ABSTRACT
The innate immune system relies on a germ-line-encoded repertoire of pattern recognition receptors (PRRs), activated by deeply conserved pathogen signatures, such as bacterial cell wall components or foreign nucleic acids. To enable effective defence against invading pathogens and prevent from deleterious inflammation, PRR-driven immune responses are tightly controlled by a dense network of nuclear and cytoplasmic regulators. Long non-coding RNAs (lncRNAs) are increasingly recognized as important components of these regulatory circuitries, providing positive and negative control of PRR-induced innate immune responses. The present review provides an overview of the presently known roles of lncRNAs in human and murine innate antiviral and antibacterial immunity. The emerging roles in host defence and inflammation suggest that further mechanistic insights into the cellular functions of lncRNAs will decisively advance our molecular understanding of immune-associated diseases and open new avenues for therapeutic intervention.
1. Introduction
By protecting from malignant cell transformation, participating in tissue repair upon injury and defeating infectious agents, the immune system plays a central role in the maintenance of organismal homoeostasis. While the adaptive immune system confers long-lasting, but delayed protection against newly emerging threats, the innate immune system provides immediate responsiveness to conserved pathogen- and danger-associated molecular patterns (PAMPs and DAMPs)Citation[1,Citation2]. The resulting inflammatory response, however, can cause irreversible tissue damage and even organ failure when not properly controlled [Citation3]. Besides protein regulators, non-coding RNAs are increasingly recognized as important control-elements of the immune system enabling the adequate adjustment of the inflammatory response relative to the detected threat.
Regulatory non-coding RNAs are typically divided into two major groups. While microRNAs are approx. 20–24 nt long and typically act through miRISC-complex dependent recruitment of proteins decreasing target mRNA translation and stability, long non-coding RNAs (lncRNAs) are regulatory RNAs ≥ 200 nt and act through a plethora of different mechanisms and interactors [Citation4–6]. A role of microRNAs in the immune system was already revealed in the first decade of the 2000s, at a time when long non-coding RNAs were still hardly explored [Citation7]. Upon sensing of bacterial components by mammalian phagocytes, for instance, which play a central role in the innate immune system, microRNAs 146 and 155 were found to act as inducible feedback-regulators of the inflammatory response [Citation8–11]. Other microRNAs adopt feed-forward regulatory functions during innate immune activation of phagocytes [Citation12]. Similar regulatory implications in immune cells have recently been reported for lncRNAs. Between 2012 and 2017, the results of comprehensive transcriptome cataloguing approaches by the ENCODE and FANTOM consortia became publically available and revealed ~20.000 human lncRNA genes [Citation13,Citation14], most of which still remain functionally uncharacterized. Many basic questions, e.g. as to common modes of operation, subcellular localization patterns or the evolutionary conservation of lncRNAs, are still subject to intense debates. However, it is becoming increasingly clear that lncRNAs adopt important functions in a variety of cellular processes and in human diseases [Citation4,Citation15,Citation16]. The already available mechanistic insights into the functions of immune-associated lncRNAs suggest important roles in the adjustment of pro-inflammatory signal-transduction events and in the differentiation and polarization of immune cells. The present review attempts to comprehensively depict the known contributions of lncRNAs to innate immunity in humans and mice and discusses emerging principles of lncRNA-based control in PAMP-triggered inflammation.
2. LncRNA classification and functional heterogeneity
Compared to mRNAs, lncRNAs are typically expressed at lower levels and are less conserved, but often undergo similar maturation steps, including splicing, 5ʹ-capping and 3ʹ-polyadenylation [Citation13]. LncRNAs can be retained in in the nucleus or exported to the cytoplasm and consequently adopt a variety of different cellular functions, including transcriptional or translational regulation and control of protein and RNA stability [Citation4,Citation17]. In the literature, lncRNAs are often sub-classified according to their mode of operation or their site of transcription relative to coding genes. In this context, antisense lncRNAs, encoded on the reverse complement strand of an mRNA gene, intronic lncRNAs encoded within an intron of another gene, enhancer lncRNAs near or within an active enhancer element and intergenic long non-coding RNAs have been discriminated [Citation5]. Intergenic lncRNAs (lincRNAs) form the largest of these sub-groups [Citation13]. Mechanistically, lncRNAs may act in cis or in trans through interactions with nucleic acids or proteins. Their interactions with DNA, RNA or protein partners can fulfil decoying, guiding or scaffolding functions [Citation4,Citation5]. LncRNA PACER for instance acts as a decoy, binding to the p50 subunit of the NFκB transcription factor to prevent formation of repressive p50 homodimer complexes [Citation18]. XIST and HOTAIR are classical examples of lncRNAs guiding chromatin-modifiers, such as the Polycomb Repressive Complex 2 (PRC2) to chromatin target sites, thereby regulating dosage compensation and developmental programmes, respectively [Citation19,Citation20]. In addition to its guiding function, HOTAIR also functions as a scaffold, bringing PRC2 and the LSD1-CoREST demethylase into close proximity during gene silencing [Citation21]. Further lncRNAs bind several interaction partners at a time through different modular domains [Citation5,Citation21]. Besides the recruitment of chromatin-modifying enzymes, such interactions e.g. contribute to the assembly of multi-protein complexes during protein-ubiquitination or liquid-liquid phase-separation [Citation5,Citation17,Citation22–24].
Despite the continuous improvement of mammalian gene catalogues, it remains debated how many of the annotated lncRNA genes are truly non-coding or functional. While the latter can only be determined individually through careful mutagenesis and biochemical studies, the former has recently been addressed by high-throughput analysis of ribosome-occupation. ~20-40% of lncRNAs turned out to associate and co-sediment with ribosomes in pull-down, ribosome-profiling and density gradient experiments [Citation25–29]. Several of the lncRNAs in question were furthermore found to encode functional peptides, including in immune cells [Citation25,Citation26]. Coding potential analysis therefore remains a critical step during the experimental dissection of cellular lncRNA-mechanisms. Besides exclusion of protein-coding potential, the mechanistic investigation of lncRNAs typically involves the identification of lncRNA binding partners through affinity purification approaches, the determination of the subcellular localization by fractionation or in situ hybridization and loss-of-function studies using in vitro or in vivo models [Citation17,Citation30]. In the context of the innate immune system, the already reported molecular functions and interactors of lncRNAs illustrate a high degree of heterogeneity, ranging from control of signalling protein activity to mRNA stabilization and regulation of chromatin accessibility [Citation27,Citation31,Citation32]. In the following sections, we first introduce the key principles of innate immune recognition, followed by the illustration of known functions of lncRNAs in professional phagocytes and in immune-activated tissue-anchored cell-types.
3. Key components and pathways of the innate immune system
Innate recognition of PAMPs and DAMPs is achieved through pattern recognition receptors (PRRs) spanning the plasma-membrane or locating to endosomes or the cytosol [Citation1]. Different PRR classes can be discriminated based on their protein domain-structure, including the Toll-like receptors (TLRs), the Rig-I like receptors (RLRs), the NOD-like receptors (NLRs) and the C-type lectin receptors (CLRs) [Citation1]. The pathogen structures sensed by the individual members of the different PRR classes range from bacterial cell wall components, such as lipopolysaccharides (TLR4), bacterial lipoproteins (TLR2) and peptidoglycans (NLRs), to bacterial and viral nucleic acids, such as dsRNA (e.g. TLR3, Rig-I, MDA5), CpG DNA (TLR9) or cytosolic DNA (cGAS) [Citation33,Citation34]. Different PRRs can activate distinct signalling cascades, culminating in the activation of master-transcription factors of the immune response, such as NFκB or the IRF protein family [Citation1,Citation33] (). While NFκB activates the majority of the acute pro-inflammatory genes, the IRF transcription factors selectively trigger the expression of interferons. The latter counteract intracellular infections by STAT transcription factor dependent activation of interferon-stimulated genes (ISGs), the products of which for instance interfere with viral transcription and translation pathways [Citation1,Citation33,Citation35,Citation36].
Figure 1. Roles of lncRNAs in PRR-triggered phagocyte immunity. LncRNAs have been implicated in PRR-MyD88-NFκB, PRR-TRIF-IRF3 and PRR-MAVS-IRF3 signalling (TLR4 and Rig-I pathway shown exemplarily), as well as in JAK/STAT-dependent ISG expression programmes triggered upon type I IFN release and auto- or paracrine IFNAR stimulation. Nuclear and cytoplasmic lncRNAs acting as positive and negative regulators of these pathways in murine (green) and human (blue) phagocytes are indicated
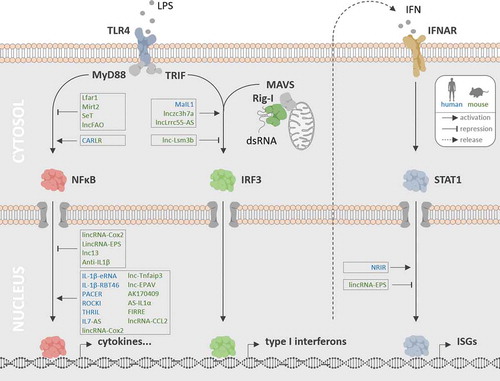
3.1. Pro-inflammatory gene regulation via NFκB
The lipopolysaccharide (LPS) sensor TLR4 is a prototypic plasma membrane spanning PRR and the only TLR capable of simultaneous activation of the intracellular MyD88 and TRIF signalling pathways [Citation1,Citation33] (). TLR4-signalling through the intracellular adaptor MyD88 triggers a complex ubiquitin-ligase (e.g. TRAF6) and kinase (e.g. IKK) dependent cascade culminating in the phosphorylation and degradation of the cytosolic NFκB-adaptor IκBα. Degradation of IκBα leads to NFκB nuclear translocation and pro-inflammatory gene expression [Citation33]. A MAPK-dependent branch downstream of MyD88-TRAF6 furthermore activates the AP1 complex, a transcriptional co-activator of NFκB target genes [Citation33]. Unlike the TLR4-MyD88 pathway, TLR4-signalling through the intracellular adapter TRIF is induced upon endocytosis of activated TLR4 [Citation1]. TRIF-activation triggers a pathway which culminates in the activation of the IRF3 transcription factor (see below), but also of TRAF6, thereby fostering NFκB activation [Citation33]. Different from TLR4, the remaining TLRs exclusively signal either through MyD88 or through TRIF. MyD88-dependent human TLRs and their agonists are TLR2 in conjunction with TLR1 or 6 (lipoproteins), TLR5 (flagellin), TLR7 and 8 (ssRNA) and TLR9 (CpG DNA). TLR3 signals through TRIF and senses dsRNA [Citation33]. Other PRRs activate NFκB independent of MyD88 or TRIF. The NLR family members and cytosolic peptidoglycan receptors NOD1 and NOD2, for instance, signal through the adaptor protein RIP2 to trigger IκBα degradation and MAPK signalling dependent NFκB and AP1 activation [Citation33]. Irrespective of the upstream pathway, nuclear translocation of NFκB leads to the transcription of a cell-type specific set of immune genes. This includes inflammation mediators, such as IL1β and TNFα, which in turn feedback-activate NFκB by autocrine cytokine receptor stimulation and signalling through the intracellular MyD88 and RIPK1 adapters, respectively [Citation3,Citation37,Citation38]. NFκB induces the expression of numerous other soluble immune-mediators. Examples are IL8, which recruits immune cells from the periphery, COX2, which elicits diverse systemic adaptations, including fever and elevated pain sensitivity or IL6, which triggers acute phase protein production by hepatocytes and regulates B- and T-cell differentiation [Citation38–40].
