as receptor activator of nuclear factor-κB ligand (RANKL) and macrophage colony-stimulating factor (CSF1), are required for differentiating progenitor cells into osteoclasts [Citation4,Citation5]. RANKL can be considered an essential cytokine in differentiation of osteoclasts, and CSF1 plays a critical role in osteoclast precursor survival and osteoclastogenesis [Citation6]. Moreover, in ovariectomized (OVX)-induced osteoporosis model, deletion of CSF1 attenuated the bone loss, suggesting that CSF1 play a key role in estrogen-deficiency bone loss [Citation7]. Monocyte osteoclasts begin to express specific markers, such as tartrate-resistant acid phosphatase (TRAP) [Citation8,Citation9], PU.1, a hematopoietic-specific member of the ets family which expresses during different phases in osteoclast differentiation [Citation10], nuclear factor of activated T cells c1 (NF-ATc1) [Citation8,Citation11], Cathepsin K (CTSK) [Citation12]. Mononuclear osteoclasts also have the function of bone resorption, but the most significant characteristic of mature osteoclasts is the multinucleation induced by cell-cell fusion. During the past decades, some progress has been achieved in the research on the function of non-coding RNAs, natural compounds, epigenetic regulations, and so on [Citation13–Citation15]. However, more is to be explored about the mechanism of osteoclast differentiation to develop an in-depth understanding of the pathogenesis of osteolytic diseases, including osteoporosis.
Circular RNAs (circRNAs), a family of non-coding RNAs, extensively exist within animal cells. circRNAs were previously regarded as nonfunctional byproducts, but their biogenesis and potential roles have been proposed recently [Citation16,Citation17]. Previous studies have demonstrated that there are some circRNAs with microRNA ‘sponges’ function, which can offset microRNA-mediated mRNA inhibition [Citation18–Citation20], therefore participating in epigenetic regulation, transcriptional and posttranscriptional regulation by different mechanisms. The expression patterns of circRNAs profiles in the process of osteoclast differentiation have been reported. More importantly, by analyzing the relationship between differentially expressed RNAs, we constructed the circRNA-miRNA co-expression networks, providing a systematic perspective upon the underlying roles of circRNA-miRNA axis in the process of osteoclastogenesis [Citation21,Citation22]. hsa_circ_0007873 enhanced, while hsa_circ_0010763 and hsa_circ_0015622 inhibited miR-103 within the co-regulatory network [Citation21]. miR-335-5p can downregulate DKK1 (an inhibitor of Wnt signaling), therefore not only enhancing Wnt signaling but also promoting the formation and development of osteoblasts [Citation23]. miR-29a regulates Wnt signaling pathway via a positive feedback loop, thus promoting the formation of osteoblasts [Citation24]. These data indicate that circRNA-miRNA synergistic regulation is essential for the formation of osteoclasts.
To explore potential molecular regulation of non-coding RNAs for osteoclast differentiation and subsequent osteolytic diseases, differentially expressed patterns of circRNAs were examined in non-treated and RANKL + CSF1-treated bone marrow monocyte/macrophage (BMM) cells. We performed real-time PCR to examine the expression of the top significantly differentially-expressed circRNAs and selected circRNA_28313. Next, we examined the roles of circRNA_28313 in BMM cell osteoclast differentiation in vitro and bone resorption in ovariectomized (OVX)-induced osteoporosis model in mice in vivo. As for the underlying mechanism, we downloaded and analyzed the microarray profiling of differentially-expressed miRNAs during osteoclast differentiation. After cross-check with miRNAs related to osteoclast differentiation (mmu04380), a target network between candidate miRNAs and their potential target circRNAs was constructed. Next, a ceRNA network consists of circRNA-miRNA-mRNA was constructed, and miR-195a was selected for further experiments. As confirmed by luciferase reporter and RIP assays, the predicted bindings between miR-195a and circRNA_28313 and CSF1 were validated. The final step was to examine the dynamic effects of circRNA_28313 and miR-195a upon CSF1. And then the osteoclast differentiation of BMM cells was examined. In summary, we identify differentially-expressed circRNAs during BMM cell osteoclast differentiation and provide a novel mechanism of circRNA-miRNA-mRNA network modulating BMM cell osteoclast differentiation and OVX-induced bone resorption in mice.
Results
Microarray profiling representing differentially-expressed circRNAs in differentiated and non-differentiated bone marrow macrophage (BMM) cells
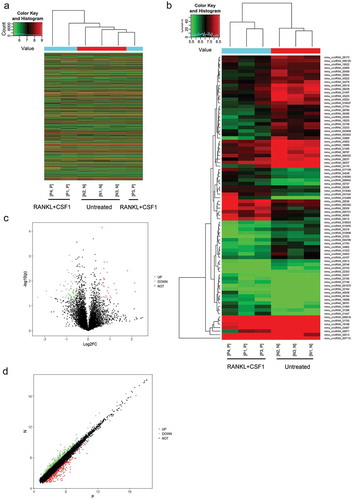
Before the microarray profiling analyses were performed, we treated BMM cells with 50ng/ml CSF1 + 100 ng/ml RANKL for osteoclast differentiation induction, as identified by TRAM staining. TRAP-positive cells with more than 3 nuclei could be considered as osteoclasts. Figure S1 shows that the red arrow indicates the appearance of TRAP+ multinucleated. Afterward, microarray analyses were performed on differentiated and non-differentiated BMM cells to identify differentially-expressed genes. As shown by the clustering, a total of 5449 circRNAs were upregulated, and 6259 were downregulated after induction (). As shown by the Hierarchical Clustering, the expression of 81 circRNAs in total could be remarkably different (Fold change >1.5 or < 0.67, P < 0.05), 29 of which were increased (Fold change > 1.5, P < 0.05) while 52 of which were reduced (Fold change < 0.67, P < 0.05) (). The category of these circRNAs is mainly exonic. We used Volcano Plots to visualize differential expression before and after differentiation of macrophages based on p-values and expression fold values. We constructed this plot with fold change values (log2FC) and p values for analyzing the relationship of fold change (variation magnitude) and statistical significance. The red plot indicates the significantly upregulated circRNAs, and the green plot indicates the significantly downregulated circRNAs (). We used a scatter plot to evaluate the difference in CircRNA expressions of two comparative samples or sample sets. The values of the X and Y axes in the scatter plot are the normalized signal values of the samples (log2 scaling) or the average normalized signal values of the sample sets (log2 scaling). The green plots at the upper part and red at the bottom represent the upregulated or downregulated circRNAs with a fold-change of > 1.5 ().
