ABSTRACT
U1 snRNP is one of the most abundant ribonucleoprotein (RNP) complexes in eukaryotic cells and is estimated to be approximately 1 million copies per cell. Apart from its canonical role in mRNA splicing, this complex has emerged as a key regulator of eukaryotic mRNA length via inhibition of mRNA 3ʹ-end processing at numerous intronic polyadenylation sites, in a process that is also termed ‘U1 snRNP telescripting’. Several reviews have extensively described the concept of U1 telescripting and subsequently highlighted its potential impacts in mRNA metabolism. Here, we review what is currently known regarding the underlying mechanisms of this important phenomenon and discuss open questions and future challenges.
1. Introduction
The phenomenon of mRNA splicing was discovered in the late 1970s [Citation1,Citation2]. Splicing of introns, from precursor mRNA (pre-mRNA), requires a ribonucleoprotein (RNP) machinery, namely spliceosome [Citation3–5]. Since the discovery of spliceosome, numerous efforts have been made to characterize its biochemical composition and action mechanisms. For instance, U1 snRNP, a subcomplex of the spliceosome that plays a crucial role in the initial step of mRNA splicing by directly interacting with RNA-RNA between 5ʹ-splice site (5ʹ-SS) and U1 snRNA’s 5ʹ-end, has been described [Citation6,Citation7]. Consequently, characterization of U1 snRNP has revealed that it plays a vital role in mRNA splicing [Citation8–10].
Apart from splicing, 3ʹ-end processing represents another major mRNA processing event [Citation11]. In the past, both events, together with transcription, have been investigated independently [Citation12]. Since the 1990s, more focus has been on their coupling and cross-regulations [Citation12–15]. Consequently, U1 snRNP was found to inhibit mRNA 3ʹ-end processing, with this inhibitory effect found to regulate gene expression in some viruses [Citation16–18]. In 2010, Dreyfuss laboratory pioneered a new method for investigating the cellular function of U1 snRNP by introducing U1 snRNA antisense morpholino oligonucleotides (AMOs), coupled with high-density genomic tiling arrays [Citation19]. They found that thousands of pre-mRNA transcripts were terminated at cryptic polyadenylation sites (PASs) within introns. Furthermore, their results demonstrated that this phenomenon was general, hence named it ‘U1 telescripting’ [Citation20,Citation21]. To date, however, the underlying molecular mechanism of U1 telescripting remains poorly understood, in comparison with U1 function in splicing. Given that U1 telescripting plays a critical role in mRNA metabolism and directly impacts transcript output, such as mRNA length, abundance, and alternative polyadenylation (APA) [Citation22,Citation23], we sought to first summarize the current knowledge on U1 telescripting by combining traditional in vitro biochemical assays with recent high-throughput sequencing analysis and then discuss potential mechanisms in the context of transcription. Compared to previous articles on U1 telescripting [Citation22,Citation23], the present review focuses on its underlying molecular mechanisms and the latest progress in this emerging field.
2. The assembly, dynamics and regulation of U1 snRNP
Assembly
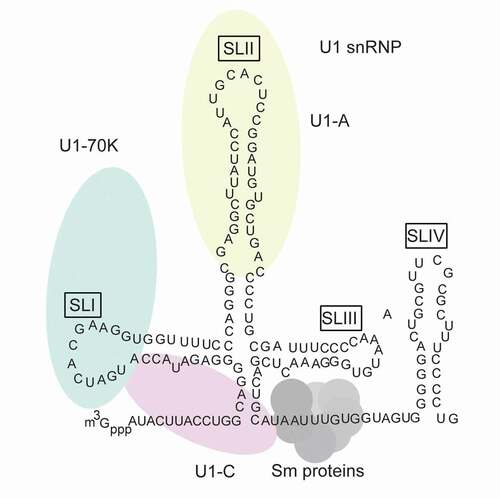
U1 snRNP is one of the most intensively studied eukaryotic protein–RNA complexes to date. In human, it is comprised of 3 U1-specific proteins (U1A, U1-70 K and U1C), 7 Sm proteins (SmB or SmB’, SmD1, SmD2, SmD3, SmE, SmF and SmG) and 165 nt U1 snRNA () [Citation24,Citation25]. The assembly of U1 snRNP is dominated by several protein–RNA interactions, including the interaction of U1-70 K RNA-binding domain (RBD) with the U1 snRNA stem loop I (SLI), the interaction of U1A RBD with the stem loop II (SLII) and the interaction of Sm core with U1 snRNA Sm sites (AUUUGUG or AUUUCUG) [Citation26–28]. The Sm core forms a stable ring structure comprising of seven Sm proteins [Citation25,Citation28], its assembly is initiated in the cytoplasm and is catalysed by the survival of motor neuron (SMN) complex [Citation29–31]. It is worth mentioning that Sm core is shared by other spliceosomal snRNPs (U2, U4 and U5) [Citation10,Citation24]. Apart from protein–RNA interaction, protein–protein interaction could also contribute to U1 snRNP assembly. For instance, U1C is believed to be recruited to U1 snRNP via its interaction with U1-70 K and Sm-D3 [Citation25,Citation32,Citation33], and Sm core formation is facilitated by the protein–protein interaction between Sm proteins and SMN complex [Citation31].
Figure 1. Schematic representation of components of U1 small nuclear ribonucleoprotein (snRNP) complex. The U1 small nuclear RNA (snRNA) is illustrated in black with secondary structures. m3Gppp shows U1 snRNA trimethyl cap. U1-specific proteins, including U1-70 K (green), U1-A(red) and U1-C(yellow), are shown with ellipses. Their positions correspond to their specific inter-molecular interactions (U1-70 K interacts with SL1; U1-A interacts with SLII; U1-C does not interact with U1 snRNA, while it is close to U1 snRNA 5ʹ-end and has contact with U1-70 K and Sm protein) . Sm proteins are shown with circles, they form a core and bind AUUUGUG sequence within U1 snRNA
Dynamics
In vivo, U1 snRNP is relatively stable in the nucleus, and much of our knowledge about U1 snRNP dynamics has been gained through the study of co-transcriptional mRNA splicing. U1 snRNP is intrinsically associated with RNA polymerase II (RNAP II) [Citation34,Citation35], and a recent study has demonstrated that this association is mediated by protein fused-in-sarcoma (FUS) [Citation36]. Therefore, it is plausible that U1 snRNP might be recruited to gene promoter region when transcription starts. Once 5ʹ-splice site (5ʹ-SS) is transcribed, U1 snRNP could immediately recognize this sequence via its free U1 snRNA 5ʹ-end [Citation9]. Moreover, it has been reported that this base-pairing is also required for U1 telescripting [Citation19,Citation20]. While many studies have described the regulatory roles of SR proteins and hnRNP proteins in regulating the U1 positioning at 5ʹ-SS through direct targeting U1-specific proteins [Citation37–40], such as U1-70 K [Citation41], it remains poorly understood whether similar mechanisms could also be at play in U1 telescripting. After splicing activation, U1 snRNP dissociates from the 5ʹ-SS early in spliceosome assembly and is recycled for next round of splicing catalysis [Citation42].