3.2. Interferon induction through IRF activation
In addition to the MyD88-NFκB dependent pathway, TLR4 can induce the expression of type I interferons (IFNs) through the TRIF-dependent pathway [Citation1] (). TLR4 interaction with TRIF triggers TRAF3 dependent activation of the TBK1 kinase and phosphorylation of IRF3. This is followed by IRF3 dimerization, translocation to the nucleus and IRF3-dependent activation of type I IFN genes [Citation1]. Unlike TLR4, dsRNA sensor TLR3 exclusively signals through TRIF and thus preferentially activates type I IFN transcription [Citation1]. Different from TLR4 and TLR3, the RLR family members and dsRNA sensors Rig-I and MDA5 upon ligand binding activate type I IFN expression through a TRIF-independent pathway. In this case, ligand-induced receptor oligomerization is followed by binding to the adaptor protein MAVS at mitochondrial surfaces (), which triggers TRAF3-TBK1-IRF3 signal transduction and type I IFN expression [Citation1]. Upon secretion, type I IFNs IFNβ and IFNα bind to the common receptor IFNAR on target cells, to induce pro-apoptotic programmes in infected cells or an antiviral and antimicrobial state within bystander cells through Janus kinase/signal transducers and activators of transcription (JAK/STAT) signalling [Citation41].
3.3. Interferon-dependent JAK/STAT pathway activation
JAKs are a family of tyrosine kinases, which, except for Jak3, are ubiquitously expressed in mammalian cells and interact with the intracellular domains of diverse receptors, including the type I IFN-receptor IFNAR [Citation35,Citation42]. Upon IFNα or β binding to IFNAR, the dimeric receptor undergoes a conformational switch, which brings JAKs associated with each receptor monomer into special proximity, leading to JAK transphosphorylation and JAK-dependent STAT transcription-factor phosphorylation [Citation43]. Activated STAT dimers translocate from the cytosol to the nucleus and promote expression of a variety of target genes [Citation43] (). Similar to type I IFNs, the type II IFN IFNγ, which is produced primarily by T- and NK cells, activates a JAK-STAT dependent signalling cascade upon binding to its receptor IFNGR [Citation44]. IFNAR and IFNGR activation typically triggers the phosphorylation and nuclear translocation of STAT1, which activates transcription of IFN stimulated genes (ISGs), such as IFIT1, OAS or ADAR1 [Citation45]. JAK-STAT activation also amplifies expression of primarily NFκB-dependent inflammatory cytokines, such as IL6 and of class I and II HLAs, thus contributing to increased antigen presentation [Citation41,Citation44].
3.4. Division of labour in the innate immune system
Phagocytes, such as resident and recruited macrophages, dendritic cells or circulating monocytes play a central role in the orchestration of PAMP-triggered innate immune responses. They express a wide range of PRRs and are usually among the first cells to respond to an infection and transmit immune signals to the periphery. Phagocytes represent a major source of various soluble mediators such as IL6 and type I IFN [Citation41,Citation46]. Moreover, phagocytes are in constant exchange with surrounding cells, such as epithelial or endothelial cells, to produce and receive substances and signals that contribute to innate defence. Type I IFN, for example, stimulates an antiviral state in bystander cells surrounding infected phagocytes [Citation41]. Via TNFα, phagocytes furthermore stimulate pro-inflammatory activation of epithelial and endothelial cells at the alveolar barrier [Citation47]. Additionally, NK cells are recruited by phagocyte signals and can in turn boost the antimicrobial activity and antigen presentation capacity of phagocytes through IFNγ release [Citation44]. Maintenance of the tissue phagocyte niche in turn involves recruitment- and differentiation-signals produced by endothelial cells and epithelial cells [Citation48]. LncRNAs seem to vitally participate in the immune crosstalk of endothelial and epithelial cells, NK cells and phagocytes [Citation49]. Thus, a comprehensive understanding of lncRNA functions in innate immunity and inflammation requires their mechanistic investigation not only in professional immune cells but also in cell types acting in the periphery of phagocytes during immune activation.
4. LncRNAs in phagocyte-driven innate immunity
Blood-derived (BDM) and bone-marrow-derived (BMDM) macrophages represent important phagocyte models, often used in basic immunology research. PRR-stimulation, e.g. with TLR4-agonist LPS, alters the expression of dozens of lncRNAs in these cells [Citation27,Citation50]. The functions of most of these lncRNAs presently remain unknown. Of note, attempts to systematically narrow-down phagocyte lncRNA functions suggest that in human and murine macrophages, ~23 and ~35% of the annotated lncRNAs associate with ribosomes, respectively [Citation26,Citation27]. Thus, investigation of the coding potential should represent a key step in the mechanistic dissection of the cellular functions of these lncRNAs. In human macrophages, lncRNAs absent from ribosomes are present both in the nucleus and cytoplasm and co-sediment with diverse macromolecular RNA-protein machineries on density gradients [Citation27]. This fosters the concept that phagocyte lncRNAs, unlike other classes of non-coding RNA, exert their functions through a multitude of different regulatory RNA-protein interactions. In line with this notion, the already reported implications of lncRNAs in phagocyte immunity involve a variety of different nuclear and cytoplasmic mechanisms (), as outlined below. Of note, only few immune-associated phagocyte lncRNAs seem to be conserved at the primary sequence level between humans and mice [Citation50]. We therefore separately depict lncRNAs involved in human and murine phagocyte immunity, respectively, in the following text.
Table 1. lncRNAs in mammalian phagocyte immunity
4.1. Control of interferon-dependent phagocyte immunity
The magnitude and kinetics of type I IFN expression is tightly regulated, to ensure adequate activation of ISG-dependent cellular defence against intracellular viruses and bacteria [Citation41,Citation45]. Recently, several lncRNAs were implicated in type I IFN production and ISG activation in professional cells of the innate immune system. In human macrophages, activation of the TLR4-MyD88-NFκB pathway by LPS triggers a rapid increase in MaIL1 lncRNA expression. MaIL1 constitutes an inducible component of the TLR4-TRIF signalling arm, which activates type I IFN production. Within the TLR4-TRIF pathway, MaIL1 forms a complex with the OPTN protein in the cytoplasm to promote ubiquitin-dependent aggregation of the OPTN signalling platform, which promotes TBK1 kinase-dependent IRF3 transcription factor phosphorylation [Citation27]. Thus, up-regulation of lncRNA MaIL1 in human macrophages serves the MyD88-dependent positive control of TRIF-TBK1-IRF3-induced type I IFN expression. Suggesting an in vivo relevance in type I IFN immunity, MaIL1 levels linearly correlate with IFNβ levels in bronchoalveolar lavage from pneumoniae patients and loss of MaIL1 impedes type I IFN dependent cell-autonomous antibacterial defence in macrophages [Citation27]. Like MaIL1, lncRNA NRIR is among the 10 most highly induced lncRNAs in LPS-activated human macrophages [Citation27]. In monocytes, TLR4-dependent NRIR induction was reported to promote ISG (i.e. CXCL10) expression through a yet unknown mechanism. Expression of NRIR was found to be elevated in monocytes from systemic sclerosis patients and positively correlated with the patients ISG expression score [Citation51]. Thus, both MaIL1 and NRIR function to leverage IFN-associated immune responses in TLR4-activated primary human phagocytes ().
In murine macrophages, lncRNA lnczc3h7a is induced in response to viral infection and acts as a molecular scaffold, bringing TRIM25 ubiquitin ligase and Rig-I into spacial proximity. This triggers TRIM25-dependent Rig-I ubiquitination and downstream IRF3-dependent type I IFN production [Citation52]. Thus, reminiscent of the function of MaIL1 in human cells, lnczc3h7a regulates ubiquitin-dependent progression of PRR-signalling in the cytoplasm to promote type I IFN production by phagocytes. The response of murine macrophages to IFN stimulation is subsequently fine-tuned by additional lncRNAs, induced by the IFN-JAK-STAT signalling axis (). lncLrrc55-AS for instance, upon STAT-dependent activation, further promotes type I IFN production by supporting demethylation and inactivation of the IRF3 inhibitor PP2A by the PME1 protein [Citation53]. Another murine lncRNA activated by type I IFN is lnc-Lsm3b, which binds to Rig-I monomers, this time however as a negative regulator, interfering with Rig-I signalling to limit further IFN production [Citation54]. LncRNAs can also adopt broader regulatory functions that span both the IFN-dependent and the NFκB-dependent gene expression networks in murine phagocytes. LincRNA-EPS for instance is a suppressor of cytokine, chemokine and ISG expression. Downregulation of lincRNA-EPS upon TLR-MyD88-NFκB pathway activation thus lifts repression of immune gene activation. Mechanistically, lincRNA-EPS alters nucleosome positioning at target gene promoters through an interaction with the hnRNPL protein, to foster a repressive chromatin state [Citation32]. LncRNA Lfar1 is also downregulated upon murine macrophage activation, however only upon IFNγ and not upon LPS stimulation, suggesting a JAK-STAT dependent regulation. Similar to lincRNA-EPS, Lfar1 functions as a negative regulator of the inflammatory response and its downregulation upon IFNγ stimulation relieves suppression of NFκB-dependent genes and NLRP3 inflammasome mediated pyroptosis [Citation55].
Taken together, lncRNAs in human and murine phagocytes both positively and negatively regulate IFN-production and IFN-receptor triggered immune responses through a variety of mechanisms, ranging from control of protein ubiquitination, stability and activity in the cytoplasm to chromatin remodelling in the nucleus ().