Figure 1. Microarray profiling representing differentially-expressed circRNAs in differentiated and non-differentiated bone marrow monocyte/macrophage (BMM) cells (a) Hierarchical clustering of gene expression in differentiated and non-differentiated BMM cells. (b) Hierarchical clustering of differentially-expressed circRNAs in differentiated and non-differentiated BMM cells. (c) Volcano plot diagram showing these differentially-expressed circRNAs. (d) Scatter diagram showing the expression correlation of these circRNAs.
Among differentially-expressed circRNAs, we select-ed circRNA_012460, circRNA_28313, circRNA_28312, circRNA_28309, circRNA_001034, circRNA_21447, circ-RNA_40206, and circRNA_28236, which obtained a fold-change > 3, for further validation. BMM cells were induced toward osteoclast differentiation and examined for the expression of the circRNAs mentioned above. As shown in Figure S2(a), the expression of circRNA_012460, circRNA_28313, circRNA_28312, circRNA_28309, circRNA_40206, and circRNA_28236 was significantly upregulated in the induction group, circRNA_28313 more upregulated. Thus, circRNA_28313 was selected for further experiments.
Before investigating the role of circRNA_28313 on BMM cell osteoclast differentiation, Through two analyses, we could exclude the possibility of trans-splicing/genome recombinations and demonstrate the existence of head-to-tail splicing. On the basis of osteoclast cDNA and genomic DNA, we designed convergent and divergent primers for the amplification of linear and circular RNA. Figure S2(b) shows that only the divergent primers within cDNA, rather than in gDNA can amplify circRNA_28313. Moreover, we pre-treated RNAs with RNase R, which demonstrated that cirRNAs presented resistance to RNase R, whereas a significant decrease in linear RNA was induced by RNase R treatment (RT) (Figure S2(c)).
In vitro effects of circRNA_28313 knockdown upon BMM cell osteoclast differentiation
To investigate the role of circRNA_28313 in BMM cell osteoclast differentiation, we first transfected Lsh1-circRNA_28313 or Lsh2-circRNA_28313 to achieve circRNA_28313 knockdown, and performed real-time PCR to verify the transfection efficiency (). After that, we treated transfected BMM cells with 30 ng/ml CSF1 and 100 ng/ml RANKL to conduct osteoclast differentiation, then performed TRAP staining to evaluate. As shown in , circRNA_28313 knockdown remarkably downregulated TRAP+ multinuclear cell number. Moreover, Lsh2-circRNA_28313 was selected for further experiments because of better transcription efficiency.
Figure 2. Effects of circRNA_28313 knockdown on the differentiation of BMM cells to osteoclasts in vitro (a) The knockdown of circRNA_28313 in BMM cells was achieved by transfection of Lsh1-circRNA_28313 or Lsh2-circRNA_28313, as confirmed by real-time PCR. (b) BMMs were then cultured in the presence of 30 ng/ml M-CSF and 100 ng/ml RANKL for 5 days, followed by TRAP staining to identify osteoclasts. The number of TRAP+ multinuclear osteoclasts was counted. (c) BMM were induced to osteoclast differentiation, transfected with Lsh-circRNA_28313, and stained for actin ring formation. Representative images are shown. Quantification of actin ring count using Image J software. (d-e) BMM cells were treated and transfected as above-described and examined for the protein levels of CSF1, PU.1, TRAP, NF-ATc1, and CTSK. the statistical analysis were shown in (e).
*P < 0.05, **P < 0.01, compared to Lsh NC or control group; #P < 0.05, ##P < 0.01, compared to Lsh NC group under RANKL +CSF1 treatment.
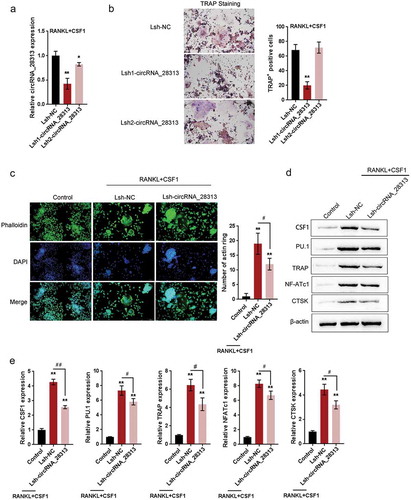
Next, we observed the formation of actin ring and actin-positive cells within actin ring-positive multinuclear cells and then stained them with TRITC-phalloidin (red) and DAPI (blue). The knockdown of circRNA_28313 significantly reduced actin ring formation (), thus playing a critical role in osteoclast differentiation and bone resorption. As a further confirmation, the protein levels of CSF1, PU.1, a hematopoietic-specific member of the Ets family that expresses during different phases in osteoclast differentiation [Citation10], and three osteoclast markers, including TRAP, NF-ATc1, and CTSK, were examined. As shown in –, CSF1 and RANKL stimulation induced dramatical increases in the protein levels of CSF1, PU.1, TRAP, NF-ATc1, and CTSK, while circRNA_28313 knockdown significantly reversed the inducible effect of M-CSF and RANKL on these factors, indicating that circRNA_28313 knockdown could suppress M-CSF + RANKL-induced differentiation of osteoclasts within BMM cells.