A recent study has reported that U1 snRNP may function in tethering and mobilizing long noncoding RNAs (lncRNA) to chromatin [Citation43], suggesting there is at least a subset of cellular U1 snRNP associates with nuclear RNAs, independent of its mRNA processing activity. Following the same line of reasoning, other reported noncanonical functions of U1 snRNP might be attributed to its association with corresponding interaction targets. For instance, U1 snRNP could potentially associate with TFIIH and TAF-15 through U1 snRNA [Citation44,Citation45], thereby implicating its role in transcription initiation [Citation46]. Overall, it is plausible that different subsets of U1 snRNP may have divergent roles. However, little is understood regarding their distributions, cross-regulations and dynamic exchanges, if any.
Regulation
As mentioned earlier, U1 snRNP is abundant and stable in eukaryotic cells and plays an essential role in mRNA metabolism. Therefore, eukaryotic cells must have evolved a variety of mechanisms for maintaining its high expression level and homoeostasis. Firstly, cells have developed specific mechanisms for high expression of U1 snRNP individual component. For instance, snRNA gene promoters, including U1 snRNA promoter, contain an essential proximal sequence element that could promote its high-level transcription [Citation47,Citation48]. Furthermore, there is no intron in U1 snRNA-encoding region, and the transcription elongation process is independent of positive transcription elongation factor b, and the cis-element for U1 snRNA 3ʹ-end formation is a 3ʹ-box rather than a general human mRNA PAS signal [Citation47]. Secondly, U1-70 K could promote U1 snRNP Sm core assembly through highjacking SMN-Gemin2-Sm, suggesting a U1 snRNP–specific assembly pathway to promote its overabundance in comparison with other snRNPs [Citation49]. Thirdly, U1 snRNP–specific proteins may have autoregulation or cross-regulation mechanism to maintain its homoeostasis. For instance, U1A has been shown to autoregulate its expression by inhibiting the polyadenylation of its own pre-mRNA [Citation50,Citation51], and U1C could regulate U1-70 K isoform expression and thereby controls U1-70 K/U1C homoeostasis[Citation52].
Several studies have shown that, in addition to its canonical form, different isoforms and a variety of amino acid/nucleotide modifications of U1 components can be found in cellular U1 snRNP repertoire [Citation53–57]. Although the biological significance of these variants/modifications has not been determined, they are thought to be associated with U1 snRNP assembly and functions [Citation58]. Another hypothesis is that the diversity of U1 snRNP variants might be associated with its tissue-specific regulation, adding another layer of complexity of U1 snRNP in gene regulatory network [Citation59].
3. U1 snRNP telescripting: insights from study of individual PASs
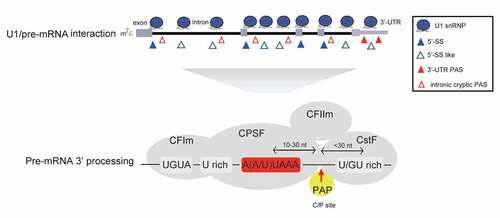
U1 telescripting relies on U1 snRNP inhibition of mRNA 3ʹ-end processing at thousands of PASs, most of which are located inside introns (). As mentioned earlier, research history of cross-regulation of splicing and mRNA 3ʹ-end processing dates back to the 1990s [Citation60,Citation61], when scientists were interested in how the two major mRNA processing events are coordinately regulated, at the end of mRNA transcription. However, most early studies tended to only decipher the mechanism for U1 snRNP inhibition of mRNA 3ʹ-end processing using in vitro and biochemical assays in the context of individual PAS RNAs, owing to technical limitations. Since canonical mRNA 3ʹ-end processing comprises two tightly connected processes, namely cleavage at the PAS and subsequent addition of a stretch of A to the cleaved pre-mRNA (cleavage and polyadenylation) [Citation11], we discuss how U1 snRNP inhibits cleavage or polyadenylation steps separately.
Figure 2. U1 snRNP inhibits cryptic PAS processing. U1 snRNP associates with pre-mRNA at many 5ʹ-SS or 5ʹ-SS like sites through its free U1 snRNA 5ʹ-end, which may inhibit the 3ʹ-end processing of downstream PAS and prevent PCPA. In addition to canonical PASs at 3ʹ-UTR, pre-mRNAs harbour many intronic cryptic PASs. Based on current knowledge, mRNA 3ʹ-end processing is carried out within mRNA 3ʹ-end processing complex, which includes trans-acting 3ʹ processing factors and core PAS cis-elements. It is generally believed that 3ʹ-UTR canonical PASs and intronic cryptic PASs share similar core cis-elements (AAUAAA motif, U/GU-rich sequences etc.), and the specific protein–RNA interactions within the 3ʹ processing complex are crucial for this process. (CPSF: cleavage and polyadenylation specificity factor; CstF: cleavage stimulation factor; CFIm: cleavage factor Im; CFIIm: cleavage factor IIm; PAP: poly(A) polymerase)
3.1 U1 snRNP inhibition of the cleavage step of mRNA 3ʹ-end processing
Previous studies have shown that human canonical mRNA 3ʹ-end processing is performed by a 3ʹ processing complex (), comprising four core protein sub-complexes (CPSF, CstF, CFIm and CFIIm), a polyA polymerase and dozens of other loosely associated proteins [Citation11,Citation62]. Functionally, CPSF recognizes the PAS core cis-element AAUAAA, via WDR33 and CPSF30 [Citation63–65], and cleaves the reaction through CPSF73 [Citation66]. Moreover, CstF binds U/GU-rich PAS region ~20 nt, downstream of the cleavage site, and stimulates the cleavage reaction, possibly by promoting CPSF recruitment or stabilizing 3ʹ processing/PAS structures poised for cleavage reaction [Citation66–68]. In addition, CFIm binds UGUA sequence and serves as an enhancer-dependent activator that directly functions by interacting with CPSF [Citation69–71]. In contrast to other sub-complexes, CFIIm’s function remains poorly characterized, although some reports have suggested that it connects mRNA 3ʹ-end processing with transcription termination, in a process that involves RNAP II disassembly from a DNA template [Citation72–74].
If U1 snRNP inhibits the cleavage step of canonical mRNA 3ʹ-end processing, one may expect that it may either interfere with the 3ʹ processing complex assembly on PAS cis-elements or directly inhibit the cleavage reaction. Indeed, evidences from several studies have corroborated this assumption (). For example, Niwa et al. (1992) evaluated an exon-scanning mechanism for splice site selection and observed that CstF64, a component of CstF subcomplex, was less recruited if a 5ʹ-SS was inserted into a last terminal exon pre-mRNA substrate [Citation60]. This effect was largely dependent on the position and direction of 5ʹ-SS relative to AAUAAA element. Interestingly, a recent study using another endogenous PAS RNA substrate from wdr26 gene showed similar results [Citation75]. However, these studies did not answer several questions. For example, in both cases, addition of a 5ʹ-SS to the 3ʹ-end of the RNA substrate did not affect CstF64 binding and had no inhibitory effect, indicating a simple U1 snRNP steric hindrance model does not fully explain U1 inhibition of CstF64 recruitment. One possible explanation for this orientation of 5ʹ-SS effect is that U1 snRNP’s binding to 5ʹ-SS might generate downstream effects that function in a 5ʹ->3ʹ direction and impact CstF64 recruitment. Moreover, in the case of wdr26 PAS RNA, U1A appears to directly inhibit PAS cleavage via its RNA-binding ability. Nevertheless, it is technically difficult to fully exclude the possibility that this inhibition might be caused by snRNP-free U1A protein (). Additionally, there is no evidence that U1A depletion may reproduce U1 AMO effect. Another example comes from the study involving a complex immunodeficiency syndrome caused by downregulated p14/robld3 [Citation76]. Specifically, a point mutation upstream of p14/robld3 PAS region reportedly created a U1-binding site, causing inefficient cleavage of p14 pre-mRNA at the PAS site. However, the mechanism through which U1 snRNP interferes with 3ʹ-end cleavage remains unknown. Notably, the orientation of 5ʹ-SS and PAS in the aforementioned RNA substrates is similar to what has been observed in U1 telescripting. Despite the flaws, it is evident that these studies provide relevant insights into the mechanism of U1 telescripting.