4.2. Positive regulation of TLR-NFκB driven phagocyte immunity
Large parts of the pro-inflammatory innate defence programmes rely on TLR-triggered NFκB and AP1 transcription-factor activation [Citation1]. Similar to the IFN-associated immune circuitries, lncRNAs have been implicated in both positive and negative control of TLR-NFκB signalling events in human phagocytes, to confer productive immune-activation levels. In human monocytes for instance, the lncRNAs IL-1β-eRNA and IL-1β-RBT46, originating from the IL1β gene locus, are induced following TLR4-MyD88-NFκB activation by bacterial LPS and promote expression of the pro-inflammatory cytokine and MyD88-signalling amplifier IL1β [Citation56]. Another TLR4-inducible nuclear lncRNA is PACER, which decoys NFκB p50 to suppress p50 homodimer formation and promote NFκB p50/p65 heterodimer formation and thus NFκB-dependent immune gene expression in human macrophages [Citation18]. Similarly, lncRNA CARLR is induced in human macrophages upon LPS-treatment and promotes pro-inflammatory gene expression by binding to NFκB, this time, however, to the p65 subunit in the cytoplasm [Citation57]. Other lncRNAs involved in positive control of TLR-MyD88 dependent gene expression in human macrophages are ROCKi and IL7-AS [Citation58,Citation59]. IL7-AS is one of the few lncRNAs implicated in both human and murine phagocyte immunity [Citation50]. Upon TLR-MAPK/NFκB activation, it associates with p300 and SWI/SNF and contributes to histone acetylation and promoter remodelling to foster the expression of soluble immune mediators such as CCL2 and IL6 [Citation59].
Like in human cells, several lncRNAs were found to confer positive control of TLR-NFκB responses in murine macrophages. Besides IL7-AS and CARLR, which are involved both in human and murine phagocyte immunity, lincRNA-Tnfaip3 is induced in a TLR4-NFκB dependent manner and promotes expression of various soluble mediators, such as IL6. Mechanistically, lincRNA-Tnfaip3 associates with chromatin regulator HMGB1 to form a lincRNA/HMGB1/NFκB complex and promote NFκB binding at target gene loci [Citation60]. Similar to CARLR and lincRNA-Tnfaip3, another TLR-inducible lncRNA, lnc-EPAV, directly controls NFκB activity in murine macrophages. Lnc-EPAV is encoded within an endogenous retrovirus locus and induced upon stimulation with viral dsRNA mimic poly(I:C). Lnc-EPAV supports the production of immune mediators, such as IL6, TNFα or type I IFN by decoying the inhibitory SFPQ protein from the p65 promoter, thereby leveraging NFκB p65 dependent gene expression [Citation61]. Using an NFκB-GFP reporter system, further lncRNAs were found to regulate NFκB-dependent gene expression in murine macrophages [Citation62]. While nuclear TLR-induced lncRNA AK170409 was found to foster NFκB activity below the IκBα step, the partially cytosolic lncRNA lincRNA-Cox2 was found to promote IκBα degradation, which causes NFκB-nuclear translocation and pro-inflammatory gene expression [Citation62]. LincRNA-Cox2 had previously been shown to be highly induced upon TLR-MyD88 pathway activation, to negatively regulate TLR-induced ISG CCL5 expression, independent of type I IFN. Mechanistically, linc-Cox2 binds to hnRNPA/B and A2/B1 in the nucleus and inhibits RNA polymerase II recruitment to the CCL5 promoter. In the same study, lincRNA-Cox2 was also shown to promote TLR-dependent expression of a subset of pro-inflammatory genes [Citation63]. In another follow-up study, positive control of NFκB-dependent gene expression by lincRNA-Cox2 was reported to involve SWI/SNF dependent chromatin remodelling at target gene promoters [Citation64]. Together, these reports suggest that lincRNA-Cox2 both positively and negatively regulates NFκB-dependent gene expression in murine macrophages through different, cytoplasmic and nuclear mechanisms. Another lncRNA acting at the chromatin level is AS-IL1α, which is induced upon TLR-activation with LPS, Pam3CSK4 or poly(I:C) and recruits RNA polymerase II to the IL1α promoter [Citation65]. Expression of lncRNA FIRRE is also increased upon macrophage activation with LPS and promotes hnRNPU dependent stabilization of immune-associated mRNAs, including IL1β, IL12b and VCAM1 [Citation66]. In vivo, mice overexpressing FIRRE displayed increased pro-inflammatory cytokine levels (TNFα, IL12-p40, MIP-2) when compared to WT mice [Citation67]. Surprisingly, in FIRRE knockout mice cytokine levels remained unaffected, questioning the physiological relevance of FIRRE in this context [Citation67]. Haematopoietic phenotypes of ΔFIRRE mice, however, argue for an in vivo role of this lncRNA in leukocyte driven immunity [Citation67]. Another murine lncRNA reported to promote activation of the macrophage inflammatory response is lncRNA-CCL2 [Citation68]. The mechanism, through which LPS-inducible lncRNA-CCL2 supports expression of inflammation markers such as IL1β, TNFα or IL6, however, remains to be determined. Expression of this lncRNA is counter regulated by SIRT1, a protein downregulated upon LPS stimulation and under septic shock conditions [Citation68].
Besides PRR-inducible lncRNAs, several lncRNAs are downregulated upon TLR-activation of murine macrophages. As described above (4.1), downregulation of lincRNA-EPS upon TLR-MyD88-NFκB activation lifts repression of inflammatory gene activation [Citation32]. In line with this observation, macrophages from lincRNA-EPS deficient mice, infected with L. monocytogenes display increased expression of pro-inflammatory genes and nitric oxid production and are less susceptible to Listeria monocytogenes infection [Citation69]. Another lncRNA downregulated in a TLR-MyD88-NFκB dependent manner is lnc13. Downregulation of lnc13 relieves suppression of a subset of NFκB-dependent genes. Thereby, lnc13 contributes to murine macrophage immune activation very similar to lincRNA-EPS. Mechanistically, lnc13 regulates its target genes by fostering hnRNPD and HDAC1 induced repressive chromatin states [Citation70]. Of note, decreased lnc13 levels were observed in intestinal biopsy samples from coeliac disease patients [Citation70], suggesting a role of this lncRNA in human inflammatory diseases as well.
In summary, the reports available so far suggests that lncRNAs vitally contribute to human and murine phagocyte activation, as induced positive-regulators and supressed negative regulators of PRR-triggered pro-inflammatory gene expression programmes ().
4.3. Negative regulation of TLR-NFκB driven phagocyte immunity
Besides supporting the activation of TLR-NFκB dependent gene expression, lncRNAs are also involved in processes restraining the activity of this signalling axis. In a human macrophage cell line model, lncRNA THRIL, for instance, was reported to be downregulated upon Pam3CSK4 or TNFα stimulation. It was suggested that a THRIL/hnRNPL ribonucleoprotein complex positively regulates TNFα expression at the promoter level [Citation71]. In this case, THRIL downregulation would counter-regulate TNFα production. Independent expression profiling studies, however, failed to confirm the presence of this lncRNA in primary human phagocytes [Citation27,Citation50].
In murine LPS-treated macrophages, lncRNA Mirt2 was found to be induced both via the TLR-MyD88 and the TLR-TRIF signalling pathway. Mirt2 was shown to negatively regulate TLR-triggered pro-inflammatory gene expression by interacting with the TLR-MyD88 pathway component TRAF6 and negatively regulating TRAF6 auto-ubiquitination and oligomerization in the cytoplasm [Citation72]. This observation puts Mirt2 on par with other cytoplasmic lncRNAs such as MaIL1 or lnczc3h7a, which regulate the immune response via ubiquitination of PRR-signalling components. Another TLR4-induced negative regulator, besides Mirt2, is Anti-IL1β, which inhibits IL1β production, probably through deposition of promoter H3K4 tri-methylation marks [Citation73]. LncRNA SeT is also induced in murine macrophages upon LPS stimulation and supresses biallelic TNFα expression [Citation74]. In SeT deficient mice, increased steady-state TNFα levels and biallelic expression seem to result in increased mortality upon LPS-injection [Citation75], suggesting a protective role of SeT in endotoxemia. LncRNA lncFAO is another negative regulator of murine macrophage immune responses, which governs mitochondrial energy balance – a process that is tightly linked to macrophage inflammatory activity [Citation76]. LncFAO is induced late upon LPS-stimulation to counter-regulate expression of pro-inflammatory mediators such as IL1β and contribute to inflammation resolution. Mechanistically, lncFAO associates with the fatty acid oxidation promoting enzyme HADHB at mitochondria and stabilizes HADHB protein levels. LncFAO binding promotes mitochondrial HADHB activity and fatty acid oxidation, which in turn decreases macrophage pro-inflammatory activity [Citation77].
Thus, lncRNAs confer both positive and negative control of TLR-NFκB driven immunity in human and murine phagocytes through control of mitochondrial metabolism, protein and mRNA stability, and chromatin modification and remodelling ().
4.4. LncRNAs in phagocyte differentiation and polarization
Many lncRNAs display highly tissue- and cell-type specific expression patterns, which suggests their implication in cell lineage specification and differentiation [Citation13,Citation78]. In the immune system, besides their well-established roles in PRR-triggered immunity, lncRNAs indeed contribute to haematopoiesis and peripheral blood cell differentiation. Myeloid-specific human transcript HOTAIRM1, for instance, is up-regulated in a PU.1 transcription factor dependent manner during granulocytic differentiation and promotes expression of the myeloid differentiation markers CD11b and CD18 [Citation79,Citation80]. Both proteins together form the myeloid-specific integrin αMβ2, which regulates myeloid cell adhesion and induction of which is also PU.1-dependent [Citation81]. Another lncRNA involved in myeloid cell differentiation is Morrbid. This lncRNA is up-regulated in murine macrophages upon LPS-treatment and inhibits macrophage apoptosis. Mechanistically, Morrbid negatively regulates expression of the pro-apoptotic factor Bim in cis, by binding to PRC2 component EZH2 and promoting PRC2 binding to the Bim promoter and deposition of repressive H3K27me3 chromatin marks [Citation82]. In Morrbid-deficient mice, eosinophil, neutrophil, and monocyte counts were reduced, suggesting a general role of this lncRNA in the control of myeloid cell life-span. Morrbid expression was found to be elevated in eosinophils of hyper-eosinophilic syndrome patients, possible prolonging eosinophil life-span [Citation82]. Another lncRNA involved in the granulocytic lineages is EGO. This lncRNA is generated from an intronic region within the ITPR1 gene and is highly expressed in mature eosinophils. RNA silencing revealed EGO to promote the expression of eosinophil markers such as MBP and EDN [Citation83]. A function in the differentiation of dendritic cells from human monocytes was reported for lnc-DC. This lncRNA binds to STAT3, a transcription factor required for dendritic cell differentiation, and promotes STAT3 phosphorylation by preventing its interaction with and dephosphorylation by SHP1 [Citation84]. Lnc-DC additionally increases IL12 production by dendritic cells upon activation with LPS [Citation84]. Furthermore, NEAT1 was found to be required for proper LPS-dependent maturation of dendritic cells [Citation85].