In vivo effects of circRNA_28313 knockdown on an osteoporosis mice model
We first evaluated evaluating the in vitro effect of circRNA_28313 upon osteoclasts, and then used an OVX mouse model to examine the in vivo effect of circRNA_28313 upon bone loss. The expression of circRNA_28313 was significantly upregulated in non-infected OVX mice and Lsh-NC-infected OVX mice compared to the sham group, while significantly downregulated in Lsh-circRNA_28313-infected mice compared to Lsh-NC-infected OVX mice ()).
Figure 3. Effects of circRNA_28313 knockdown on osteoporosis mice model in vivo (a) Ovariectomized (OVX) mouse model was established and injected with Lsh-NC or Lsh-circRNA_28313 and examined for the expression of circRNA_28313 by real-time PCR. (b) The body weight of mice in four groups was determined on 0, 2, 4, 6, 8 weeks. (c) Right tibias of all mice were harvested and subjected to a high-resolution Micro-CT scanning. Representative three-dimensional (3D) reconstructed images are shown. (d) Bone volume to tissue volume (BV/TV, %), trabecular thickness (Tb.Th, mm), trabecular number (Tb.N, 1/mm), and the trabecular separation (Tb.Sp, mm) of each sample were measured and analyzed.
*P < 0.05, **P < 0.01, compared to sham group; #P < 0.05, ##P < 0.01, compared to OVX + Lsh-NC group.
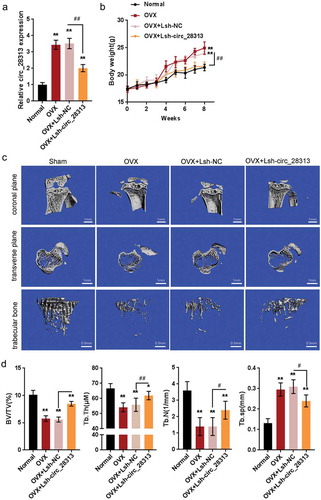
After ovariectomization, the body weight of mice was significantly increased in non-infected OVX mice and Lsh-NC-infected OVX mice than that in the sham group, while significantly decreased in Lsh-circRNA_28313-infected mice compared to Lsh-NC-infected OVX mice (). In –, Micro-CT with the 3D reconstruction of the tibia reveals significant bone loss within non-infected OVX mice and Lsh-NC-infected OVX mice. In Lsh-circRNA_28313-infected mice, more trabecular bone was observed. Furthermore, As shown in –, we examined BV/TV (%), Tb.Th (µM), Tb.N (1/mm), and Tb.Sp (mm) through the histomorphometric analyses. All four parameters showed significant increases in bone resorptive function in the vehicle group than that in the sham group. In addition, BV/TV, Tb.N, and Tb.Th presented the increases within the Lsh-circRNA_28313 infected group than in the Lsh-NC-infected OVX group, whereas Tb.Sp showed significant decreases within the Lsh-circRNA_28313 infected group than in the Lsh-NC-infected OVX group.
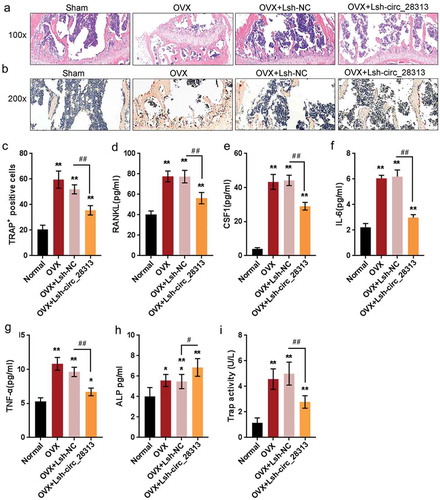
As revealed by HE and TRAP staining, circRNA_28313 knockdown had the ability to prevent bone loss. The number of mature osteoclasts (TRAP-positive osteoclast) could be increased within the non-infected or Lsh-NC-infected OVX group, resulting in remarkable bone loss and damaged trabecular microarchitecture (-). The Lsh-circRNA_28313 knockdown group showed bone mass maintenance and reduced quantities of mature osteoclasts than that in the Lsh-NC-transfected OVX group (). Since the factors in gut-derived serum participate in bone metabolism, it has also been determined by ELISA the serum levels of RANKL, CSF1, IL-6, and TNF-α. demonstrated that RANKL, CSF1, IL-6, and TNF-α serum concentrations could be significantly upregulated within non-infected or Lsh-NC-infected OVX group, while partially reduced in Lsh-circRNA_28313-infected OVX group. Similarly, the activities of ALP and TRAP were also significantly increased in non-infected or Lsh-NC-infected OVX group, while partially reduced in Lsh-circRNA_28313-infected OVX group ( and ). Consistent with these data, circRNA_28313 knockdown partly prevents OVX-induced bone loss.
Figure 4. Effects of circRNA_28313 knockdown on osteoporosis mice model in vivo (a-b) Decalcified bone tissue was paraffin-embedded and sectioned for H&E and TRAP staining. Representative microscopic images are shown at the indicated magnification. (c) The number of TRAP positive cells was measured. (d-g) Serum levels of RANKL, CSF1, IL-6, and TNF-α were determined by mouse serum ELISA kits. (H and I) The activities of ALP and TRAP in serum were determined by mouse serum ELISA kits.
*P < 0.05, **P < 0.01, compared to sham group; #P < 0.05, ##P < 0.01, compared to OVX + Lsh-NC group.
