ABSTRACT
Epstein-Barr virus (EBV) was the first human cancer-causing virus to be discovered over fifty years ago. Given its relatively large genome size for a virus and hence the capacity to store more than mere protein-coding information, EBV also harbours genetic material for producing an array of distinct noncoding (nc)RNAs. The double-stranded nature of its DNA genome allows the utilization of the whole gamut of ncRNA types, including both RNA polymerase II and III transcripts, in devising a sophisticated strategy to ensure its replication upon infection in host cells and evasion of host immune responses. Owing to the development of sensitive technologies in recent years, mostly entailing next-generation sequencing, the list of ncRNA types generated by EBV has expanded now to include two RNAs (EBER1 and EBER2) best categorized as long ncRNAs, dozens of microRNAs, one small nucleolar RNA, stable intronic sequence RNAs, and the most recently discovered circular RNAs. With the application of cutting-edge technology, the molecular mechanisms of some of these noncoding transcripts are beginning to emerge, while others remain yet to be elucidated. As viruses often take advantage of existing molecular pathways established by the host, it is likely that further novel concepts of the greatly unexplored noncoding world can be learned from studying the many EBV ncRNAs.
Epstein-Barr virus (EBV) is a human lymphotropic gamma1-herpesvirus, which was first detected in electron microscopy studies by Michael Epstein and Yvonne Barr in collaboration with Denis Burkitt, who provided patient samples of what is now referred to as Burkitt’s lymphoma [Citation1]. While EBV is widely known to the public as the causative agent for infectious mononucleosis or the ‘kissing disease’, it is also a major global cause for cancer, causing nasopharyngeal carcinoma, Burkitt’s lymphoma and other non-Hodgkin’s lymphomas as well as contributing to a significant fraction of Hodgkin’s lymphomas and gastric cancers [Citation2]. The genome of EBV consists of ~172 kb of double-stranded DNA, which in virions is linear and flanked on both ends by a variable number of so-called terminal repeat (TR) units and circularizes upon infection to reside in the host nucleus as an episome [Citation3,Citation4]. Infection with EBV is mainly asymptomatic in healthy individuals, as the virus persists in a stage of latency, in which only a minimal set of viral genes (up to nine of its over 85 open reading frames) are translated into protein. Only sporadically, EBV reactivates in response to cues that are poorly understood to enter the lytic cycle and express the vast majority of viral proteins, which are necessary for producing progeny virions. In theory, the double-stranded DNA genome could act as a template for both RNA polymerase II and III to generate a plethora of noncoding (nc)RNAs, in addition to messenger RNAs for protein production, to implement these distinct viral gene expression programmes. Indeed, EBV utilizes transcripts generated by both polymerases to benefit its own life cycle. This heterogeneous transcriptome is made possible in part by the relatively large genome size of EBV and hence a lenient capsid space constraint compared to other viruses, such as the workhorses of molecular biology (lentiviruses or adeno-associated viruses) that have a much smaller genome size of barely exceeding 10 kb, allowing the storage of more than viral structural and non-structural protein information in the genome.
Several well-characterized types of ncRNAs execute a single specific cellular function: some orchestrate fundamental cellular processes, such as translation (ribosomal (r) and transfer (t)RNAs) or splicing (small nuclear (sn)RNAs); others guide important regulatory processes, such as RNA modification (small nucleolar (sno)RNAs) or the rate of protein translation (micro (mi)RNAs) [Citation5,Citation6]. The high abundance of these classes of ncRNAs facilitated their discovery and the elucidation of their raison d’être decades ago, whereas low abundant ncRNA species entered the spotlight only recently with the advent of sensitive technology, such as next-generation sequencing. Of these, the class of long ncRNAs (lncRNAs), which have arbitrarily been defined as RNAs of more than 200 nucleotides (to distinguish them from the more abundant smaller RNAs such as tRNAs, snRNAs, or snoRNAs), has garnered great attention in the last decade due to their diverse and intricate modes of action to regulate gene expression [Citation7]. Unlike other types of ncRNAs that have one specific function, such as rRNA, tRNA, snRNA, or snoRNA, lncRNAs cannot be grouped into a single category in terms of molecular mode of action, as they associate with diverse protein interactors depending on context to execute their function.