Figure 3. U1 snRNP or U1 component inhibits pre-mRNA 3ʹ-end processing in the context of multiple PASs. (A) U1 snRNP inhibits the cleavage step of mRNA 3ʹ-end processing for the PASs of indicated genes. 5ʹ-SS and C/P site are indicated with filled rectangle and arrow, respectively, and their orientations and distances are also indicated. (B) U1 snRNP inhibits the polyadenylation step in the context of BPV/L3 PASs, which is mediated by direct interaction between U1-70 K and poly(A) polymerase (PAP). (C) U1A inhibits the cleavage or polyadenylation step of mRNA 3ʹ-end processing in the context of indicated PASs. Cleavage/polyadenylation (C/P) sites are indicated with red arrows
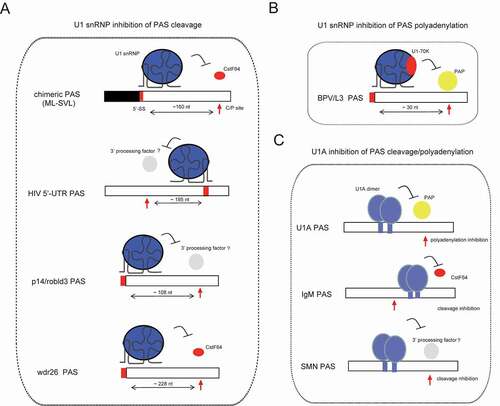
Notably, orientation of U1 effect might be sequence-dependent. For example, HIV-1 pre-mRNA’s 5ʹ-untranlsated region (5ʹ-UTR) harbours a U1-binding site and a promoter-proximal PAS, with the direction of 5ʹ-SS relative to PAS shown to be opposite to that of the aforementioned substrates [Citation77]. During the HIV life cycle, this PAS cleavage must be inhibited to maximize downstream gene expression [Citation17,Citation18]. Interestingly, previous studies have suggested that this effect is caused by a sequence between cap and PAS core element AAUAAA, although the actual underlying mechanism remains unknown [Citation78]. Insertion of 5ʹ-SS, downstream of cleavage site of adenovirus L3 PAS, has been shown to inhibit the PAS cleavage reaction, in a similar fashion to HIV-1 5ʹ-UTR PAS [Citation79]. Although this study found that U1 snRNP did not impair the binding of characterized cleavage factors, such as CPSF, CstF and CFIm, other possibilities could not be excluded. For example, it is possible that U1 snRNP might affect the assembly process or change their spatial conformation around PAS, indicating that the mechanisms through which U1 snRNP inhibits mRNA 3ʹ-end processing might not be limited to steric hindrance [Citation60,Citation75]. On the other hand, several reports have shown that U1 snRNP proteins may directly interact with 3ʹ-end cleavage factors, such as U1A/CPSF160 and U1-70 K/CFIm25 [Citation80,Citation81], providing an attractive mechanism for U1 inhibition of the PAS cleavage. However, the functional impact of this interaction remains elusive. For instance, previous evidence indicates that U1A may be facilitating, rather than inhibiting, mRNA polyadenylation by interacting with CPSF160 in the context of the canonical SVL PAS RNA [Citation80].
3.2 U1 snRNP inhibits the polyadenylation step of mRNA 3ʹ-end processing
In contrast to U1 snRNP inhibition of the cleavage step, the mechanism for U1 snRNP’s inhibition of the polyadenylation step of mRNA 3ʹ-end processing has been solely attributed to improper recruitment/positioning of polyA polymerase (PAP), which is the only 3ʹ processing factor executing the function in polyadenylation step based on in vitro models [Citation62,Citation82]. Particularly, U1A/U1-70 K specifically interacts with PAP, and this is sufficient to block polyadenylation in the context of several PASs, including BPV and L3 PAS () [Citation51,Citation83]. Based on these, the interaction between U1A/U1-70 K and PAP is believed to be responsible for U1 snRNP–mediated inhibition of mRNA polyadenylation. Although U1 telescripting mainly depends on U1 snRNP inhibition of the cleavage step of intronic PASs to maintain full-length pre-mRNA transcription, it might also provide another layer of mRNA quality control mechanism through U1 snRNP-mediated inhibition of polyadenylation. In this scenario, pre-mRNAs might be cleaved, but not polyadenylated, with non-polyadenylated products forming targets for the RNA exosome [Citation84]. To support the generality of U1 snRNP-mediated inhibition of polyadenylation, a U1 interference method was developed to knock down gene expression by targeting sequences near the PAS region [Citation85,Citation86].
Overall, previous studies have demonstrated that U1 snRNP inhibits mRNA 3ʹ-end processing both in vitro and in cells in the context of multiple PAS RNAs, possibly through an interaction between U1 snRNP with PASs or core 3ʹ processing factors. Although results from in vitro assays at a given RNA context are useful, these studies have obvious limitations in deciphering the underlying molecular mechanisms of U1 telescripting. For example, most, if not all, of U1 telescripting events are accompanied by transcription and mRNA splicing [Citation22,Citation23], although the mechanism through which U1 snRNP-mediated splicing and telescripting are coordinately regulated remains poorly understood. In addition, U1 inhibits cleavage of thousands of cryptic PASs in mammalian cells, which implies the existence of a unified mechanism, whereas conclusions from studies using individual PASs tend to be sequence-dependent.
4. U1 snRNP telescripting: insights from transcriptomic analysis
Transcriptome-wide analyses have affirmed that U1 telescripting is a general and widespread phenomenon in mammalian gene regulation. This process has been comprehensively characterized in publications from Dreyfuss laboratory [Citation19–21,Citation87]. Recently, several groups have accurately mapped these cryptic PASs using 3ʹ-end sequencing (3ʹ-seq) [Citation88–90]. Generally, 3ʹ-seq complements microarray/RNA-seq and provides valuable information for U1-regulated PASs, regarding their sequences and positions. As expected, these cryptic PASs, often utilized in normal tissues, share similar core cis-element with canonical PASs located in 3ʹ-UTR [Citation88,Citation91]. More importantly, the upstream nature 5ʹ-SS is often within 1000 nt and peaks around 250 nt [Citation88], suggesting an interplay between mRNA splicing and inhibition of intronic PAS. However, it is not known whether U1 snRNP binding at 5ʹ-SS plays dual roles in splicing and telescripting and, if so, how are both processes coordinated (hereafter called splicing-dependent U1 telescripting)? In other cases, especially for large introns, U1 snRNP might bind non-5ʹ-SS within pre-mRNA intron to prevent premature cleavage and polyadenylation (PCPA) (hereafter called splicing-independent U1 telescripting). Given the range of U1 protection of cryptic PASs [Citation19,Citation20,Citation88], we discuss how existing models may explain both scenarios. Furthermore, we highlight their strengths and potential shortcomings.