Selection of miRNAs related to osteoclast differentiation and circRNA_28313
As we have mentioned, circRNAs are associated with relevant miRNAs, while the axes of circRNA-miRNA participate in a series of disease pathways [Citation25]. To investigate the underlying mechanism of circRNA_28313 function in BMM osteoclast differentiation and OVX-induced bone loss, next, we analyzed online data to identify miRNAs related to BMM osteoclast differentiation and circRNA_28313. We downloaded and analyzed two online microarray profiling, GSE772478 and GSE53017, revealing differentially-expressed miRNAs during CSF1 + RANKL -induced osteoclast differentiation, and a total of 12 miRNAs were downregulated after CSF1 + RANKL induction. In the meantime, the MirPath V3 tool of DIANA tools, including microT-CDS v5.0, Tarbase v7.0, and Targetscan, were used to screen miRNAs related to osteoclast differentiation (mmu04380) and a total of 126 miRNAs were identified. After cross-checking, we selected 4 miRNAs, miR-181a, miR-338, miR-223, and miR-195a, for further validation (). Next, the lncTar online tool was used to construct a target network between these four miRNAs and their potential target circRNAs (); miR-338 and miR-195a were predicted to target circRNA_28313 (). Afterward, the DIANA tool was used to predict osteoclast differentiation genes that could be targeted by miR-195a and miR-338 and construct a ceRNA network consist of circRNA-miRNA-mRNA. However, in CSF1 + RANKL-treated BMM cells, miR-338 expression was significantly increased while miR-195a expression was decreased (). Thus, miR-195a was selected for further experiments. In Lsh-circRNA_28313-transfected BMM cells, miR-195a expression was significantly upregulated (). We transfected miR-195a mimics/inhibitor to achieve miR-195a expression, and performed real-time PCR to verify the transfection efficiency (); after transfection, CSF1 protein levels were remarkably increased via miR-195a inhibition whereas decreased via miR-195a overexpression (). These data suggest that circRNA_28313, miR-195a, and CSF1 may form a circRNA-miRNA-mRNA network to modulate BMM cell osteoclast differentiation.
Figure 5. Selection of miRNAs related to osteoclast differentiation and circRNA_28313 (a) A schematic diagram showing the miRNAs differentially-expressed during osteoclast differentiation and miRNAs related to osteoclast differentiation |mmu04380. After cross-check, 4 miRNAs were selected for further validation. (b) The lncTar online tool was used to predict the circRNAs targeting the 4 candidates 4 miRNAs, and the targeting network was constructed using Cytoscape. miR-338 and miR-195a were predicted to target circRNA_28313. (c) The DIANA tool was used to predict the osteoclast differentiation genes which could be targeted by miR-195a and miR-338, and a ceRNA network of circRNA-miRNA-mRNA was constructed. (d) BMM cells were treated with RANKL + CSF1 and examined for the expression of miR-195a and miR-338. (e) BMM cells were transfected with Lsh-NC or Lsh-circRNA_28313 and treated with RANKL + CSF1, and examined for the expression of miR-195a. (f) miR-195a expression was achieved in BMM cells by transfection of miR-195a mimics or miR-195a inhibitor, as confirmed by real-time PCR. (g) BMM cells were transfected with miR-195a mimics or miR-195a inhibitor examined for the protein levels of CSF1. **P < 0.01.
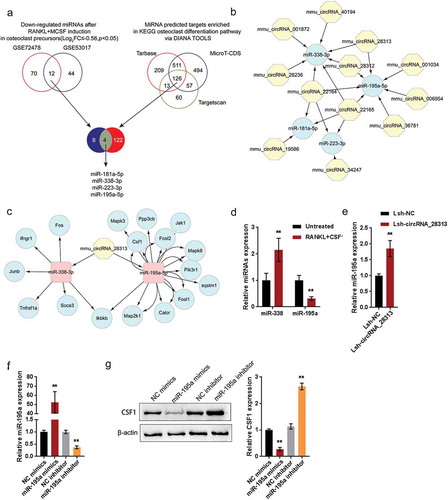
miR-195a directly targets circRNA_28313 and CSF1 3ʹUTR
To further confirm the existence of the circRNA-miRNA-mRNA network mentioned above, we performed luciferase reporter and RIP assays. We constructed two different types of luciferase reporter vectors, wild- and mutant-type, namely wt-/mut-circRNA_28313 and wt-/mut-CSF1 3ʹUTR. Within the putative miR-195a binding site of mutant-type vectors, 5 or 7 bases have been mutated (–). We co-transfected these vectors into HEK293 cells with miR-195a mimics or miR-195a inhibitor, then examined the luciferase activity. – showed that the luciferase activity of wild-type vectors could be significantly reduced via the overexpression of miR-195a while increased via the silence of miR-195a; mutating the putative miR-195a binding site could eliminate the alterations in the luciferase activity (–). As is known to all, miRNAs commonly not only induce the silence of gene expression via binding to the AGO2 protein, but also form the RNA-induced silencing complex (RISC). Under the conditions of the ceRNA mechanism, it could be a common phenomenon for AGO2 to simultaneously bind to circRNAs and miRNAs [Citation26–Citation28]. Furthermore, we confirmed the binding of circRNA_28313 to miR-195a via performing the RIP assays with AGO2 or IgG antibodies. Indeed, anti-AGO2 could significantly pull down endogenous circRNA_28313 and miR-195a (). These data indicate that miR-195a could directly target circRNA_28313 and CSF1 3ʹUTR. They could form a ceRNA network to modulate the osteoclast differentiation of BMM cells.
Figure 6. miR-195a directly targets circRNA_28313 and CSF1 3ʹUTR (a) A schematic diagram showing the predicted binding site between circRNA_28313 and miR-195a. Wild- and mutant-type circRNA_28313 luciferase reporter vectors were constructed and co-transfected with miR-195a mimics/inhibitor in HEK293 cells and the luciferase activity was determined. (b) A schematic diagram showing the predicted binding site between CSF1 3ʹUTR and miR-195a. Wild- and mutant-type CSF1 3ʹUTR luciferase reporter vectors were constructed and co-transfected with miR-195a mimics/inhibitor in HEK293 cells and the luciferase activity was determined. (c) The levels of circRNA_28313 and miR-195a precipitated by anti-AGO2 antibody were determined using RIP assays.