EBV astonishingly leverages the activities of multiple types of ncRNAs to its own benefit (). The conspicuous EBV-encoded RNAs EBER1 and EBER2 were the first ncRNAs identified in this virus [Citation8]. While both RNAs are slightly shorter than 200 nucleotides (167 and 173 nts for EBER1 and EBER2, respectively), they are best categorized as lncRNAs. These transcripts, which in terms of copy number are on par with other essential cellular ncRNAs, such as snRNAs, and thus likely carry out an important function, have long resisted molecular scrutiny and only recently receded their defensive stance owing to the application of sensitive technology. In addition, EBV expresses a single snoRNA (v-snoRNA1) [Citation9], whose function is still unclear, and at least 44 functional miRNAs based on their interaction with Argonaute protein and their incorporation into the RNA-induced silencing complex [Citation10,Citation11]. The viral miRNAs are encoded in two separate genomic loci, of which the majority and the most abundantly expressed miRNAs originate from two BART (BamHI A Rightward Transcript) clusters, and only six other miRNAs expressed at much lower levels arise from the BHRF1 locus. Transcriptome-wide studies indicated that EBV miRNAs mainly target and antagonize cellular mRNAs that are involved in pathways adverse to viral propagation, such as apoptosis and cell cycle regulation [Citation10–12]. EBV miRNAs and their functions have been reviewed in detail elsewhere [Citation13] and are not further discussed here. In this review, we focus on recent advances in the study of EBER1 and EBER2. Furthermore, we briefly discuss two novel classes of EBV ncRNAs that are also produced by this versatile virus, i.e. stable intronic sequence RNAs (sisRNAs) and circular RNAs (circRNAs).
Figure 1. EBV expresses several types of ncRNAs. The gene loci for the many EBV ncRNAs are indicated within the circular viral episome, which is ~172 kb long. The location of the TR regions, the origin of replication (oriP) necessary for genome replication during latency, and the BamHI A fragment are indicated. The promoters for the LMP2A and LMP1 genes as well as the major latency promoter Cp, from which all EBNA genes are expressed, are also shown (please see Young and Rickinson [Citation87] for further details on the EBV genome organization). The ticks represent exons W1 and W2 of the BamHI W repeats, of which more than five copies are present in the genome and constitute the internal repeat region. Ebv-sisRNA-1 originates from the intron between exon W1 and W2, likely from each of the intronic regions of W repeats (please note that only three lines are shown here for simplicity). Most EBV miRNAs (>38) are expressed from two BART clusters (brackets); 6 annotated miRNAs are expressed from the BHRF1 gene, which are not indicated here. The gene locus from which the predominant circRNAs during latency (circBART/circRPMS1) originate is also indicated; other circRNA loci are not shown
![Figure 1. EBV expresses several types of ncRNAs. The gene loci for the many EBV ncRNAs are indicated within the circular viral episome, which is ~172 kb long. The location of the TR regions, the origin of replication (oriP) necessary for genome replication during latency, and the BamHI A fragment are indicated. The promoters for the LMP2A and LMP1 genes as well as the major latency promoter Cp, from which all EBNA genes are expressed, are also shown (please see Young and Rickinson [Citation87] for further details on the EBV genome organization). The ticks represent exons W1 and W2 of the BamHI W repeats, of which more than five copies are present in the genome and constitute the internal repeat region. Ebv-sisRNA-1 originates from the intron between exon W1 and W2, likely from each of the intronic regions of W repeats (please note that only three lines are shown here for simplicity). Most EBV miRNAs (>38) are expressed from two BART clusters (brackets); 6 annotated miRNAs are expressed from the BHRF1 gene, which are not indicated here. The gene locus from which the predominant circRNAs during latency (circBART/circRPMS1) originate is also indicated; other circRNA loci are not shown](/cms/asset/40b7470e-230e-4605-9ef1-1b8346df73c6/krnb_a_1875184_f0001_oc.jpg)