Besides their roles in differentiation and longevity, lncRNAs were also reported to affect the polarization state of phagocytes. Unlike classically activated macrophages, M2-like macrophages are generated in response to Th2 cell cytokines (see section 7) and adopt rather anti-inflammatory and tissue repair functions [Citation86]. Upon human macrophage differentiation with M-CSF and Th2 cytokines IL4 and IL13, expression levels of lncRNA lnc-M2 were found to be increased compared to non-activated, M-CSF-differentiated M0 macrophages. Lnc-M2 is induced by Th2 cytokines in a STAT3-dependent manner and forms a complex with PKA to promote PKA phosphorylation and PKA/CREB pathway-dependent M2 polarization [Citation87]. In mice, the lncRNA AK085865 was reported to support M2 polarization. AK085865 deficient animals display fewer M2 macrophages, reduced eosinophil counts and attenuated allergic airway inflammation in an asthmatic mouse model [Citation88].
Taken together, besides their emerging roles in PRR-driven phagocyte immunity, lncRNAs adopt important functions in human and murine myeloid cell differentiation and polarization, thereby contributing to pathogen eradication and tissue repair, but also to inflammation-associated disorders when falsely regulated.
5. LncRNAs in innate immune responses beyond the phagocyte system
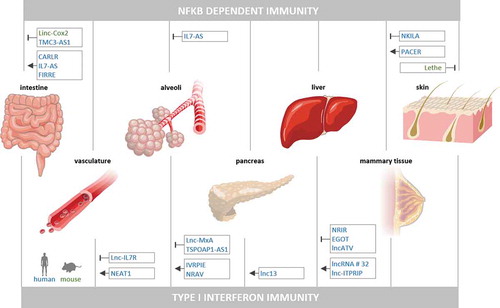
Professional immune cells, such as monocytes or macrophages are key to the adequate activation and subsequent resolution of innate inflammatory responses to pathogen- and danger-signals. These cells however do not act autonomously but rather receive a multitude of signals from the periphery, including their surrounding tissue environment [Citation47,Citation48]. To capture the complex intercellular communication events within human tissue under infection conditions, we recently developed an engineered model of the human intestinal barrier, comprising an epithelial and an endothelial compartment equipped with peripheral blood leukocytes. RNA-seq analysis of epithelial cells, endothelial cells, monocytes and natural killer cells isolated from epithelially infected (Salmonella enterica Typhimurium) or control-treated tissue models, revealed dozens of up- and downregulated lncRNAs in all cell types during innate immune crosstalk at the intestinal barrier [Citation49]. While the majority of these lncRNAs currently remains uncharacterized, several studies have already provided insights into the roles of individual lncRNAs in tissue-anchored cell types, such as endothelial cells, epithelial cells or fibroblasts, during immune activation and inflammation (, ).
Table 2. lncRNAs in mammalian immune responses beyond the phagocyte system
Figure 2. LncRNAs in NFκB- and type I IFN-activating PRR responses outside the phagocyte system. Murine (green) and human (blue) lncRNAs, reported to positively or negatively control activation of NFκB- or type I IFN-driven innate immune responses in intestinal, vascular, alveolar, pancreatic, hepatic, mammary or fibroblast cells are shown
5.1. Positive control of interferon-immunity in epithelial, endothelial and β-cells
While phagocytes represent a predominant source of type I IFN during acute immune responses, cells of the endo- and epithelia may produce IFNs as well. In addition, the endo- and epithelia represent important targets of type I IFN induced metabolic adaptations and desensitization against viral and intracellular bacterial infections [Citation35,Citation41,Citation89]. IFN production by endothelial cells during viral infection is under positive control by the nuclear lncRNA NEAT1. Upon infection, a ribonucleoprotein particle, containing NEAT1 and the Hexim1 protein as core components, undergoes extensive remodelling, leading to release of paraspeckle proteins, such as SFPQ, and recruitment of STING [Citation90]. The latter functions as a cyclic dinucleotide sensor and protein component of antiviral IFN-induction pathways [Citation1,Citation34,Citation91]. Consequently, NEAT1-Hexim1 complex remodelling upon infection results in STING-dependent IRF3 phosphorylation and type I IFN expression [Citation90]. During Hantavirus infection of endothelial cells NEAT1 was found to be upregulated via the PRR Rig-I to promote type I IFN expression by displacing SFPQ from the Rig-I promoter [Citation92]. Thus, dynamic nuclear NEAT1-SFPQ complexes are involved in different IFN-induction pathways in cells of the blood vessel linings during viral infection. In lung epithelial cells, human lncRNA IVRPIE is upregulated upon Influenza A virus infection and poly(I:C) stimulation to promote IFNβ and ISG expression. Mechanistically, IVRPIE fosters the deposition of activatory H3K4 tri-methylation marks at target gene promoters, potentially through an interaction with hnRNPU [Citation93]. Also in lung epithelial cells, lncRNA NRAV is down-regulated upon IAV infection through a yet to be determined pathway. NRAV down-regulation promotes expression of several ISGs, including the antiviral protein MxA. Mechanistically, NRAV interacts with the protein ZONAB, which functions as a positive regulator of MxA expression. Thus, NRAV down-regulation likely serves to withdraw a repressive interaction of the lncRNA with ZONAB and promote antiviral defence [Citation94]. Of note, viruses such as IAV may also utilize lncRNAs has host-factors, promoting replication in lung epithelial cells, as exemplified by lncRNA-ACOD1 [Citation95,Citation96]. A closer investigation of the endogenous roles of such lncRNAs, e.g. in resolution of antiviral responses, could provide further insights into the implications of lncRNAs in lung epithelial immunity. In human hepatocytes, lncRNA ITPRIP was reported to be upregulated upon type I IFN stimulation and HCV infection to promote oligomerization of the PRR MDA5, thereby enhancing type I IFN production [Citation97]. Thus, several lncRNAs function as positive regulators of type I IFN production in the cytoplasm and nucleus of human endothelial and epithelial cells during viral infections.
Upon their release, type I IFNs bind to the ubiquitous surface receptor IFNAR to trigger JAK-STAT signalling and ISG expression [Citation35,Citation45]. In human pancreatic β cells, lnc13 is transferred from the nucleus to the cytoplasm upon stimulation with a viral dsRNA mimic. Lnc13 subsequently stabilizes STAT1 mRNA in the cytoplasm by promoting its interaction with the PCBP2 RNA-binding protein [Citation31]. Thus, different from its reported function in phagocytes (4.2), in β cells, lnc13 does not act in the nucleus as a repressor but rather as a cytoplasmic positive-regulator of the STAT-pathway, suggesting cell-type specific roles of this lncRNA.
5.2. Negative control of interferon-immunity in epithelial cells
Uncontrolled IFN immunity can have detrimental effects, by, for example, contributing to the development of autoimmune diseases and cellular exhaustion [Citation35,Citation41]. Besides the contribution of lncRNAs to the activation of type I IFN immunity, several lncRNAs therefore act as negative regulators of type I IFN production and signalling within and beyond the leukocyte system. In human lung epithelial cells, lncRNA TSPOAP1‐AS1 for instance is induced following poly(I:C) stimulation in an NFκB-dependent manner. TSPOAP1‐AS1 is largely retained in the nucleus and counteracts Influenza A virus-induced IFNβ expression [Citation98]. Similarly, human lnc-MxA is upregulated upon Influenza A infection and poly(I:C) or IFNβ stimulation of lung epithelial cells to restrain IFNβ expression. This involves lnc-MxA association with the IFNβ promoter and interference with IRF3 and p65 transcription factor binding [Citation99]. Like in lung epithelial cells, lncRNAs contribute to negative control of type I IFN production and signalling in human hepatocytes. LncATV negatively regulates type I IFN and ISG expression by associating with Rig-I and preventing downstream signal transduction [Citation100]. Rig-I induced nuclear lncRNA EGOT restrains ISG expression through an as yet unknown mechanism. Knockdown of EGOT decreases HCV replication and expression of EGOT was found to be increased in liver biopsies from HCV patients [Citation101]. NRIR is induced upon IFNα stimulation of hepatocytes and negatively regulates ISG expression, again through an unknown mechanism [Citation102]. This regulation mode differs from its reported role in phagocytes, thus similar to lnc13, NRIR might adopt cell-type specific functions. Different from LncATV, EGOT and NRIR, lncRNA # 32 is downregulated upon poly(I:C) or type I IFN stimulation in human hepatocytes. LncRNA # 32 promotes ISG expression through interaction with the ATF2 transcription factor. Thus, the downregulation of lncRNA # 32 counteracts type I IFN induced ISG expression [Citation103]
Taken together, type I IFN production and downstream signalling is promoted and limited by a complex network of lncRNAs during immune-activation of cells of the blood vessels, lung, liver and pancreas (). Further lncRNAs acting as regulators of type I IFN immunity in other organ systems are to be expected.