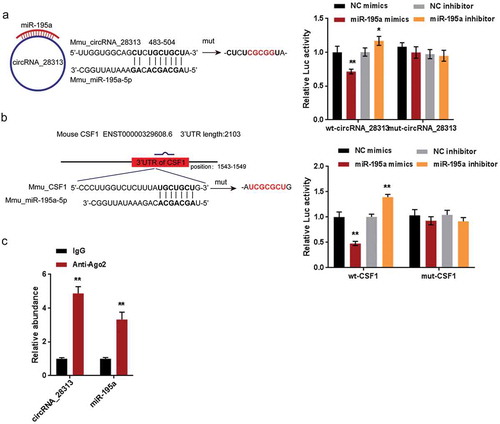
Dynamic effects of circRNA_28313 and miR-195a on BMM cell osteoclast differentiation
After confirming the ceRNA network, the dynamic impact of circRNA_28313 and miR-195a on BMM cell osteoclast differentiation were evaluated. We co-transfected BMM cells with Lsh-circRNA_28313/miR-195a inhibitor, then evaluated CSF1, PU.1, TRAP, NF-ATc1, and CTSK protein levels, TRAP+ multinuclear cell quantities, as well as actin-ring formation. As shown in ), the protein levels of CSF1, PU.1, TRAP, NF-ATc1, and CTSK (), the number of TRAP+ multinucleated cells (), and the actin-ring formation () were significantly suppressed by circRNA_28313 silence whereas promoted via the inhibition of miR-195a, by which the effects of circRNA_28313 silence were significantly reversed. These data indicate that circRNA_28313 relieves miR-195a-mediated suppression on CSF1 via acting as a ceRNA, therefore modulating BMM cell osteoclast differentiation.
Figure 7. Dynamic effects of circRNA_28313 and miR-195a on BMM cell osteoclast differentiation BMM cells were co-transfected with Lsh-circRNA_28313 and miR-195a inhibitor and examined for (a) the protein levels of CSF1, PU.1, TRAP, NF-ATc1, and CTSK by Immunoblotting, (b) TRAP+ multinucleated cells by TRAP staining, and (c) actin-ring formation.
Discussion
Herein, we performed microarray profiling analyses on RANKL + CSF1-treated and non-treated BMM cells to identify differentially-expressed circRNAs and found that circRNA_28313 was significantly induced by RANKL + CSF1 treatment. circRNA_28313 knockdown significantly inhibited RANKL + CSF1-induced differentiation of osteoclasts within BMM cells in vitro, while suppressed OVX-induced bone resorption in mice in vivo. Via bioinformatics analyses, it has been demonstrated that miR-195a might bind to circRNA_28313 and 3ʹUTR of CSF1 to form a circRNA-miRNA-mRNA network. circRNA_28313 relieves miR-195a-mediated suppression on CSF1 via acting as a ceRNA, therefore modulating the osteoclast differentiation in BMM cells.
CircRNAs, previously thought to be the product of abnormal gene splicing, have been regarded as non-functional transcriptional noise for a long time [Citation29,Citation30]. Nevertheless, there exist a significant amount of circRNAs within the human transcriptome. In addition, there is increasing evidence that circRNAs can exert essential effects on the regulation of cell functions and on many human diseases [Citation20,Citation31–Citation33]. It hasn’t been completely explained the role of circRNA within BMM cell osteoclast differentiation and subsequent bone resorption during osteoporosis and the underlying mechanism. Herein, a subset of circRNAs highly-expressed within RANKL + CSF1-treated BMM cells have been confirmed; among them, circRNA_28313 was dramatically upregulated upon RANKL + CSF1 stimulation. As confirmed by loss-of-function experiments, impaired circRNA_28313 expression impeded RANKL + CSF1-induced osteoclast formation in BMM cells via reducing TRAP+ multinucleated cell numbers, inhibiting the actin ring formation, and decreasing the protein levels of osteoclast markers, including CSF1, PU.1, TRAP, NF-ATc1, and CTSK. These data indicate that circRNA_28313 is functionally associated with the osteoclast differentiation in mice BMM cells.
Bone absorption is an essential function of osteoclasts in the body [Citation34,Citation35]. The maintenance of the healthy bone structure, morphology, and bone volume requires the dynamic balance between osteoblasts and osteoclasts. When osteoclast function exceeds compensation by osteoblasts, bone destruction and absorption follows [Citation36], resulting in osteolytic diseases, including osteoporosis [Citation2]. Interestingly, several researches have reported that preosteoclast showed totally different functions from mature osteoblasts. Preosteoclast barely resorb bone matrix but could promote angiogenesis [Citation13,Citation37]. Therefore, depletion of mature osteoclasts while preserving preosteoclasts will be a more effective treatment strategy in osteoporosis. In the present study, circRNA_28313 knockdown significantly blocks the osteoclast differentiation in BMM cells, we also evaluated its effects on OVX-induced bone absorption in mice. Consistent with its cellular function, circRNA_28313 knockdown improved the bone absorption in OVX-induced osteoporosis model in mice.
As we have mentioned, circRNAs could serve as sponges of miRNAs and form a ceRNA network with miRNA downstream mRNA to modulate several cellular processes [Citation25]. Similar as circRNAs, the dysregulation and function of miRNAs during osteoclast differentiation has also been reported [Citation38–Citation40]. According to GSE772478 and GSE53017, a total of 12 miRNAs were differentially expressed upon RANKL + CSF1 induction; among them, 4 miRNAs were related to osteoclast differentiation pathways. Moreover, the prediction of miRNA targets revealed that circRNA_28313 might target both miR-338 and miR-195a, suggesting that circRNA_28313 can serve as a sponge of miRNA. However, in BMM cells upon RANKL + CSF1 treatment, only the expression of miR-195a was significantly downregulated. Consistent with the online tools prediction, circRNA_28313 negatively regulates miR-195a and miR-195a negatively regulates CSF1. Therefore, miR-195a might be involved in circRNA_28313 function in osteoclast differentiation and bone absorption.