Stable intronic sequence RNAs
In general, intronic sequences as part of pre-mRNAs are degraded rapidly in the nucleus following splicing and debranching of intron lariat structures. However, some functional ncRNAs are embedded within introns, such as snoRNAs or miRNAs [Citation14,Citation15], and these intronic fragments, assisted by the protective action of interacting proteins, resist rapid turnover. As first reported in Xenopus oocytes and subsequently shown in other biological systems as well, the accumulation of stable introns or intron fragments is more widespread than previously appreciated [Citation16,Citation17]. Moreover, an expanding body of evidence supports the notion that sisRNAs are not mere transcriptional noise, but may represent regulatory entities in various settings [Citation18]. By applying sequence homology and secondary structure conservation analyses of the EBV genome together with related primate gamma1-herpesviruses, several putative sisRNAs have been predicted in the EBV genome [Citation19]. In particular, ebv-sisRNA-1, which originates from the BamHI W repeat region, was confirmed to be expressed in RNA-seq experiments and to accumulate in EBV-infected cells to high copy numbers that parallel the abundance of EBER2 (~2x105 copies) [Citation19]. Based on the original study that described sisRNAs in Xenopus oocytes and reported a copy number of ~1% compared to cognate exons [Citation16], the presence of ebv-sisRNA-1 at such high copy number is unexpected and strongly suggests an essential function during the viral life cycle. Intriguingly, the number of W repeats in EBV strains has been shown to be variable, but a minimum of five repeats is required in EBV strains to assure optimal B-cell transformation and lower numbers of W repeats correlate with diminished transforming ability [Citation20]. While the number of W repeats is mainly believed to determine the final size of the latent EBNA (EBV nuclear antigen)-LP protein, as the W repeat region contains two exons (i.e. exons W1 and W2) that encode functionally active repeat domains of EBNA-LP, the dependence of multiple W repeats may also be attributed to the requirement of a sufficient number of templates to generate ebv-sisRNA-1 above a certain threshold. In a subsequent study, the 81-nucleotide long ebv-sisRNA-1 was shown to interact in vitro with the RNA-binding proteins (RBPs) hnRNP L (heterogenous nuclear ribonucleoprotein L) and hnRNP D using streptavidin pulldown assays of biotinylated sisRNAs as well as RNA-IP experiments [Citation21]. Given the long stretch of an unstructured CA-rich region within ebv-sisRNA-1, the in vitro interaction with hnRNP L is not surprising, and further studies will need to be conducted to show that these RNA-protein interactions occur in vivo as well to contribute to the as-yet unknown function of ebv-sisRNA-1. The putative interactions with RBPs implicated in the regulation of splicing and the locus of origin may point towards a possible role for ebv-sisRNA-1 in contributing to alternative splicing events at the W repeat region. Transcription of all mRNAs encoding the six latent EBNA proteins is driven by the same promoter and traverses the W repeats to produce a primary transcript from which each mature mRNA isoform (i.e. EBNA 1, 2, 3A, 3B, 3 C, and LP) is generated by extensive alternative splicing. Here, ebv-sisRNA-1 may act in cis in concert with splicing regulators to determine the ratio of EBNA mRNA isoforms. In addition, other viral sisRNAs, such as ebv-sisRNA-2 [Citation21], albeit less abundant, may also execute vital functions to secure EBV maintenance in host cells.