5.3. Positive control of TLR-NFκB driven immunity in epithelial and endothelial cells
Besides IFN immunity, classic NFκB-driven pro-inflammatory gene expression needs to be tightly controlled, not only in cells of the immune system, to prevent from ineffective immune responses but also from vascular damage or disruption of the epithelial barriers [Citation104,Citation105]. Several lncRNAs seem to assist in the activation of NFκB-induced gene expression in epithelial cells. PACER for instance is not only active in phagocytes (4.2) but also in human mammary epithelial cells and promotes expression of pro-inflammatory mediator COX2 [Citation18]. In human intestinal epithelial cells, CARLR is induced upon LPS-treatment. In human intestinal biopsy cells from coeliac disease compared to control patients CARLR is furthermore transferred from the nucleus to the cytoplasm. Its NFκB p65 binding activity and role as a positive regulator of inflammatory gene expression in macrophages predicts that CARLR adopts a similar function in the intestinal epithelium [Citation57]. Again in human intestinal epithelial cells, lncRNA FIRRE, which is also active in macrophages (4.2), is induced upon TLR4-NFκB activation and stabilizes immune-associated mRNAs (IL1b, IL12b, VCAM1) through interaction with hnRNPU [Citation66]. Furthermore, expression of IL7-AS is elevated upon TLR-MAPK/NFκB pathway activation in intestinal epithelial cells, to promote expression of a set of cytokines (but not IFNs) through the association with p300 and SWI/SNF dependent histone acetylation and promoter remodelling complexes [Citation59]. In lung epithelial cells, IL7-AS is induced upon stimulation with the pro-inflammatory, NFκB activating cytokine IL1β and promotes IL6 expression, similar to its role described in intestinal epithelial cells [Citation50]. In HeLa cells, NEAT1 was found to be induced upon TLR3-p38 pathway activation to dislocate SFPQ from the IL8 promoter and tether it into paraspeckles, thereby promoting IL8 expression [Citation106]. This function is reminiscent of the displacement of SFPQ from the Rig-I promoter by NEAT1 in endothelial cells [Citation92], (5.1). Also in HeLa cells, NEAT1, is stabilized upon Salmonella infection through diminished nuclear RNA decay and promotes paraspeckle formation and expression of immune-associated genes such as TNFSF9 or CCL2, thereby contributing to antibacterial defence [Citation107]. Thus, positive control of the TLR-NFκB axis in human mammary, intestinal and cervical epithelial cell models is provided by several lncRNAs directly acting on NFκB, promoting permissive chromatin states or stabilizing mRNAs ().
5.4. Negative control of TLR-NFκB driven immunity in epithelial cells, endothelial cells and fibroblasts
Failure to restrain NFκB activity may not only provoke inflammation-associated tissue damage [Citation104,Citation105] but also promote oncogenesis [Citation108]. In a breast cancer cell line model, human cytoplasmic lncRNA NKILA is induced by LPS and acts as a negative feedback regulator, interacting with NFκB and blocking IκB phosphorylation. NKILA levels are decreased in breast cancer patients, which predicts a role of NKILA in NFκB activation in this disease [Citation109]. Similarly, in human intestinal epithelial cells, lncRNA TMC3-AS is induced by LPS and interacts with NFκB p65 to prevent from p65 binding to the IL10 promoter [Citation110]. In human endothelial cells, lnc-IL7R is upregulated upon LPS-treatment and negatively regulates inflammatory gene expression (e.g. IL6, VCAM-1 or E-selectin) by promoting H3K27 tri-methylation at gene promoters [Citation111]. Thus, several human lncRNAs prevent from exaggerated NFκB activation in human epithelial and endothelial cells.
Among the few lncRNAs implicated in immune-modulation in murine cells outside the phagocyte system, Lethe was described as a negative regulator induced upon TNFα stimulation of mouse embryonic fibroblasts. Lethe functions as a negative feedback regulator, restraining NFκB activity by interacting with NFκB p65 and inhibiting p65 binding to DNA [Citation112]. Also upon TNFα stimulation, linc-Cox2 is upregulated in murine intestinal epithelial cells to suppress IL12b induction through Mi2/NuRD repressor complex recruitment to the IL12b promoter [Citation113].
Taken together, similar to their functions in professional immune cells, lncRNAs are engaged in positive and negative control circuitries of epithelial cells, endothelial cells and fibroblasts (), promoting and restraining inflammatory gene expression through a variety of nuclear and cytoplasmic regulatory mechanisms.
6. Emerging patterns of lncRNA-mediated control in the innate immune system
The above-depicted functions of lncRNAs in the immune system suggest recurring higher-level regulatory principles through which the inflammatory process is modulated and adapted to external signals. Irrespective of their subcellular localization and molecular interactors, regulatory RNAs can be sub-grouped into feedback and feed-forward regulators. Feedback-regulators help to stabilize a given signalling pathway at an optimal level through either negative or positive feedback [Citation114]. Feed-forward regulators on the other hand may cause a bi-phasic or ultrasensitive response to an immune stimulus [Citation114]. LncRNA Mirt2, for instance, acts as a negative feedback regulator, which inhibits PRR-signal transduction upon its TLR-dependent induction, by interfering with TRAF6 activation in the cytoplasm. PACER on the other hand provides positive feedback, upon NFκB-dependent activation, by promoting NFκB p50/p65 heterodimer formation. Several other lncRNAs introduced above, similarly act as negative or positive feedback regulators, helping to stabilize PRR, cytokine and IFN signalling networks (). Besides lncRNAs providing direct feedback to their upstream pathways, feed-forward control seems to be a major principle of lncRNA-mediated regulation of inflammatory responses. The majority of innate-immune associated lncRNAs described today seems to be engaged in coherent feed-forward control, where a positive regulator is induced or a repressor is withdrawn, to amplify the response to a given stimulus, not necessarily involving a direct feedback function (). Examples are MaIL1 and linc-Cox2, but also lnc13 and lincRNA-EPS (). Finally, incoherent feed-forward control, e.g. involving the induction of a repressor of a given pathway, is another prominent regulation mode, through which for instance linc-Cox2, IL7-AS or SeT impact the innate immune response ().
Figure 3. LncRNA-based feedback and feed-forward mechanisms in PRR-triggered immunity. LncRNAs can provide positive or negative feedback to their upstream PRR-, cytokine- or interferon-signalling pathways (left). Feed-forward control promotes, sensitizes or re-shapes receptor triggered immune-signalling programmes. In this context, lncRNA have been implicated in coherent (elevated positive-regulator or down-regulated repressor) and incoherent (induced repressor) feed-forward control (right). Murine lncRNAs shown in green, human lncRNAs in blue
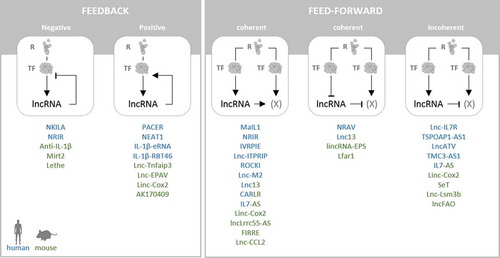
The majority of immune-associated lncRNAs characterized to date seems to be involved in positive control (feedback or feed-forward) of PRR, cytokine- and IFN-receptor signalling pathways (), suggesting an important contribution of lncRNAs to the initiation and amplification of the immune response. This is reminiscent of the previously known feed-forward control of the macrophage inflammatory response by microRNAs of the let-7 family [Citation12]. Negative regulators, including several lncRNAs, such as lncFAO, but also microRNAs, such as miR-146 [Citation10,Citation11], subsequently level the inflammatory response or contribute to inflammation resolution. Thus, it is becoming increasingly clear, that mammalian innate immune responses to pathogen- and danger-signals are tightly controlled and modulated by a dense network of regulatory RNAs, preventing both from overshooting and attenuated inflammation through feed-back and feed-forward control mechanisms (, ).
7. LncRNAs at the interface between innate and adaptive immunity
Besides the above-portrayed functions of lncRNAs in the innate immunity, lncRNAs vitally participate in the adaptive immune system by affecting differentiation and activation of T- and B-lymphocytes [Citation115]. Phagocytes actively communicate with T- and B-cells to regulate their activity. Among others, this involves the secretion of cytokines affecting T-cell differentiation, or the presentation of antigens recognized by T-cell receptors (TCRs) [Citation116]. T-cell activation upon TCR ligation to antigens presented e.g. by dendritic cells or on the surface of various other cell-types through MHC-complexes involves a calcium-signalling dependent pathway leading to the activation of the transcription factor NFAT [Citation116,Citation117]. NFAT nuclear translocation is counter-regulated by lncRNA NRON [Citation118]. This lncRNA serves as a scaffold redirecting NFAT to a cytoplasmic complex consisting of the IQGAP protein and several kinases, which prevent NFAT from being dephosphorylated and imported into the nucleus [Citation119]. Resolution of T cell responses involves the process of activation-induced cell death (AICD), which is regulated by lncRNA NKILA. TCR activation triggers STAT-dependent expression of NKILA, which sensitizes cytotoxic T-lymphocytes to AICD [Citation120]. In epithelial cells, NKILA was reported counteract LPS-induced NFκB activation (see 5.4). Thus, NKILA is an example of an lncRNA engaged both in innate and adaptive immunity.
T-cell polarization is actively shaped by macrophages, dendritic cells and other cell types producing cytokines such as IL6 or IL12 [Citation116]. Via IL12 release, activated macrophages and dendritic cells stimulate NK- and T-cells for production of interferon-γ (IFNγ) [Citation116], which in turn triggers ISG expression in phagocytes and promotes increased antigen presentation and antimicrobial responses [Citation121]. Production of IFNγ by CD4+ Th1-polarized cells and CD8 + T-cells is under positive control by the lncRNA IFNG-AS1 (also known as NeST, or Tmevpg1) [Citation122–125]. Mechanistically, murine IFNG-AS1 interacts with the histone-methyltransferase WDR5 to promote deposition of activating histone methylation marks at the IFNγ locus [Citation126]. Supporting a physiological role in inflammation, the IFNG-AS gene overlaps with an Inflammatory Bowel Disease susceptibility SNP and its expression is increased in ulcerative colitis patients [Citation123,Citation127]. Moreover, in mice, IFNG-AS1 was shown to abate Salmonella enterica induced pathologies and reduce bacterial burden in an IFNγ dependent manner [Citation126]. Besides its role in CD4+ Th1 cells and CD8 + T-cells, IFNG-AS1 also promotes IFNγ production by NK cells, which participate both in innate and adaptive immunity [Citation128] (see below). Another lncRNA regulating IFNγ production is MORRBID. Originally described to regulate phagocyte differentiation and longevity (see 4.4), in CD8 + T-cells MORRBID is induced upon TCR activation and type I IFN stimulation during viral infection [Citation129]. Unlike IFNG-AS1, MORRBID functions to restrain IFNγ production [Citation129].
While CD8 + T-cells and CD4+ Th1 cells regulate phagocyte activity via IFNγ, CD4+ Th2 cells, upon their activation produce IL4, which promotes macrophage polarization towards an anti-inflammatory and tissue-repair associated phenotype [Citation86,Citation116]. Similar to IFNγ-production by Th1 cells, IL4 generation by Th2 cells depends on several lncRNAs, including lincR-Crr2-5ʹAS, GATA3-AS1 and TH2-LCR [Citation115]. In macrophages, IL4 dependent polarization in turn is regulated by lncRNA lnc-M2 (see 4.4). Thus, the communication between T-cells and phagocytes is controlled by lncRNAs on both sides.