Reportedly, circRNAs could interact with miRNAs via direct targeting to exert its biological function. Li et al. reported that circular RNA CDR1as uses miR-7/GDF5/SMAD and p38 MAPK pathways to modulate osteoblast differentiation of periodontal ligament stem cells [Citation41]. Here, it has been proved that circRNA_28313 could form an interaction with and inhibit miR-195a via targeting. Moreover, miR-195a could inhibit the protein levels of CSF1 via targeting its 3ʹUTR of CSF1. Therefore, circRNA_28313, miR-195a, and CSF1 might form a ceRNA network to modulate the osteoclast differentiation. As expected, circRNA_28313 knockdown significantly suppressed RANKL + CSF1-induced BMM cell osteoclast differentiation, which could be remarkably enhanced by miR-195a inhibition via significantly increasing the protein levels of CSF1, PU.1, TRAP, NF-ATc1, and CTSK, increasing the number of TRAP+ multinucleated cells, and promoting the actin ring formation. More importantly, the inhibition of miR-195a might significantly reverse the impact of circRNA_28313 silence, indicating that circRNA_28313 could relieve miR-195a-mediated suppression on CSF1 via acting as a ceRNA, therefore modulating RANKL + CSF1-induced osteoclast differentiation.
In conclusion, circRNA_28313, miR-195a, and CSF1 form a ceRNA network to function in RANKL + CSF1-induced osteoclast differentiation, thus affecting OVX-induced bone absorption in mice. However, whether this ceRNA network affects preosteoclasts differentiation and function needs further investigation.
Materials and methods
Cell culture, bone marrow monocyte/macrophage (BMM) cells differentiation, and cell transfection
As previously described [Citation42], we isolated BMM cells from the femurs and tibias of C57BL/6 mice (4–6 weeks of age) and cultured in a complete α-MEM medium supplemented with CSF1 (30 ng/ml) and 10% fetal bovine serum. Twenty-four hours later, the non-adherent cells were discarded and the adherent cells were cultured at 37°C in 5% CO2.
For in vitro BMM cells differentiation, we cultured BMM cells (8 × 103 cells/well) in a 96-well plate in α-MEM medium supplemented with RANKL (50 ng/ml) and CSF1 (30 ng/ml). A series of doses of Pra-C (0, 5, 10, or 20 mM) was added to the culture medium, respectively. Cells were cultured for 8 days and the medium was changed every 2 days. Then the cells were fixed and stained using a Diagnostic Acid Phosphatase staining kit for TRAP analysis, photographed for the TRAP+ multinucleated cells (possessing more than 3 nuclei), and analyzed for the percentage of TRAP+ multinucleated cells following a method described previously [Citation43].
To knockdown circRNA_28313, shRNA1-circRNA_28313 or shRNA2-circRNA_28313 was cloned into pLVX-IRES-Puro vector and lentivirus was constructed by Genechem Co.ltd. (Shanghai, China). The transfection was conducted following a method previously described according to the manufacturer’s instructions [Citation44]. The expression of miR-195a was achieved by transfection of miRNA mimics or miRNA inhibitor (Genepharma, Shanghai, China) with the help of Lipofectamine 2000 (Invitrogen).
Microarray analyses on differentiated and un-differentiated BMM cells
Mouse circular RNA Array V2.0 (Cat.AS-SCR-M-2.0, Arraystar lnc, MD, USA) was performed on BMM-derived osteoclasts and un-differentiated BMM cells. To select the differentially expressed genes (mRNAs and lncRNAs), we used threshold values of |Log2FC| > 1 and a Benjamini–Hochberg corrected P value of 0.05. Next, the data were processed and analyzed following the methods described previously [Citation45] and applied for KEGG signaling annotation. The final visualization was performed using Cytoscape [Citation46].
Real-time PCR
BMMs were seeded in a 6-well plate at a density of 1 × 105 cells/well in complete α-MEM and induced for differentiation until mature osteoclasts formed. Total RNA from cells or tissue samples was isolated using TRIzol (Invitrogen, USA). cDNA was synthesized from 1 mg total RNA using reverse transcriptase (TaKaRa Biotechnology, Otsu, Japan). For circular RNAs expression measurement, total RNA was incubated with 5U/μg RNase-R for 20 min at 37°C to degrade the linear RNAs. The total RNA was purified using a RNeasy Mini kit (Qiagen, Valencia, CA, USA). Real-time PCR was then conducted with the ABI 7500 Sequencing Detection System (Applied Biosystems, Foster City, CA, USA) and the SYBR Premix Ex Taq kit (TaKaRa Biotechnology).
Actin ring formation analysis
The target cells were processed following the methods described before [Citation47], stained with DAPI (Sigma-Aldrich), and subsequently observed under a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Immunoblotting assays
Protein samples were separated by SDS-PAGE and transferred to Polyvinylidene difluoride (PVDF) membrane (Membrane Solutions, Shanghai, China). Membranes were incubated overnight at 4°C with the appropriate primary antibodies and then washed and incubated with HRP-conjugated secondary antibody. The primary antibodies were as follows: anti-CSF1 (ab9693, Abcam, Cambridge, MA, USA), anti-PU.1 (ab76543), anti-TRAP (ab2391, Abcam), anti-NF-ATc3 (ab93628, Abcam), anti-CTSK (ab19027), and anti-β-actin (ab8226, Abcam). Protein levels in each lane were normalized to the levels of β-actin.