Circular RNAs
For long, there has only been anecdotal evidence for closed circular RNA molecules within the transcriptome [Citation22–25], and the few known cases were considered at first to be the product of faulty splicing events with no physiologically relevant function. The vast majority of circRNAs are generated through a process called back-splicing, where a downstream splice donor is joined to an upstream splice acceptor site. Alternatively, intronic circRNAs are generated by a different mechanism in that they are remnants of intronic lariat structures resulting from canonical splicing events, which have undergone trimming but evaded debranching and carry an internal 2′-5′ phosphodiester bond. As circRNAs constitute only a minute fraction of the transcriptome (estimated to be a low single digit percentage of the cellular mRNA pool [Citation26]), conventional next-generation sequencing library preparation protocols that include only rRNA depletion and often poly(A) selection are insufficient in eliciting their detection. An additional step to eliminate linear RNAs, commonly by treating RNA samples with the RNase R exonuclease, greatly enriches the circRNA fraction within deep sequencing libraries and allows their detection by specialized search algorithms [Citation26,Citation27].
In recent years, it has become evident that the expression of circRNAs is widespread in metazoans as well as in yeast and plants [Citation28]. In line with the pervasiveness of circRNAs across several kingdoms of life, this relatively novel class of RNAs was also reported to be expressed by EBV [Citation29–31]. Similarly, the genomes of other gamma-herpesviruses, such as rhesus lymphocryptovirus, Kaposi’s sarcoma-associated herpesvirus (KSHV), and murine gammaherpesvirus 68 (MHV68), also retain the ability to produce circRNAs [Citation29,Citation32,Citation33]. In higher eukaryotes, intronic complementary sequences in the form of inverted Alu-repeat elements are associated with the biogenesis of circRNAs [Citation34], but the absence of such genetic elements in the EBV genome suggests another mechanism of biogenesis. During latency, the predominant circRNAs originate from the BART locus and are referred to as circBARTs or circRPMS1 [Citation29–31]; other sites, such as the LMP2, BHLF1, and EBNA loci act as additional sources of viral circRNAs, and their presence or abundance is cell line and latency type-dependent [Citation29,Citation30]. Some cellular circRNAs, lacking a m7G-cap and hence eIF4F-mediated translation initiation, have been shown to act as templates for non-canonical translation through the action of internal ribosome entry sites [Citation35,Citation36]. Thus far, there is no evidence that the EBV latent circRNAs participate in protein synthesis, based on the lack of ribosome association in polysome fractionation experiments [Citation29] or by analysing existing ribosome profiling data [Citation30]. Other cellular circRNAs have been reported to act as sponges for miRNAs [Citation28], and overexpression studies of circRPMS1 indicated that miRNA sequestration may be achieved under conditions of artificially high levels [Citation31]. However, it should be noted that non-physiologically high levels may be prone to in vitro artefacts, and it remains questionable as to whether the usually low copy numbers of endogenous circRNAs may amount to sufficient levels to act as an adequate sink for cognate miRNAs.
The sensitivity of next-generation sequencing greatly facilitated the detection of the low-abundant circRNAs in various systems, but the elucidation of function is lagging behind for most circRNAs. Functional characterization is complicated by the fact that circRNAs share an identical sequence with their linear parental mRNA. Thus, depletion/knockdown studies are limited in scope, as only the narrow backsplice junction site is available for specific targeting, which may not be amenable to RNA interference or antisense oligonucleotide (ASO)-entailing degradation approaches. An alternative depletion strategy would be to target factors that have been implicated in circRNA biogenesis, such as NF90/NF110 or QKI [Citation37,Citation38], and examine whether the circRNA of interest is affected while other circRNAs remain greatly untouched. Overexpression studies are also feasible with the utilization of vectors containing inverted repeat sequences that promote circRNA generation [Citation39]; however, as alluded above, such approach is prone to producing non-physiological levels of circRNAs and hence artifactual phenotypes. Moreover, if a circRNA of interest requires nearby genomic elements for functionality, which would not be included in the overexpression vector, or acts in cis at the endogenous locus, ectopic expression studies would likely not uncover their function. Regardless of the difficulties associated with deciphering circRNA function, their resistance to exonucleases and hence extraordinary stability positions them well as suitable candidates for cancer biomarkers [Citation40]. In proof-of-principle experiments, EBV circRNAs have indeed been detected in patient samples [Citation29,Citation30], and thus represent a viable alternative for the detection of EBV infection in disease aetiology. While EBERs are routinely designated for detection in disease tissue in EBV pathology, which relies on in situ hybridization methods, targeting circRNAs instead may prove advantageous if tissue degradation is an issue.