U1–CPAF (cleavage and polyadenylation factors) model
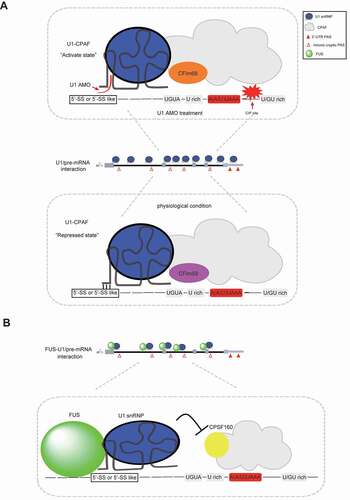
Dreyfuss and colleagues proposed a U1–CPAF model for elucidating the mechanism of U1 telescripting, based on findings from several key experiments () [Citation92]. Firstly, they captured a U1–CPAF protein complex by cross-linked immunoprecipitations and mass spectrometric analysis. Secondly, they performed RNA Immunoprecipitation (RIP-seq) analysis to reveal that U1 and CPAF share similar binding region at intronic PCPA sites. Thirdly, they demonstrated that U1 AMO treatment promotes PAS processing activity of the U1–CPAF complex, and this process is partly achieved through enhanced CFIm68 recruitment. In this model, U1–CPAF bind 5ʹ-SS/5ʹ-SS-like sequence and cryptic PAS through U1 and CPAF, respectively, while U1 and CPAF have a direct protein–protein interaction. This proposal expands our knowledge regarding U1 snRNP telescripting and provides an explanation for most of the data reported in previous works. However, several questions, including the following, remain unanswered: (i) What are the actual modes of U1–CPAF assembly and specific changes of U1–CPAF following U1 AMO treatment? (ii) How does U1–CPAF bind to both 5ʹ-SS and PCPA sites in the scenario of splicing-dependent U1 telescripting, especially when these two sites are not adjacent to each other? (iii) How strong is the interaction between U1 and CPAF, and does their co-localization at PCPA sites fully exclude the possibility that U1 in suppressing PCPA through steric hindrance? (iv) Treatment of transcription elongation inhibitor, including DRB or flavopiridol, could elicit PCPA [Citation22], suggesting U1 telescripting mechanisms involve transcription regulation, while the mechanism for U1–CPAF regulation of transcription remains largely unknown. Nevertheless, it is possible that U1–CPAF might function as an entity, under some circumstances, especially when U1- and CPAF-binding sites are close to each other. In addition, some factors, such as RNA secondary structures and RNA-binding proteins, might bring the binding sites together if they are far away from each other. Its function in transcription may be attributed to its association with other proteins, although a detailed experimental exploration is required to confirm this.
Figure 4. Schematic representation of reported models in elucidating U1 telescripting mechanism. (A) U1–CPAF (U1–cleavage and polyadenylation factors) model. Under physical conditions, U1–CPAF forms a complex near intronic cryptic PASs region, and its PAS processing activity is inhibited. In contrast, U1–CPAF becomes active upon U1 AMO treatment, resulting in efficient C/P at intronic PASs. This U1 AMO-mediated activation of U1–CPAF may be at least partially caused by the altered CPAF components. For instance, CFIm68, instead of CFIm59, accumulates in CPAF upon U1 AMO treatment. (B) FUS–U1 co-inhibition model. FUS and U1 snRNP form a complex, bind sequences upstream of intronic cryptic PASs and consequently inhibit the downstream PAS processing. This inhibitory effect may be caused by impaired recruitment of core 3ʹ processing factors, such as CPSF160
Co-transcriptional FUS–U1 inhibition model
Protein FUS is a member of the FET family, which comprise ubiquitously expressed RNA-binding proteins associated with human cancers and neurological disorders [Citation93,Citation94]. Previous studies have shown that FUS plays various roles in gene transcription and mRNA processing, through interactions with some core mRNA transcription and processing factors [Citation95–97]. Specifically, FUS interacts with U1 snRNP and RNAP II machinery, playing a general role in the coupling of splicing and transcription [Citation36]. Recently, Ohno and colleagues proposed a co-transcriptional FUS–U1 inhibition model, thereby revealing a new mechanism of U1 telescripting () [Citation89]. Specifically, their experimental works revealed the following conclusions: (i) targeted RNA immunoprecipitation sequencing revealed that FUS and U1 snRNP share similar or neighbouring binding sites, upstream of cryptic PASs. (ii) FUS depletion caused a similar global PCPA effect, in a similar fashion to that observed following U1 AMO–mediated functional depletion of U1 snRNP. (iii) Knockdown of either FUS or U1 snRNP reduced the binding of its counterpart to target region upstream of PCPA. Although this model does not fully explain how FUS or U1 snRNP represses the usage of cryptic PASs, it provides several interesting insights into guide future studies. For example, considering that U1 AMO and FUS siRNA treatment generate similar PCPA effects, what is nature of the relationship between them? In other words, is it possible that one is the cause or consequence of the other? This hypothesis is important as could provide relevant insights into the underlying mechanism of U1 telescripting. In addition, this model indicates a direct blocking model, where FUS–U1 inhibits recruitment of a core 3ʹ processing factor CPSF160 on cryptic PASs. However, the underlying mechanism remains unknown, especially considering that the distance between FUS–U1 binding and CPSF recognition sites within pre-mRNA cannot be neglected. Furthermore, it would be interesting to know whether recruitment of CPSF160 on DNA is also activated upon U1 AMO/FUS siRNA treatment. The answer is expected to differentiate between the following two scenarios: (i) whether FUS/U1 directly blocks co-transcriptional recruitment of core 3ʹ processing factors and (ii) whether FUS/U1 blocks termination of transcription at cryptic PASs, thereby indirectly inhibiting assembly of core 3ʹ processing factors on cryptic PASs. Aside from CPSF160, a comprehensive understanding of DNA/RNA binding profiles of other core 3ʹ processing factors, at cryptic PASs, would be helpful in elucidating the assembly of 3ʹ processing complex, which is a key functional unit in canonical mRNA 3ʹ processing. Lastly, the model fails to mention how these processes are coordinated despite the key roles played by both FUS and U1 in splicing and U1 telescripting [Citation98]. Although the details remain sketchy, the co-transcriptional FUS–U1 inhibition model suggests an intricate interplay among FUS, U1 snRNP, 3ʹ processing factors and RNAP II movement along pre-mRNA.