Of note, naïve CD4 + T-cell activation critically depends on dendritic cell presented antigens [Citation116]. Dendritic cell differentiation and activation by innate immune agonists such as LPS is controlled by lncRNAs lnc-DC and NEAT1 (see 4.4). It remains to be determined, whether lncRNAs participating in the PRR-responses of other phagocytes, including macrophages (see section 4), are also active in dendritic cells and contribute to their bridging functions in innate and adaptive immunity. Likewise, the roles of lncRNAs in NK cells, a type of innate lymphoid cells, that do not rearrange immunoglobulin and TCR genes but can kill target cells through immunoglobulin-binding [Citation130,Citation131], are still poorly understood. Besides the role of IFNG-AS1 in IFNγ production by NK cells (see above), Lnc-CD56 was found to promote expression of the important NK-cell marker and adhesion molecule CD56 and to contribute to NK-cell differentiation [Citation132]. Further lncRNAs are expressed and dynamically regulated under infection conditions in NK cells [Citation49,Citation132] but presently remain uncharacterized. Functional characterization of the still poorly studied lncRNA repertoires of dendritic cells and NK cells should further improve our understanding of the regulatory implications of lncRNAs at the interface between innate and adaptive immunity.
8. Conclusions
LncRNAs are critically involved in many cellular processes [Citation4]; thus, it is not surprising that these molecules also play a central role in innate immune responses of professional phagocytes and other cell types in their periphery. The literature available so far suggests a plethora of different mechanisms through which lncRNAs can interfere with or promote cellular inflammatory responses. These mechanisms range from nuclear processes, such as transcription factor positioning and chromatin remodelling to cytoplasmic processes, including protein ubiquitination and mRNA stabilization. However, despite the recent progress in our understanding of the roles of lncRNAs in innate immunity, the field is still at its infancy and the majority of immune-associated lncRNAs remains mechanistically uncharacterized. Moreover, only few of the known immune-relevant lncRNAs seem to be conserved between mice and humans at the primary sequence level [Citation50]. This could hint at the redundancy of many immunoregulatory lncRNAs, as regulatory RNAs whose function is redundant with other regulators are expected to display little evolutionary sequence conservation [Citation133]. LncRNA redundancy, however, does not necessarily indicate lack of importance at an evolutionary scale. Rather, the volatility of lncRNA repertoires was speculated to contribute to robustness and evolvability of species [Citation134]. This might be particularly true for immune-modulatory lncRNAs, since infectious agents constitute an important driver of evolution [Citation135]. Besides the possibility of redundancy as an explanation for little lncRNA conservation, lncRNA genes might also accumulate neutral mutations that dissipate the primary sequence over the course of evolution without affecting RNA secondary structure and function [Citation136]. Such structure-neutral mutations are well documented for ribosomal RNAs, which display higher conservation at the structural than at the primary sequence level [Citation137]. Besides sequence- and structure-conservation, the simple act of transcription from syntenic loci, independent of the lncRNA sequence, might explain the function of some lncRNAs. Combinations of sequence- and structure-conservation and syntenic transcription could contribute to an lncRNAs overall functional conservation [Citation138]. An important task in the coming years will be to determine structure-conserved orthologues, functional equivalents and syntenic loci corresponding to immune-regulatory lncRNAs such as linc-Cox2 and MaIL1, the study of which is presently limited to rodents and primates, respectively.
Independent of their evolutionary conservation, the already obtained mechanistic insights into the roles of lncRNAs in human and murine cells suggest a vital participation in the TLR-TIRF-, RLR-MAVS- and STING-dependent type I IFN induction pathways and in JAK-STAT- dependent responses to type I IFNs. Furthermore, lncRNAs participate in TLR-MyD88, TLR-TRIF and cytokine-receptor dependent activation of the inflammation master regulator NFκB. Of note, lncRNA-mediated feedback and feed-forward control of cellular immune responses is not limited to professional innate immune cells, but also contributes to the adequate immune activation of epithelial cells, endothelial cells and fibroblasts in different organ systems. Many of the immune-regulatory lncRNAs identified to date, seem to interact with chromatin-regulatory (e.g. PRC2 or SWI/SNF) and paraspeckle proteins (e.g. SFPQ). Further lncRNAs impact the immune response through interaction with RNA binding proteins controlling mRNA stability (e.g. hnRNPU or PCBP2) or ubiquitination dependent signalling (e.g. TRIM25 or OPTN). It would be of great interest to the community to learn about the global RNA substrates of these RNA binding proteins (RBPs), e.g. through unbiased CLIP-seq methods which could reveal larger RBP-lncRNA networks and lncRNA binding hubs involved in cellular innate immunity. Co-sedimentation analysis using density gradients indeed suggest that lncRNAs in immune-activated phagocytes form discriminable subgroups, potentially interacting with shared protein components [Citation27]. Besides their functions in response to PRR agonists, relatively little is known about the role of lncRNAs in the differentiation and polarization of immune cells. Many lncRNAs exhibit highly tissue- and cell-type-specific expression patterns [Citation13,Citation78] and these lncRNAs might play important roles in leukocyte differentiation and in shaping the cellular responsiveness to immune stimuli. Further efforts are required to map lineage specific lncRNAs involved in leukocyte differentiation and function and potentially contributing to haematological disorders.
Besides the important progress already made, many open questions as to the functions of lncRNAs in the innate immune system and the strategies to study lncRNA candidates remain. The lower copy numbers of lncRNAs per cell compared to mRNAs, for example, raise questions about possible lncRNA mechanisms. Whereas individual lncRNAs, such as MALAT1 are highly expressed in many cell types [Citation138], the median expression levels reached by lncRNAs are ~10-fold below those reached by mRNAs [Citation139]. This should, for instance affect the decoying capacity of lncRNAs such as PACER, which occludes NFκB p50 from the COX-2 promoter. Besides serving as protein decoys, several lncRNAs were described to function as competing endogenous RNAs, which capture microRNAs and reduce their ability to bind to other target RNAs [Citation4,Citation140]. The copy number of an lncRNA relative to mRNAs targeted by the same microRNA would be highly relevant to the determination of its ability to serve as a ceRNA. So far, however, such stoecheometries are poorly documented for immune-associated lncRNAs. Apart from the decoy function, low-level lncRNA expression does not necessarily indicate little regulatory potential, as exemplified by lncRNA VELUCT, which profoundly affects cell survival despite very low copy numbers [Citation141]. Mechanistically, lowly expressed lncRNAs could for instance serve as starting points for the assembly of larger signalling complexes, such as the OPTN-TBK1 platform [Citation27] or Myddosomes [Citation142]. Once triggered, such complexes might continue to assemble independent of additional RNA copies. Besides their lower expression compared to mRNAs, the association of a substantial number of lncRNAs with ribosomes [Citation26,Citation27] raises questions about their potential cellular functions. Both in human and murine cells, annotated lncRNAs were found to encode previously unknown peptides, including in phagocytes [Citation25,Citation26]. Of note, ribosome-association alone does not predict coding potential. Long read-sequencing and ribosome-profiling data are therefore required to fine-map lncRNA transcript architectures and ribosome-occupied regions. Thereby, falsely annotated lncRNAs encoding peptides could be discriminated from true lncRNAs associating with ribosomes to control immune-relevant ribosomal functions. In addition to the protein-coding potential of lncRNAs, it remains unclear, to which degree the already published lncRNA functions hint towards recurring regulatory principles in the innate immune system, especially since most immune-regulated lncRNAs in phagocytes and other cell types have not yet been functionally characterized [Citation27,Citation49]. One reason for this could be the challenging de novo identification of RNA interaction partners, which renders mechanistic lncRNA research tedious. However, emerging technologies, such as gradient-sequencing and mass spectrometry (Grad-seq), RNA antisense affinity purification and mass-spectrometry (RAP-MS) or proximity labelling methods promise to accelerate the fine-mapping of lncRNA-protein interactions [Citation27,Citation143–145]. Together with the recent improvements of the ENCODE eCLIP, ChIP-seq and chromatin 3D structure catalogues [Citation146], these methodologies may substantially facilitate the dissection of lncRNA-mechanisms involved in the control of immune mediator production and inflammation. The already available mechanistic insights predict that further research on inflammation-associated lncRNAs will decisively advance our understanding of the control-mechanisms of the innate immune system and thus of the pathomechanisms underlying prevalent human diseases.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kagan JC, Barton GM. Emerging principles governing signal transduction by pattern-recognition receptors. Cold Spring Harb Perspect Biol. 2014;7:a016253.
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295.
- Liu T, Zhang L, Joo D, et al. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2. DOI:10.1038/sigtrans.2017.23
- Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18:206.
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166.
- Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet. 2015;16:421–433.
- Mehta A, Baltimore D. MicroRNAs as regulatory elements in immune system logic. Nat Rev Immunol. 2016;16:279–294.
- O’Connell RM, Taganov KD, Boldin MP, et al. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA. 2007;104:1604–1609.
- Taganov KD, Boldin MP, Chang KJ, et al. NF-kappa B-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486.
- Schulte LN, Westermann AJ, Vogel J. Differential activation and functional specialization of miR-146 and miR-155 in innate immune sensing. Nucleic Acids Res. 2013;41:542–553.
- Janga H, Aznaourova M, Boldt F, et al. Cas9-mediated excision of proximal DNaseI/H3K4me3 signatures confers robust silencing of microRNA and long non-coding RNA genes. PloS One. 2018;13:e0193066.
- Schulte LN, Eulalio A, Mollenkopf HJ, et al. Analysis of the host microRNA response to Salmonella uncovers the control of major cytokines by the let-7 family. Embo J. 2011;30:1977–1989.
- Derrien T, Johnson R, Bussotti G, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789.
- Hon CC, Ramilowski JA, Harshbarger J, et al. An atlas of human long non-coding RNAs with accurate 5ʹ ends. Nature. 2017;543:199–204.
- Giral H, Landmesser U, Kratzer A. Into the wild: GWAS exploration of Non-coding RNAs. Front Cardiovasc Med. 2018;5:181.
- Castellanos-Rubio A, Ghosh S. Disease-associated SNPs in inflammation-related lncRNAs. Front Immunol. 2019;10:420.
- Carlevaro-Fita J, Johnson R. Global positioning system: understanding long noncoding RNAs through subcellular localization. Mol Cell. 2019;73:869–883.