Construction of an ovariectomized (OVX) mouse model
Following a method described before, we established an OVX model in mouse [Citation48] according to the guiding principles of the Animal Care Committee of Second Xiangya Hospital of Central South University to analyze the effects of selected circRNA and miRNA on the osteoporosis in vivo. A total of 32 C57BL/6 mice (8 weeks old, weighted 21.51 ± 0.87 g) were divided into four groups: non-OVX control mice, OVX mice not transfected, OVX mice tail vein injected with Lsh NC (negative control, 100 μl, 1*109 TU/ml), and OVX mice injected with (Lsh circRNAs_28313 100 μl, 1*109 TU/ml). Mice were bred under a standard condition and the ovaries of the mice were removed following methods previously described [Citation48–Citation50]. Four weeks later, the mice were euthanized and their tibias were harvested for further experiments.
Micro CT scanning
A high definition Micro-CT was performed using a mCT80 system (Scanco Medical, Switzerland) to analyze the fixed tibias. The scanning protocol was set at a 10 mm equidistant definition, 70 kV and 70 mA X-ray energy settings, and a voxel size of 10 mm in three-dimensional (3D) form. After reconstruction, a region of interest (i.e., 200 slices below the aspect 0.1 mm to the growth plates at the proximal tibia) was selected for further trabecular bone analysis. For the investigation of trabecular structure, we detected the volume of bone to tissue (BV/TV, %), trabecular number (Tb.N, 1/mm), trabecular separation (Tb.Sp, mm), and trabecular thickness (Tb.Th, mm) [Citation51].
Histological analyses by hematoxylin and eosin (H&E) and TRAP staining
For histological analyses, the tibia samples were decalcified in 10% EDTA with continuous shaking for 3 weeks and then embedded in paraffin. For H&E staining, the sections were stained with hematoxylin for 5 min and eosin for 2 min at 22–24°C and then visualized and photographed under a high-resolution microscope. TRAP staining was performed on sections following a previously described method [Citation43].
Serum collection and serum ELISA assay
Whole blood samples were collected from mice and centrifuged at 1000 × g for 15 min at room temperature within 2 h of collection. Then, the serum supernatant was aspirated, transferred to a new tube, aliquoted into Eppendorf tubes, and stored at – 80°C until analyzed. Mouse RANKL ELISA Kit (TNFSF11) (ab100749, abcam), mouse M-CSF ELISA Kit (ab155457, abcam), mouse IL-6 ELISA Kit (ab100712, abcam), mouse TNF-α ELISA Kit (ab208348, abcam), mouse ALP ELISA Kit and mouse TRAP ELISA Kit were used to measure RANKL, CSF1, IL-6, TNF-α, TRAP and ALPlevels or activities in the serum following the protocols.
RNA immunoprecipitation
The EZMagna RIP-Kit (Millipore Burlington, MA, USA) was used in accordance to the protocols. Target cells were lysed in a complete RNA immunoprecipitation (RIP) lysis buffer and the cell extract was incubated with magnetic beads conjugated with anti-Argonaute 2 (AGO2) or control anti-IgG antibody (Millipore) for 6 h at 4°C followed by an incubation with Proteinase K to remove the proteins. Purified RNA was analyzed using real-time PCR.
Luciferase reporter assays
The MSF1 3ʹUTR- or circRNAs_28313-binding sites of miRNA were predicted by microT-CDS v5.0, Tarbase v7.0, Targetscan. The different fragment sequences (wild-type and mutant-type) were synthesized and then inserted into the psiCHECK-2 vector (Promega, Fitchburg, WI, USA). Then a Dual-Luciferase Assay Kit (Promega) was used to detect the luciferase activity.
Statistical analysis
Data from at least three independent experiments are processed using SPSS17.0 (Armonk, NY, USA) and shown as mean ± S.D. The differences between two groups were compared using a Student t-test. The differences among more than two groups were compared using a one-way ANOVA. *P < 0.05; **P < 0.01.
Supplemental Material
Download Zip (1 MB)Acknowledgments
This study was supported by Key Research and Development Program of Hunan Province Science & Technology Department (Grant No. 2017WK2062).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
Supplementary data for this article can be accessed here
Additional information
Funding
References
- Alliston T, Derynck R. Medicine: interfering with bone remodelling. Nature. 2002;416:686–687.
- Tanaka S. RANKL-independent osteoclastogenesis: a long-standing controversy. J Bone Miner Res. 2017;32:431–433.
- Soysa NS, Alles N, Aoki K, et al. Osteoclast formation and differentiation: an overview. J Med Dent Sci. 2012;59:65–74.
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342.
- Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649.
- Takahashi N, Udagawa N, Tanaka S, et al. Generating murine osteoclasts from bone marrow. Methods Mol Med. 2003;80:129–144.
- Yao GQ, Troiano N, Simpson CA, et al. Selective deletion of the soluble colony-stimulating factor 1 isoform in vivo prevents estrogen-deficiency bone loss in mice. Bone Res. 2017;5:17022.
- Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602.
- Arai F, Miyamoto T, Ohneda O, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190:1741–1754.
- Scott EW, Simon MC, Anastasi J, et al. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577.
- Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176.
- Kiviranta R, Morko J, Alatalo SL, et al. Impaired bone resorption in cathepsin K-deficient mice is partially compensated for by enhanced osteoclastogenesis and increased expression of other proteases via an increased RANKL/OPG ratio. Bone. 2005;36:159–172.
- Dou C, Li J, Kang F, et al. Dual effect of cyanidin on RANKL-induced differentiation and fusion of osteoclasts. J Cell Physiol. 2016;231:558–567.
- Dou C, Zhang C, Kang F, et al. MiR-7b directly targets DC-STAMP causing suppression of NFATc1 and c-Fos signaling during osteoclast fusion and differentiation. Biochim Biophys Acta. 2014;1839:1084–1096.
- Yasui T, Hirose J, Aburatani H, et al. Epigenetic regulation of osteoclast differentiation. Ann N Y Acad Sci. 2011;1240:7–13.
- Ashwal-Fluss R, Meyer M, Pamudurti NR, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66.
- Conn SJ, Pillman KA, Toubia J, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134.