A preliminary U1-centred steric hindrance model
As mentioned earlier, another interpretation of overlapping binding for U1 and CPAF complex at PCPA sites is that U1 might compete with CPAF in binding cryptic PAS region [Citation92], thereby suppressing CPAF’s PAS processing activity. In keeping with this, individual-nucleotide resolution UV crosslinking and immunoprecipitation, coupled with RNA sequencing analysis using U1A antibody, have revealed moderate enrichment of U1A binding near PAS site [Citation75]. This U1A–RNA interaction is believed to play a direct role in PAS suppression. This preliminary U1-centred steric hindrance model may further extend to the scenario of co-transcriptional FUS–U1 inhibition model, as evidenced by elevated CPSF160 binding signal at cryptic PASs upon FUS or U1 depletion [Citation89]. In fact, this preliminary model aims to confirm that U1, together with other interaction partners, such as FUS, RNAP II, and other associated factors, might form a molecular juggernaut that prevents assembly of the functional core 3ʹ processing complex on cryptic PASs during transcription [Citation99]. Previous studies have shown that some factors, such as RNA structures and phase separation of individual components, might increase the enormity of this U1-centred structure. This attractive model might partially explain why specific transcription inhibitors could also elicit PCPA [Citation22]. Plausibly, transcription error may disrupt the U1-centred complex across cryptic PAS, thereby making PAS core cis-elements accessible to the 3ʹ processing machinery and ultimately leading to efficient PCPA. In addition, it might also provide an explanation for the variations among available U1-RIP-seq data. For example, U1 snRNP binding across pre-mRNA might be sensitive to treatment difference during RIP-seq library preparation, given the dynamic feature of U1-centred complex in the context of transcription. However, the U1-centred steric hindrance model requires additional experimental validation, just like many other theoretical models. For example, since accumulating evidences suggest that U1 AMO may disrupt U1 snRNP structure [Citation19,Citation36,Citation92], unravelling the details of structural rearrangement within U1-associated complex would be an interesting prospect. Apart from FUS, discovery of any other U1-interacting proteins that play similar roles in PCPA as well as unravelling the mechanisms through which U1 and U1-interacting proteins occupy cryptic PAS positions under normal physiological conditions would enrich the current body of knowledge.
5. U1 snRNP telescripting: a perspective from co-transcriptional mRNA 3ʹ processing complex assembly and dynamics
In the context of in vivo co-transcriptional mRNA processing, three perspectives are suggested to explain the inefficient usage of intronic PASs under normal physiological conditions. Firstly, intact 3ʹ processing complex is not available near intronic PASs; secondly, 3ʹ processing complex is present near intronic PASs, but it is not functional in PAS processing and thirdly, the 3ʹ processing complex is active near intronic PAS, while the cleavage and polyadenylation reactions occur following transcription of intronic PAS. However, the polyadenylated product is immediately degraded along with intron removal. The last scenario is, however, excluded based on available evidences: particularly, (i) transcription integrity would be lost if pre-mRNA is cleaved within intron [Citation20]; (ii) there currently exists no clear polyadenylation-assisted degradation mechanism for mammalian mRNA quality control in its canonical maturation pathway [Citation100] and (iii) existence of a degradation process should be related with intron removal. However, previous works indicate that treatment of splicing inhibitors, such as U2 AMO, did not reveal any PCPA effect [Citation19]. To better understand the underlying mechanism of U1 telescripting, we propose in vivo characterization of the dynamic assembly of 3ʹ processing factors before and after PCPA induction.
In vivo assembly of 3ʹ processing factors on pre-mRNA entails co-transcription regulated by various mechanisms (). For example, CPSF interacts with transcription pre-initiation complex, through TFIID, and potentially transfers to RNAP II carboxy-terminal domain (CTD) during transcription elongation [Citation101,Citation102]. Both CstF and CFIm interact with RNAPII, thereby joining transcription elongation [Citation102–104]. Notably, CTD Ser2 phosphorylation (Ser2-P) enhances recruitment of 3ʹ processing factors towards the end of transcription [Citation14,Citation105,Citation106]. Once transcription passes the PAS, RNAP II pauses and most likely fully assembles the 3ʹ processing complex that acts on PAS [Citation102]. Although several evidences have demonstrated that some core 3ʹ processing factors bind to intronic PAS [Citation67,Citation107], little is known regarding assemblage of intact 3ʹ processing complex. Unravelling the mode of 3ʹ processing complex’s full assembly on PAS is a prerequisite for mRNA 3ʹ processing, based on current in vitro model. Insights into this are expected to provide two potential directions for investigating the underlying mechanism of U1 telescripting. Specifically, how does U1 AMO activate the assembly of intact 3ʹ processing complex on cryptic PAS, in case intact 3ʹ processing complex is not fully assembled? Alternatively, how does U1 blocking activate 3ʹ processing complex’s functional activity in the event that intact 3ʹ processing complex is present, but is clearly not functional? In other words, how does U1 inhibit PAS processing activity?
Figure 5. Recruitment of core mRNA processing factors (mRNA 3ʹ-end processing factors, U1 snRNP) in the context of co-transcriptional mRNA processing. CPSF connects with transcription pre-initiation through general transcription factors (TFIID) and transfers to RNA polymerase II (RNAP II) during transcription initiation. Other mRNA processing factors (CstF, CFIm and U1 snRNP/FUS) may also direct bind RNAP II during transcription pre-initiation/initiation/elongation. These stepwise co-transcriptional events may increase local concentration of mRNA processing factors and thereby promote the mRNA processing efficiency once RNA is transcribed. mRNA termination is coupled with mRNA 3ʹ-end processing at canoical PAS within 3ʹ-UTR, during which fully assembled 3ʹ processing complex recognizes and processes PAS efficiently
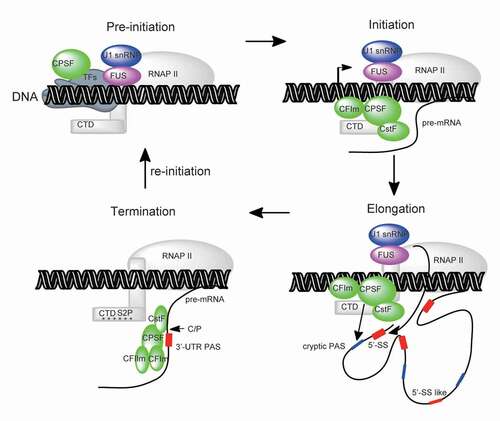
To add further complexity to the mechanism, U1 snRNP interacts with RNAP II, during transcription [Citation34,Citation35]; hence, its competition with 3ʹ processing factors for RNAP II binding might begin with transcription initiation or elongation. Given that U1 AMO treatment disrupts U1 snRNP interaction with RNAP II in vitro [Citation36], it appears plausible that U1 AMO could also produce similar effects in vivo. If so, this might increase co-transcriptional RNAP II’s chances of interacting with 3ʹ processing factors and consequently promote assembly of the 3ʹ processing complex following cryptic PAS’s transcription. Consistent with this theory, previous studies have shown that FUS depletion promotes Ser2-P of RNAP II CTD [Citation108]. In fact, this process facilitates recruitment of 3ʹ processing factors in transcription termination [Citation14,Citation106,Citation109,Citation110]. Future studies are expected to explore how different treatments, such as U1 AMO, transcription inhibitor and FUS depletion, among others, could cause similar effects on assembly of the 3ʹ processing complex and function on cryptic PASs.