- Krawczyk M, Emerson BM. p50-associated COX-2 extragenic RNA (PACER) activates COX-2 gene expression by occluding repressive NF-kappaB complexes. Elife. 2014;3:e01776.
- Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323.
- Zhao J, Sun BK, Erwin JA, et al. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756.
- Tsai MC, Manor O, Wan Y, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693.
- Chen LL. Linking long noncoding RNA localization and function. Trends Biochem Sci. 2016;41:761–772.
- Fox AH, Nakagawa S, Hirose T, et al. Paraspeckles: where long noncoding RNA meets phase separation. Trends Biochem Sci. 2018;43:124–135.
- Thapa P, Shanmugam N, Pokrzywa W. Ubiquitin signaling regulates RNA biogenesis, processing, and metabolism. BioEssays. 2020;42:e1900171.
- Chen J, Brunner AD, Cogan JZ, et al. Pervasive functional translation of noncanonical human open reading frames. Science. 2020;367:1140–1146.
- Jackson R, Kroehling L, Khitun A, et al. The translation of non-canonical open reading frames controls mucosal immunity. Nature. 2018;564:434–438.
- Aznaourova M, Janga H, Sefried S, et al. Noncoding RNA MaIL1 is an integral component of the TLR4-TRIF pathway. Proc Natl Acad Sci USA. 2020;117:9042–9053.
- van Heesch S, van Iterson M, Jacobi J, et al. Extensive localization of long noncoding RNAs to the cytosol and mono- and polyribosomal complexes. Genome Biol. 2014;15:R6.
- Carlevaro-Fita J, Rahim A, Guigo R, et al. Cytoplasmic long noncoding RNAs are frequently bound to and degraded at ribosomes in human cells. Rna. 2016;22:867–882.
- Wang HV, Chekanova JA. An overview of methodologies in studying lncRNAs in the high-throughput era: when acronyms ATTACK!. Methods Mol Biol. 2019;1933:1–30.
- Gonzalez-Moro I, Olazagoitia-Garmendia A, Colli ML, et al. The T1D-associated lncRNA Lnc13 modulates human pancreatic beta cell inflammation by allele-specific stabilization of STAT1 mRNA. Proc Natl Acad Sci USA. 2020;117:9022–9031.
- Atianand MK, Hu W, Satpathy AT, et al. A long noncoding RNA lincRNA-EPS acts as a transcriptional brake to restrain inflammation. Cell. 2016;165:1672–1685.
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820.
- Motwani M, Pesiridis S, Fitzgerald KA. DNA sensing by the cGAS-STING pathway in health and disease. Nat Rev Genet. 2019;20:657–674.
- Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063.
- Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol. 2014;32:513–545.
- Shi JH, Sun SC. Tumor necrosis factor receptor-associated factor regulation of nuclear factor kappaB and mitogen-activated protein kinase pathways. Front Immunol. 2018;9:1849.
- Turner MD, Nedjai B, Hurst T, et al. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843:2563–2582.
- Narumiya S, Furuyashiki T. Fever, inflammation, pain and beyond: prostanoid receptor research during these 25 years. Faseb J. 2011;25:813–818.
- Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295.
- Swiecki M, Colonna M. Type I interferons: diversity of sources, production pathways and effects on immune responses. Curr Opin Virol. 2011;1:463–475.
- Cai B, Cai JP, Luo YL, et al. The specific roles of JAK/STAT signaling pathway in sepsis. Inflammation. 2015;38:1599–1608.
- Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283.
- Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189.
- Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol. 2011;1:519–525.
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(Suppl 2):S3.
- Bhattacharya J, Westphalen K. Macrophage-epithelial interactions in pulmonary alveoli. Semin Immunopathol. 2016;38:461–469.
- Guilliams M, Thierry GR, Bonnardel J, et al. Establishment and maintenance of the macrophage niche. Immunity. 2020;52:434–451.
- Schulte LN, Schweinlin M, Westermann AJ, et al. An advanced human intestinal coculture model reveals compartmentalized host and pathogen strategies during salmonella infection. mBio. 2020;11:e03348–19.
- Roux BT, Heward JA, Donnelly LE, et al. Catalog of differentially expressed long non-coding RNA following activation of human and mouse innate immune response. Front Immunol. 2017;8:1038.
- Mariotti B, Servaas NH, Rossato M, et al. The long non-coding RNA NRIR drives IFN-response in monocytes: implication for systemic sclerosis. Front Immunol. 2019;10:100.
- Lin H, Jiang M, Liu L, et al. The long noncoding RNA Lnczc3h7a promotes a TRIM25-mediated RIG-I antiviral innate immune response. Nat Immunol. 2019;20:812–823.
- Zhou Y, Li M, Xue Y, et al. Interferon-inducible cytoplasmic lncLrrc55-AS promotes antiviral innate responses by strengthening IRF3 phosphorylation. Cell Res. 2019;29:641–654.
- Jiang M, Zhang S, Yang Z, et al. Self-recognition of an inducible host lncRNA by RIG-I feedback restricts innate immune response. Cell. 2018;173:906–19 e13.
- Zhang K, Shi Z, Zhang M, et al. Silencing lncRNA Lfar1 alleviates the classical activation and pyoptosis of macrophage in hepatic fibrosis. Cell Death Dis. 2020;11:132.
- NE II, Heward JA, Roux B, et al. Long non-coding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes. Nat Commun. 2014;5:3979.
- Castellanos-Rubio A, Kratchmarov R, Sebastian M, et al. Cytoplasmic form of Carlr lncRNA facilitates inflammatory gene expression upon NF-kappaB activation. J Iimmunol. 2017;199:581–588.
- Zhang Q, Chao TC, Patil VS, et al. The long noncoding RNA ROCKI regulates inflammatory gene expression. Embo J. 2019;38. DOI:10.15252/embj.2018100041.
- Liu X, Lu Y, Zhu J, et al. A long noncoding RNA, antisense IL-7, promotes inflammatory gene transcription through facilitating histone acetylation and switch/sucrose nonfermentable chromatin remodeling. J Iimmunol. 2019;203:1548–1559.
- Ma S, Ming Z, Gong AY, et al. A long noncoding RNA, lincRNA-Tnfaip3, acts as a coregulator of NF-kappaB to modulate inflammatory gene transcription in mouse macrophages. Faseb J. 2017;31:1215–1225.
- Zhou B, Qi F, Wu F, et al. Endogenous retrovirus-derived long noncoding RNA enhances innate immune responses via derepressing RELA expression. mBio. 2019;10. DOI:10.1128/mBio.00937-19.
- Covarrubias S, Robinson EK, Shapleigh B, et al. CRISPR/Cas-based screening of long non-coding RNAs (lncRNAs) in macrophages with an NF-kappaB reporter. J Biol Chem. 2017;292:20911–20920.
- Carpenter S, Aiello D, Atianand MK, et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science. 2013;341:789–792.
- Hu G, Gong AY, Wang Y, et al. LincRNA-Cox2 promotes late inflammatory gene transcription in macrophages through modulating SWI/SNF-mediated chromatin remodeling. J Iimmunol. 2016;196:2799–2808.
- Chan J, Atianand M, Jiang Z, et al. Cutting edge: a natural antisense transcript, AS-IL1alpha, controls inducible transcription of the proinflammatory cytokine IL-1alpha. J Iimmunol. 2015;195:1359–1363.
- Lu Y, Liu X, Xie M, et al. The NF-kappaB-responsive long noncoding RNA FIRRE regulates posttranscriptional regulation of inflammatory gene expression through interacting with hnRNPU. J Iimmunol. 2017;199:3571–3582.
- Lewandowski JP, Lee JC, Hwang T, et al. The firre locus produces a trans-acting RNA molecule that functions in hematopoiesis. Nat Commun. 2019;10:5137.
- Jia Y, Li Z, Cai W, et al. SIRT1 regulates inflammation response of macrophages in sepsis mediated by long noncoding RNA. Biochimica Et Biophysica Acta Mol Basis Dis. 2018;1864:784–792.
- Agliano F, Fitzgerald KA, Vella AT, et al. Long non-coding RNA LincRNA-EPS inhibits host defense against listeria monocytogenes infection. Front Cell Infect Microbiol. 2019;9:481.
- Castellanos-Rubio A, Fernandez-Jimenez N, Kratchmarov R, et al. A long noncoding RNA associated with susceptibility to celiac disease. Science. 2016;352:91–95.
- Li Z, Chao TC, Chang KY, et al. The long noncoding RNA THRIL regulates TNFalpha expression through its interaction with hnRNPL. Proc Natl Acad Sci U S A. 2014;111:1002–1007.
- Du M, Yuan L, Tan X, et al. The LPS-inducible lncRNA Mirt2 is a negative regulator of inflammation. Nat Commun. 2017;8:2049.
- Lu J, Wu X, Hong M, et al. A potential suppressive effect of natural antisense IL-1beta RNA on lipopolysaccharide-induced IL-1beta expression. J Iimmunol. 2013;190:6570–6578.
- Stratigi K, Kapsetaki M, Aivaliotis M, et al. Spatial proximity of homologous alleles and long noncoding RNAs regulate a switch in allelic gene expression. Proc Natl Acad Sci USA. 2015;112:E1577–86.
- Stathopoulou C, Kapsetaki M, Stratigi K, et al. Long non-coding RNA SeT and miR-155 regulate the Tnfalpha gene allelic expression profile. PloS One. 2017;12:e0184788.
- Thapa B, Lee K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep. 2019;52:360–372.
- Nakayama Y, Fujiu K, Yuki R, et al. A long noncoding RNA regulates inflammation resolution by mouse macrophages through fatty acid oxidation activation. Proc Natl Acad Sci USA. 2020;117:14365–14375.
- Washietl S, Kellis M, Garber M. Evolutionary dynamics and tissue specificity of human long noncoding RNAs in six mammals. Genome Res. 2014;24:616–628.
- Zhang X, Lian Z, Padden C, et al. A myelopoiesis-associated regulatory intergenic noncoding RNA transcript within the human HOXA cluster. Blood. 2009;113:2526–2534.
- Wei S, Zhao M, Wang X, et al. PU.1 controls the expression of long noncoding RNA HOTAIRM1 during granulocytic differentiation. J Hematol Oncol. 2016;9:44.
- Panopoulos AD, Bartos D, Zhang L, et al. Control of myeloid-specific integrin alpha Mbeta 2 (CD11b/CD18) expression by cytokines is regulated by Stat3-dependent activation of PU.1. J Biol Chem. 2002;277:19001–19007.