- Cortes-Lopez M, Miura P. Emerging functions of circular RNAs. Yale J Biol Med. 2016;89:527–537.
- Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388.
- Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338.
- Dou C, Cao Z, Yang B, et al. Changing expression profiles of lncRNAs, mRNAs, circRNAs and miRNAs during osteoclastogenesis. Sci Rep. 2016;6:21499.
- Jin D, Wu X, Yu H, et al. Systematic analysis of lncRNAs, mRNAs, circRNAs and miRNAs in patients with postmenopausal osteoporosis. Am J Transl Res. 2018;10:1498–1510.
- Zhang J, Tu Q, Bonewald LF, et al. Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J Bone Miner Res. 2011;26:1953–1963.
- Taipaleenmaki H, Bjerre Hokland L, Chen L, et al. Mechanisms in endocrinology: micro-RNAs: targets for enhancing osteoblast differentiation and bone formation. Eur J Endocrinol. 2012;166:359–371.
- Rong D, Sun H, Li Z, et al. An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget. 2017;8:73271–73281.
- Zheng Q, Bao C, Guo W, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215.
- Yu CY, Li TC, Wu YY, et al. The circular RNA circBIRC6 participates in the molecular circuitry controlling human pluripotency. Nat Commun. 2017;8:1149.
- Wang K, Gan TY, Li N, et al. Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ. 2017;24:1111–1120.
- Hsu MT, Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280:339–340.
- Cocquerelle C, Daubersies P, Majerus MA, et al. Splicing with inverted order of exons occurs proximal to large introns. Embo J. 1992;11:1095–1098.
- Yang W, Du WW, Li X, et al. Foxo3 activity promoted by non-coding effects of circular RNA and Foxo3 pseudogene in the inhibition of tumor growth and angiogenesis. Oncogene. 2016;35:3919–3931.
- Jeck WR, Sorrentino JA, Wang K, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. Rna. 2013;19:141–157.
- Li X, Zheng Y, Zheng Y, et al. Circular RNA CDR1as regulates osteoblastic differentiation of periodontal ligament stem cells via the miR-7/GDF5/SMAD and p38 MAPK signaling pathway. Stem Cell Res Ther. 2018;9:232.
- Q Guo, Z Cao, B Wu, et al. Modulating calcium-mediated NFATc1 and mitogen-activated protein kinase deactivation underlies the inhibitory effects of kavain on osteoclastogenesis and bone resorption. 2018;234:789–801.
- Ouyang Z, Zhai Z, Li H, et al. Hypericin suppresses osteoclast formation and wear particle-induced osteolysis via modulating ERK signalling pathway. Biochem Pharmacol. 2014;90:276–287.
- Zhang Q, Sugawara I. Immunology of tuberculosis. World J Exp Med. 2012;2:70–74.
- Ding N, Liu C, Yao L, et al. Alendronate induces osteoclast precursor apoptosis via peroxisomal dysfunction mediated ER stress. J Cell Physiol. 2018;233(9):7415–7423.
- Xia Z, Chen C, Chen P, et al. MicroRNAs and their roles in osteoclast differentiation. Front Med. 2011;5:414–419.
- Kagiya T, Nakamura S. Expression profiling of microRNAs in RAW264.7 cells treated with a combination of tumor necrosis factor alpha and RANKL during osteoclast differentiation. J Periodontal Res. 2013;48:373–385.
- de la Rica L, Garcia-Gomez A, Comet NR, et al. NF-kappaB-direct activation of microRNAs with repressive effects on monocyte-specific genes is critical for osteoclast differentiation. Genome Biol. 2015;16:2.
- Li X, Zheng Y, Zheng Y, et al. Circular RNA CDR1as regulates osteoblastic differentiation of periodontal ligament stem cells via the miR-7/GDF5/SMAD and p38 MAPK signaling pathway. Stem Cell Res Ther. 2018;9:232.
- Li H, Zhai Z, Liu G, et al. Sanguinarine inhibits osteoclast formation and bone resorption via suppressing RANKL-induced activation of NF-kappaB and ERK signaling pathways. Biochem Biophys Res Commun. 2013;430:951–956.
- Solberg LB, Brorson SH, Stordalen GA, et al. Increased tartrate-resistant acid phosphatase expression in osteoblasts and osteocytes in experimental osteoporosis in rats. Calcif Tissue Int. 2014;94:510–521.
- Cheng Y, Yang X, Deng X, et al. MicroRNA-218 inhibits bladder cancer cell proliferation, migration, and invasion by targeting BMI-1. Tumour Biol. 2015;36:8015–8023.
- Liu K, Yao H, Wen Y, et al. Functional role of a long non-coding RNA LIFR-AS1/miR-29a/TNFAIP3 axis in colorectal cancer resistance to pohotodynamic therapy. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2871–2880.
- Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504.
- Park KR, Kim EC, Hong JT, et al. Dysregulation of 5-hydroxytryptamine 6 receptor accelerates maturation of bone-resorbing osteoclasts and induces bone loss. Theranostics. 2018;8:3087–3098.
- Zhang Q, Tang X, Liu Z, et al. Hesperetin prevents bone resorption by inhibiting RANKL-induced osteoclastogenesis and Jnk mediated Irf-3/c-Jun activation. Front Pharmacol. 2018;9:1028.
- Moriwaki S, Suzuki K, Muramatsu M, et al. Delphinidin, one of the major anthocyanidins, prevents bone loss through the inhibition of excessive osteoclastogenesis in osteoporosis model mice. PLoS One. 2014;9:e97177.
- Wang X, He Y, Guo B, et al. In vivo screening for anti-osteoporotic fraction from extract of herbal formula Xianlinggubao in ovariectomized mice. PLoS One. 2015;10:e0118184.
- Peng S, Zhang G, Zhang BT, et al. The beneficial effect of icaritin on osteoporotic bone is dependent on the treatment initiation timing in adult ovariectomized rats. Bone. 2013;55:230–240.