Conclusions
Dreyfuss’ research group proposed the concept of U1 telescripting, based on U1 inhibition of PCPA, and made tremendous efforts in characterizing its impact on the mammalian transcriptome. Despite recent progress, several key questions regarding the basic underlying mechanisms of this important phenomenon, as well as its regulation and function, remain. Firstly, apart from U1/FUS’s role in global repression of PCPA, what are some of the proteins that can inhibit PCPA for specific genes? Future studies are expected to reveal more factors associated with global/gene-specific PCPA repression. It will be interesting to understand whether they share a unifying PCPA inhibition mechanism. Secondly, is U1 telescripting intrinsically linked with mRNA alternative polyadenylation (APA), a widespread phenomenon characteristic of tissue-/disease-specific expression pattern in mammalian cells [Citation111–113]? Unravelling how and to what extent U1 telescripting regulation contribute to the diverse APA patterns across different normal and diseases-associated tissues is an interesting prospect for future investigations. To address this, it will be imperative to combine individual gene analysis, with functional genomics, and cell/diseases modelling systems. Thirdly, what are the fates and functions of thousands of PCPAed mRNA products, following inactivation of U1 telescripting? This question is in line with several disciplines of RNA biology, including mRNA decay, splicing, export and translation. A combination of systematic approaches, such as ribosome profiling sequencing and quantitative mass spectrometry, with gene-specific functional studies will undoubtedly provide insights into answer these questions. Overall, the U1 telescripting concept expands our understanding of co-transcriptional mRNA processing and opens up frontiers for new research.
Disclosure statement
The authors have declared no conflicts of interest for this article.
Additional information
Funding
References
- Berget SM, Moore C, Sharp PA. Spliced segments at the 5ʹ terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977;74:3171–3175.
- Chow LT, Gelinas RE, Broker TR, et al. An amazing sequence arrangement at the 5ʹ ends of adenovirus 2 messenger RNA. Cell. 1977;12:1–8.
- Brody E, Abelson J. The “spliceosome”: yeast pre-messenger RNA associates with a 40S complex in a splicing-dependent reaction. Science. 1985;228:963–967.
- Frendewey D, Keller W. Stepwise assembly of a pre-mRNA splicing complex requires U-snRNPs and specific intron sequences. Cell. 1985;42:355–367.
- Grabowski PJ, Seiler SR, Sharp PA. A multicomponent complex is involved in the splicing of messenger RNA precursors. Cell. 1985;42:345–353.
- Mount SM, Pettersson I, Hinterberger M, et al. The U1 small nuclear RNA-protein complex selectively binds a 5ʹ splice site in vitro. Cell. 1983;33:509–518.
- Zhuang Y, Weiner AM. A compensatory base change in U1 snRNA suppresses a 5ʹ splice site mutation. Cell. 1986;46:827–835.
- Kondo Y, Oubridge C, van Roon AM, et al. Crystal structure of human U1 snRNP, a small nuclear ribonucleoprotein particle, reveals the mechanism of 5ʹ splice site recognition. In: Elife. 2015;4:e04986.
- Wilkinson ME, Charenton C, Nagai K. RNA splicing by the spliceosome. Annu Rev Biochem. 2020;89:359–388.
- Plaschka C, Lin PC, Charenton C, et al. Prespliceosome structure provides insights into spliceosome assembly and regulation. Nature. 2018;559:419–422.
- Colgan DF, Manley JL. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 1997;11:2755–2766.
- Bentley DL. The union of transcription and mRNA processing: 20 years of coupling. RNA. 2015;21:569–570.
- McCracken S, Fong N, Yankulov K, et al. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature. 1997;385:357–361.
- Licatalosi DD, Geiger G, Minet M, et al. Functional interaction of yeast pre-mRNA 3ʹ end processing factors with RNA polymerase II. Mol Cell. 2002;9:1101–1111.
- Tellier M, Maudlin I, Murphy S. Transcription and splicing: A two-way street. Wiley Interdiscip Rev RNA. 2020;11:e1593.
- Furth PA, Choe WT, Rex JH, et al. Sequences homologous to 5ʹ splice sites are required for the inhibitory activity of papillomavirus late 3ʹ untranslated regions. Mol Cell Biol. 1994;14:5278–5289.
- Ashe MP, Griffin P, James W, et al. Poly(A) site selection in the HIV-1 provirus: inhibition of promoter-proximal polyadenylation by the downstream major splice donor site. Genes Dev. 1995;9:3008–3025.
- Ashe MP, Pearson LH, Proudfoot NJ. The HIV-1 5ʹ LTR poly(A) site is inactivated by U1 snRNP interaction with the downstream major splice donor site. Embo J. 1997;16:5752–5763.
- Kaida D, Berg MG, Younis I, et al. U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature. 2010;468:664–668.
- Berg MG, Singh LN, Younis I, et al. U1 snRNP determines mRNA length and regulates isoform expression. Cell. 2012;150:53–64.
- Oh JM, Di C, Venters CC, et al. U1 snRNP telescripting regulates a size-function-stratified human genome. Nat Struct Mol Biol. 2017;24:993–999.
- Venters CC, Oh JM, Di C, et al. U1 snRNP telescripting: suppression of premature transcription termination in introns as a new layer of gene regulation. Cold Spring Harb Perspect Biol. 2019;11(2):a032235.
- Di C, So BR, Cai Z, et al. U1 snRNP telescripting roles in transcription and its mechanism. Cold Spring Harb Symp Quant Biol. 2019;84:115–122.
- Lerner MR, Steitz JA. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979;76:5495–5499.
- Pomeranz Krummel DA, Oubridge C, Leung AK, et al. Crystal structure of human spliceosomal U1 snRNP at 5.5 A resolution. Nature. 2009;458:475–480.
- Surowy CS, van Santen VL, Scheib-Wixted SM, et al. Direct, sequence-specific binding of the human U1-70K ribonucleoprotein antigen protein to loop I of U1 small nuclear RNA. Mol Cell Biol. 1989;9:4179–4186.
- Tsai DE, Harper DS, Keene JD. U1-snRNP-A protein selects a ten nucleotide consensus sequence from a degenerate RNA pool presented in various structural contexts. Nucleic Acids Res. 1991;19:4931–4936.
- Weber G, Trowitzsch S, Kastner B, et al. Functional organization of the Sm core in the crystal structure of human U1 snRNP. Embo J. 2010;29:4172–4184.
- Chari A, Golas MM, Klingenhager M, et al. An assembly chaperone collaborates with the SMN complex to generate spliceosomal snRNPs. Cell. 2008;135:497–509.
- Fischer U, Liu Q, Dreyfuss G. The SMN–SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell. 1997;90(6):1023–1029.
- Liu Q, Fischer U, Wang F, et al. The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell. 1997;90:1013–1021.
- Nelissen RL, Will CL, van Venrooij WJ, et al. The association of the U1-specific 70K and C proteins with U1 snRNPs is mediated in part by common U snRNP proteins. Embo J. 1994;13:4113–4125.
- Schwer B, Shuman S. Structure-function analysis and genetic interactions of the Yhc1, SmD3, SmB, and Snp1 subunits of yeast U1 snRNP and genetic interactions of SmD3 with U2 snRNP subunit Lea1. RNA. 2015;21:1173–1186.
- Das R, Yu J, Zhang Z, et al. SR proteins function in coupling RNAP II transcription to pre-mRNA splicing. Mol Cell. 2007;26:867–881.
- Chi B, O’Connell JD, Yamazaki T, et al. Interactome analyses revealed that the U1 snRNP machinery overlaps extensively with the RNAP II machinery and contains multiple ALS/SMA-causative proteins. Sci Rep. 2018;8:8755.