- Kotzin JJ, Spencer SP, McCright SJ, et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature. 2016;537:239–243.
- Wagner LA, Christensen CJ, Dunn DM, et al. EGO, a novel, noncoding RNA gene, regulates eosinophil granule protein transcript expression. Blood. 2007;109:5191–5198.
- Wang P, Xue Y, Han Y, et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344:310–313.
- Zhang M, Zheng Y, Sun Y, et al. Knockdown of NEAT1 induces tolerogenic phenotype in dendritic cells by inhibiting activation of NLRP3 inflammasome. Theranostics. 2019;9:3425–3442.
- Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 2018;19:1801.
- Chen Y, Li H, Ding T, et al. Lnc-M2 controls M2 macrophage differentiation via the PKA/CREB pathway. Mol Immunol. 2020;124:142–152.
- Pei W, Zhang Y, Li X, et al. LncRNA AK085865 depletion ameliorates asthmatic airway inflammation by modulating macrophage polarization. Int Immunopharmacol. 2020;83:106450.
- Kotredes KP, Thomas B, Gamero AM. The protective role of type I interferons in the gastrointestinal tract. Front Immunol. 2017;8:410.
- Morchikh M, Cribier A, Raffel R, et al. HEXIM1 and NEAT1 long non-coding RNA form a multi-subunit complex that regulates DNA-mediated innate immune response. Mol Cell. 2017;67:387–99 e5.
- Ahn J, Barber GN. STING signaling and host defense against microbial infection. Exp Mol Med. 2019;51:1–10.
- Ma H, Han P, Ye W, et al. The long noncoding RNA NEAT1 exerts antihantaviral effects by acting as positive feedback for RIG-I signaling. J Virol. 2017;91:e02250–16.
- Zhao L, Xia M, Wang K, et al. A long non-coding RNA IVRPIE promotes host antiviral immune responses through regulating interferon beta1 and ISG expression. Front Microbiol. 2020;11:260.
- Ouyang J, Zhu X, Chen Y, et al. NRAV, a long noncoding RNA, modulates antiviral responses through suppression of interferon-stimulated gene transcription. Cell Host Microbe. 2014;16:616–626.
- Wang P, Xu J, Wang Y, et al. An interferon-independent lncRNA promotes viral replication by modulating cellular metabolism. Science. 2017;358:1051–1055.
- Wang J, Cen S. Roles of lncRNAs in influenza virus infection. Emerg Microbes Infect. 2020;9:1407–1414.
- Xie Q, Chen S, Tian R, et al. Long noncoding RNA ITPRIP-1 positively regulates the innate immune response through promotion of oligomerization and activation of MDA5. J Virol. 2018;92. DOI:10.1128/JVI.00507-18.
- Wang Q, Zhang D, Feng W, et al. Long noncoding RNA TSPOAP1 antisense RNA 1 negatively modulates type I IFN signaling to facilitate influenza A virus replication. J Med Virol. 2019:1–10.
- Li X, Guo G, Lu M, et al. Long noncoding RNA Lnc-MxA inhibits beta interferon transcription by forming RNA-DNA triplexes at its promoter. J Virol. 2019;93. DOI:10.1128/JVI.00786-19.
- Fan J, Cheng M, Chi X, et al. A human long non-coding RNA LncATV promotes virus replication through restricting RIG-I-mediated innate immunity. Front Immunol. 2019;10:1711.
- Carnero E, Barriocanal M, Prior C, et al. Long noncoding RNA EGOT negatively affects the antiviral response and favors HCV replication. EMBO Rep. 2016;17:1013–1028.
- Kambara H, Niazi F, Kostadinova L, et al. Negative regulation of the interferon response by an interferon-induced long non-coding RNA. Nucleic Acids Res. 2014;42:10668–10680.
- Nishitsuji H, Ujino S, Yoshio S, et al. Long noncoding RNA #32 contributes to antiviral responses by controlling interferon-stimulated gene expression. Proc Natl Acad Sci USA. 2016;113:10388–10393.
- Lentsch AB, Ward PA. Regulation of inflammatory vascular damage. J Pathol. 2000;190:343–348.
- Luissint AC, Parkos CA, Nusrat A. Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology. 2016;151:616–632.
- Imamura K, Imamachi N, Akizuki G, et al. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol Cell. 2014;53:393–406.
- Imamura K, Takaya A, Ishida YI, et al. Diminished nuclear RNA decay upon Salmonella infection upregulates antibacterial noncoding RNAs. Embo J. 2018;37:e97723.
- Taniguchi K, Karin M. NF-kappa B, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–324.
- Liu B, Sun L, Liu Q, et al. A cytoplasmic NF-kappaB interacting long noncoding RNA blocks IkappaB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27:370–381.
- Ye M, Xie M, Zhu J, et al. LPS-inducible lncRNA TMC3-AS1 negatively regulates the expression of IL-10. Front Immunol. 2020;11:1418.
- Cui H, Xie N, Tan Z, et al. The human long noncoding RNA lnc-IL7R regulates the inflammatory response. Eur J Immunol. 2014;44:2085–2095.
- Rapicavoli NA, Qu K, Zhang J, et al. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. Elife. 2013;2:e00762.
- Tong Q, Gong AY, Zhang XT, et al. LincRNA-Cox2 modulates TNF-alpha-induced transcription of Il12b gene in intestinal epithelial cells through regulation of Mi-2/NuRD-mediated epigenetic histone modifications. Faseb J. 2016;30:1187–1197.
- Rahman A, Tiwari A, Narula J, et al. Importance of feedback and feedforward loops to adaptive immune response modeling. CPT Pharmacometrics Syst Pharmacol. 2018;7:621–628.
- Zeni PF, Mraz M. LncRNAs in adaptive immunity: role in physiological and pathological conditions. RNA Biol. 2020. DOI:10.1080/15476286.2020.1838783
- Kaiko GE, Horvat JC, Beagley KW, et al. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123:326–338.
- Hogan PG. Calcium-NFAT transcriptional signalling in T cell activation and T cell exhaustion. Cell Calcium. 2017;63:66–69.
- Willingham AT, Orth AP, Batalov S, et al. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science. 2005;309:1570–1573.
- Sharma S, Findlay GM, Bandukwala HS, et al. Dephosphorylation of the nuclear factor of activated T cells (NFAT) transcription factor is regulated by an RNA-protein scaffold complex. Proc Natl Acad Sci USA. 2011;108:11381–11386.
- Huang D, Chen J, Yang L, et al. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat Immunol. 2018;19:1112–1125.
- Ivashkiv LB. IFNgamma: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol. 2018;18:545–558.
- Vigneau S, Rohrlich PS, Brahic M, et al. Tmevpg1, a candidate gene for the control of Theiler’s virus persistence, could be implicated in the regulation of gamma interferon. J Virol. 2003;77:5632–5638.
- Padua D, Mahurkar-Joshi S, Law IK, et al. A long noncoding RNA signature for ulcerative colitis identifies IFNG-AS1 as an enhancer of inflammation. Am J Physiol Gastrointest Liver Physiol. 2016;311:G446–57.
- Collier SP, Collins PL, Williams CL, et al. Cutting edge: influence of Tmevpg1, a long intergenic noncoding RNA, on the expression of Ifng by Th1 cells. J Iimmunol. 2012;189:2084–2088.
- Petermann F, Pekowska A, Johnson CA, et al. The magnitude of IFN-gamma responses is fine-tuned by DNA architecture and the non-coding transcript of Ifng-as1. Mol Cell. 2019;75:1229–42 e5.
- Gomez JA, Wapinski OL, Yang YW, et al. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus. Cell. 2013;152:743–754.
- Mirza AH, Berthelsen CH, Seemann SE, et al. Transcriptomic landscape of lncRNAs in inflammatory bowel disease. Genome Med. 2015;7:39.
- Stein N, Berhani O, Schmiedel D, et al. IFNG-AS1 enhances interferon gamma production in human natural killer cells. iScience. 2019;11:466–473.
- Kotzin JJ, Iseka F, Wright J, et al. The long noncoding RNA Morrbid regulates CD8 T cells in response to viral infection. Proc Natl Acad Sci U S A. 2019;116:11916–11925.
- Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469.
- Lo Nigro C, Macagno M, Sangiolo D, et al. NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: biological evidence and clinical perspectives. Ann Transl Med. 2019;7:105.
- Zhang R, Ni F, Fu B, et al. A long noncoding RNA positively regulates CD56 in human natural killer cells. Oncotarget. 2016;7:72546–72558.
- Cooper GM, Brown CD. Qualifying the relationship between sequence conservation and molecular function. Genome Res. 2008;18:201–205.
- Kapusta A, Feschotte C. Volatile evolution of long noncoding RNA repertoires: mechanisms and biological implications. Trends Genet. 2014;30:439–452.
- Enard D, Cai L, Gwennap C, et al. Viruses are a dominant driver of protein adaptation in mammals. Elife. 2016;5. DOI:10.7554/eLife.12469
- Walter Costa MB, Honer Z, Siederdissen C, et al. SSS-test: a novel test for detecting positive selection on RNA secondary structure. Bmc Bioinformatics. 2019;20:151.
- Doris SM, Smith DR, Beamesderfer JN, et al. Universal and domain-specific sequences in 23S-28S ribosomal RNA identified by computational phylogenetics. Rna. 2015;21:1719–1730.
- Diederichs S. The four dimensions of noncoding RNA conservation. Trends Genet. 2014;30:121–123.
- Cabili MN, Trapnell C, Goff L, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927.
- Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358.
- Seiler J, Breinig M, Caudron-Herger M, et al. The lncRNA VELUCT strongly regulates viability of lung cancer cells despite its extremely low abundance. Nucleic Acids Res. 2017;45:5458–5469.
- Tan Y, Kagan JC. Innate immune signaling organelles display natural and programmable signaling flexibility. Cell. 2019;177:384–98 e11.
- Smirnov A, Forstner KU, Holmqvist E, et al. Grad-seq guides the discovery of ProQ as a major small RNA-binding protein. Proc Natl Acad Sci USA. 2016;113:11591–11596.
- McHugh CA, Guttman M. RAP-MS: a method to identify proteins that interact directly with a specific RNA molecule in cells. Methods Mol Biol. 2018;1649:473–488.
- Yi W, Li J, Zhu X, et al. CRISPR-assisted detection of RNA-protein interactions in living cells. Nat Methods. 2020;17:685–688.
- Consortium EP, Moore JE, Purcaro MJ, et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature. 2020;583:699–710.