- Yu Y, Reed R. FUS functions in coupling transcription to splicing by mediating an interaction between RNAP II and U1 snRNP. Proc Natl Acad Sci U S A. 2015;112:8608–8613.
- Lin S, Fu XD. SR proteins and related factors in alternative splicing. Adv Exp Med Biol. 2007;623:107–122.
- Zhong XY, Wang P, Han J, et al. SR proteins in vertical integration of gene expression from transcription to RNA processing to translation. Mol Cell. 2009;35:1–10.
- Busch A, Hertel KJ. Evolution of SR protein and hnRNP splicing regulatory factors. Wiley Interdiscip Rev RNA. 2012;3:1–12.
- Shenasa H, Movassat M, Forouzmand E, et al. Allosteric regulation of U1 snRNP by splicing regulatory proteins controls spliceosomal assembly. RNA. 2020;26:1389–1399.
- Cho S, Hoang A, Sinha R, et al. Interaction between the RNA binding domains of Ser-Arg splicing factor 1 and U1-70K snRNP protein determines early spliceosome assembly. Proc Natl Acad Sci U S A. 2011;108:8233–8238.
- Shi Y. Mechanistic insights into precursor messenger RNA splicing by the spliceosome. Nat Rev Mol Cell Biol. 2017;18:655–670.
- Yin Y, Lu JY, Zhang X, et al. U1 snRNP regulates chromatin retention of noncoding RNAs. Nature. 2020;580:147–150.
- Kwek KY, Murphy S, Furger A, et al. U1 snRNA associates with TFIIH and regulates transcriptional initiation. Nat Struct Biol. 2002;9:800–805.
- Jobert L, Pinzon N, Van Herreweghe E, et al. Human U1 snRNA forms a new chromatin-associated snRNP with TAF15. EMBO Rep. 2009;10:494–500.
- Spiluttini B, Gu B, Belagal P, et al. Splicing-independent recruitment of U1 snRNP to a transcription unit in living cells. J Cell Sci. 2010;123:2085–2093.
- Jawdekar GW, Henry RW. Transcriptional regulation of human small nuclear RNA genes. Biochim Biophys Acta. 2008;1779:295–305.
- Jensen RC, Wang Y, Hardin SB, et al. The proximal sequence element (PSE) plays a major role in establishing the RNA polymerase specificity of Drosophila U-snRNA genes. Nucleic Acids Res. 1998;26:616–622.
- So BR, Wan L, Zhang Z, et al. A U1 snRNP-specific assembly pathway reveals the SMN complex as a versatile hub for RNP exchange. Nat Struct Mol Biol. 2016;23:225–230.
- Boelens WC, Jansen EJ, van Venrooij WJ, et al. The human U1 snRNP-specific U1A protein inhibits polyadenylation of its own pre-mRNA. Cell. 1993;72:881–892.
- Gunderson SI, Beyer K, Martin G, et al. The human U1A snRNP protein regulates polyadenylation via a direct interaction with poly(A) polymerase. Cell. 1994;76:531–541.
- Rosel-Hillgartner TD, Hung LH, Khrameeva E, et al. A novel intra-U1 snRNP cross-regulation mechanism: alternative splicing switch links U1C and U1-70K expression. PLoS Genet. 2013;9:e1003856.
- Tazi J, Kornstadt U, Rossi F, et al. Thiophosphorylation of U1-70K protein inhibits pre-mRNA splicing. Nature. 1993;363:283–286.
- Cheng D, Cote J, Shaaban S, et al. The arginine methyltransferase CARM1 regulates the coupling of transcription and mRNA processing. Mol Cell. 2007;25:71–83.
- Hernandez H, Makarova OV, Makarov EM, et al. Isoforms of U1-70k control subunit dynamics in the human spliceosomal U1 snRNP. PLoS One. 2009;4:e7202.
- O’Reilly D, Dienstbier M, Cowley SA, et al. Differentially expressed, variant U1 snRNAs regulate gene expression in human cells. Genome Res. 2013;23:281–291.
- Guiro J, O’Reilly D. Insights into the U1 small nuclear ribonucleoprotein complex superfamily. Wiley Interdiscip Rev RNA. 2015;6:79–92.
- McConnell TS, Lokken RP, Steitz JA. Assembly of the U1 snRNP involves interactions with the backbone of the terminal stem of U1 snRNA. RNA. 2003;9:193–201.
- Fukumura K, Taniguchi I, Sakamoto H, et al. U1-independent pre-mRNA splicing contributes to the regulation of alternative splicing. Nucleic Acids Res. 2009;37:1907–1914.
- Niwa M, MacDonald CC, Berget SM. Are vertebrate exons scanned during splice-site selection? Nature. 1992;360:277–280.
- Wassarman KM, Steitz JA. Association with terminal exons in pre-mRNAs: a new role for the U1 snRNP? Genes Dev. 1993;7:647–659.
- Shi Y, Di Giammartino DC, Taylor D, et al. Molecular architecture of the human pre-mRNA 3ʹ processing complex. Mol Cell. 2009;33:365–376.
- Chan SL, Huppertz I, Yao CG, et al. CPSF30 and Wdr33 directly bind to AAUAAA in mammalian mRNA 3 ‘ processing. Genes Dev. 2014;28:2370–2380.
- Schonemann L, Kuhn U, Martin G, et al. Reconstitution of CPSF active in polyadenylation: recognition of the polyadenylation signal by WDR33. Genes Dev. 2014;28:2381–2393.
- Sun Y, Zhang Y, Hamilton K, et al. Molecular basis for the recognition of the human AAUAAA polyadenylation signal. Proc Natl Acad Sci U S A. 2018;115:E1419–E28.
- Mandel CR, Kaneko S, Zhang H, et al. Polyadenylation factor CPSF-73 is the pre-mRNA 3ʹ-end-processing endonuclease. Nature. 2006;444:953–956.
- Yao C, Biesinger J, Wan J, et al. Transcriptome-wide analyses of CstF64-RNA interactions in global regulation of mRNA alternative polyadenylation. Proc Natl Acad Sci U S A. 2012;109:18773–18778.
- Bai Y, Auperin TC, Chou CY, et al. Crystal structure of murine CstF-77: dimeric association and implications for polyadenylation of mRNA precursors. Mol Cell. 2007;25:863–875.
- Dettwiler S, Aringhieri C, Cardinale S, et al. Distinct sequence motifs within the 68-kDa subunit of cleavage factor Im mediate RNA binding, protein-protein interactions, and subcellular localization. J Biol Chem. 2004;279:35788–35797.
- Yang Q, Gilmartin GM, Doublie S. Structural basis of UGUA recognition by the Nudix protein CFI(m)25 and implications for a regulatory role in mRNA 3ʹ processing. Proc Natl Acad Sci U S A. 2010;107:10062–10067.
- Zhu Y, Wang X, Forouzmand E, et al. Molecular mechanisms for CFIm-mediated regulation of mRNA alternative polyadenylation. Mol Cell. 2018;69:62–74 e4.
- Meinhart A, Cramer P. Recognition of RNA polymerase II carboxy-terminal domain by 3ʹ-RNA-processing factors. Nature. 2004;430:223–226.
- Zhang Z, Gilmour DS. Pcf11 is a termination factor in Drosophila that dismantles the elongation complex by bridging the CTD of RNA polymerase II to the nascent transcript. Mol Cell. 2006;21:65–74.
- Schafer P, Tuting C, Schonemann L, et al. Reconstitution of mammalian cleavage factor II involved in 3ʹ processing of mRNA precursors. RNA. 2018;24:1721–1737.
- Deng Y, Shi J, Ran Y, et al. A potential mechanism underlying U1 snRNP inhibition of the cleavage step of mRNA 3ʹ processing. Biochem Biophys Res Commun. 2020;530:196–202.
- Langemeier J, Schrom EM, Rabner A, et al. A complex immunodeficiency is based on U1 snRNP-mediated poly(A) site suppression. Embo J. 2012;31:4035–4044.
- Weichs an der Glon C, Monks J, NJ P. Occlusion of the HIV poly(A) site. Genes Dev. 1991;5:244–253.
- Furger A, Monks J, Proudfoot NJ. The retroviruses human immunodeficiency virus type 1 and Moloney murine leukemia virus adopt radically different strategies to regulate promoter-proximal polyadenylation. J Virol. 2001;75:11735–11746.
- Vagner S, Ruegsegger U, Gunderson SI, et al. Position-dependent inhibition of the cleavage step of pre-mRNA 3ʹ-end processing by U1 snRNP. RNA. 2000;6:178–188.
- Cs Mk L, Schek N, O’Connor JP, et al. Interaction between the U1 snRNP-A protein and the 160-kD subunit of cleavage-polyadenylation specificity factor increases polyadenylation efficiency in vitro. Genes Dev. 1996;10(3):325–337.
- Awasthi S, Alwine JC. Association of polyadenylation cleavage factor I with U1 snRNP. RNA. 2003;9:1400–1409.
- Sun Y, Hamilton K, Tong L. Recent molecular insights into canonical pre-mRNA 3ʹ-end processing. Transcription. 2020;11:83–96.
- Gunderson SI, Polycarpou-Schwarz M, Mattaj IW. U1 snRNP inhibits pre-mRNA polyadenylation through a direct interaction between U1 70K and poly(A) polymerase. Mol Cell. 1998;1:255–264.
- Chiu AC, Suzuki HI, Wu X, et al. Transcriptional pause sites delineate stable nucleosome-associated premature polyadenylation suppressed by U1 snRNP. Mol Cell. 2018;69:648–63 e7.
- Abad X, Vera M, Jung SP, et al. Requirements for gene silencing mediated by U1 snRNA binding to a target sequence. Nucleic Acids Res. 2008;36:2338–2352.
- Goraczniak R, Behlke MA, Gunderson SI. Gene silencing by synthetic U1 adaptors. Nat Biotechnol. 2009;27:257–263.
- Oh JM, Venters CC, Di C, et al. U1 snRNP regulates cancer cell migration and invasion in vitro. Nat Commun. 2020;11:1.
- Shi J, Deng Y, Huang S, et al. Suboptimal RNA-RNA interaction limits U1 snRNP inhibition of canonical mRNA 3ʹ processing. RNA Biol. 2019;16:1448–1460.
- Masuda A, Kawachi T, Takeda JI, et al. tRIP-seq reveals repression of premature polyadenylation by co-transcriptional FUS-U1 snRNP assembly. EMBO Rep. 2020;21:e49890.
- Kainov YA, Makeyev EV. A transcriptome-wide antitermination mechanism sustaining identity of embryonic stem cells. Nat Commun. 2020;11:361.
- Zhang H, Lee JY, Tian B. Biased alternative polyadenylation in human tissues. Genome Biol. 2005;6:R100.
- So BR, Di C, Cai Z, et al. A complex of U1 snRNP with cleavage and polyadenylation factors controls telescripting, regulating mRNA transcription in human cells. Mol Cell. 2019;76:590–9 e4.
- Tan AY, Manley JL. The TET family of proteins: functions and roles in disease. J Mol Cell Biol. 2009;1:82–92.
- Schwartz JC, Cech TR, Parker RR. Biochemical properties and biological functions of FET proteins. Annu Rev Biochem. 2015;84:355–379.
- Yamazaki T, Chen S, Yu Y, et al. FUS-SMN protein interactions link the motor neuron diseases ALS and SMA. Cell Rep. 2012;2:799–806.
- Kwon I, Kato M, Xiang S, et al. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell. 2013;155:1049–1060.
- Sun S, Ling SC, Qiu J, Albuquerque CP, Zhou Y, Tokunaga S, et al. ALS-causative mutations in FUS/TLS confer gain and loss of function by altered association with SMN and U1-snRNP. Nat Commun. 2015;6:6171.
- Reber S, Stettler J, Filosa G, et al. Minor intron splicing is regulated by FUS and affected by ALS-associated FUS mutants. Embo J. 2016;35:1504–1521.
- Murthy AC, Dignon GL, Kan Y, et al. Molecular interactions underlying liquid-liquid phase separation of the FUS low-complexity domain. Nat Struct Mol Biol. 2019;26:637–648.
- Schmid M, Jensen TH. The nuclear RNA exosome and its cofactors. Adv Exp Med Biol. 2019;1203:113–132.
- Dantonel JC, Murthy KG, Manley JL, et al. Transcription factor TFIID recruits factor CPSF for formation of 3ʹ end of mRNA. Nature. 1997;389:399–402.
- Hirose Y, Manley JL. RNA polymerase II and the integration of nuclear events. Genes Dev. 2000;14:1415–1429.
- Bentley DL. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr Opin Cell Biol. 2005;17:251–256.
- Glover-Cutter K, Kim S, Espinosa J, et al. RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes. Nat Struct Mol Biol. 2008;15:71–78.
- Mayer A, Lidschreiber M, Siebert M, et al. Uniform transitions of the general RNA polymerase II transcription complex. Nat Struct Mol Biol. 2010;17:1272–1278.
- Lunde BM, Reichow SL, Kim M, et al. Cooperative interaction of transcription termination factors with the RNA polymerase II C-terminal domain. Nat Struct Mol Biol. 2010;17:1195–1201.
- Misra A, Ou J, Zhu LJ, et al. Global Promotion of Alternative Internal Exon Usage by mRNA 3ʹ End Formation Factors. Mol Cell. 2015;58:819–831.
- Schwartz JC, Ebmeier CC, Podell ER, et al. FUS binds the CTD of RNA polymerase II and regulates its phosphorylation at Ser2. Genes Dev. 2012;26:2690–2695.
- Davidson L, Muniz L, West S. 3ʹ end formation of pre-mRNA and phosphorylation of Ser2 on the RNA polymerase II CTD are reciprocally coupled in human cells. Genes Dev. 2014;28:342–356.
- Gu B, Eick D, Bensaude O. CTD serine-2 plays a critical role in splicing and termination factor recruitment to RNA polymerase II in vivo. Nucleic Acids Res. 2013;41:1591–1603.
- Tian B, Manley JL. Alternative polyadenylation of mRNA precursors. Nat Rev Mol Cell Biol. 2017;18:18–30.
- Wang R, Zheng D, Yehia G, et al. A compendium of conserved cleavage and polyadenylation events in mammalian genes. Genome Res. 2018;28:1427–1441.
- Sanfilippo P, Wen J, Lai EC. Landscape and evolution of tissue-specific alternative polyadenylation across Drosophila species. Genome Biol. 2017;18:229.