EBER1 and EBER2
Both EBERs are RNA polymerase III transcripts of similar length and localize exclusively to the nucleus [Citation41–43], suggesting that their functions must be nucleus-bound. Even though their length does not meet the arbitrary cut-off of 200 nts, they are best classified as lncRNAs. A remarkable feature of EBERs is their high copy number in infected cells (~106 for EBER1 and ~2.5 x 105 for EBER2), which puts them in the same ball park as other essential cellular ncRNAs such as snRNAs and allows the assumption that they execute an instrumental function in viral maintenance. A possible reason for the high copy number of EBERs could be that they have to compete with other host RNAs for binding with cellular RBPs and the abundance of EBERs would shift the kinetics in favour of their association with the limited pool of RBPs. Given their abundance, it is not surprising that EBERs were the first EBV ncRNAs to be discovered almost forty years ago [Citation8] and have been serving widely as a diagnostic tool for EBV infection in the clinic [Citation44]. Even though it appears logical that a high copy number would facilitate the uncovering of molecular mechanisms, EBERs have long been refractory to mechanistic studies, in particular EBER1 due to its inaccessibility to ASO-entailing techniques [Citation45]. Genetic depletion studies performed with EBV strains lacking the EBER locus have provided conflicting observations regarding their phenotype, as one study suggested that EBERs promote B-cell growth and transformation [Citation46], whereas other studies were not able to reproduce these findings [Citation47,Citation48]. Nonetheless, a recent genomics study clearly indicated a fundamental role for EBERs in nature unlike in a test tube setting, as both viral ncRNAs are preserved in all clinical isolates of EBV from nasopharyngeal cancer patient samples and exhibit high sequence conservation with only minor nucleotide polymorphisms [Citation19,Citation49].
An unexpected insight into EBER1 function was recently provided by studying MHV68, a gamma2-herpesvirus acting as a model system for their human counterparts. MHV68 expresses eight tRNA-miRNA encoding RNAs (TMER1-8), which are organized in the genome as a tandem array and do not share significant sequence homology with each other. Their similarities to EBERs include being RNA polymerase III transcripts, a comparable length (200–250 nts), and a high copy number in infected cells. Until recently, TMERs were also functionally enigmatic molecules, as the tRNA portion of TMERs are not aminoacylated to participate in protein translation and the TMER-derived miRNAs are only beginning to be assigned target mRNAs [Citation50–52]. Of the eight TMERs, deletion studies have shown that only TMER4 has an apparent phenotype in that strains lacking TMER4 are defective in haematogenous dissemination of MHV68-infected B cells to establish latency at peripheral sites [Citation53]. In a chance experiment, Tibbetts and co-workers examined whether EBER1 would be able to rescue the deficiency of the TMER4 mutant strain and surprisingly observed that EBER1 indeed restored wildtype levels of haematogenous dissemination [Citation54], suggesting that EBER1 and TMER4 are functional analogues. This finding is even more amazing in light of the fact that EBERs do not have any homologues outside of closely related primate gamma1-herpesviruses, not even in the human gamma2-herpesvirus KSHV. While the exact molecular mode of action of TMER4, and thus likely of EBER1, remains to be elucidated, the haematogenous dissemination phenotype allows for a readout in future functional studies to test in vivo relevance of in vitro observations.
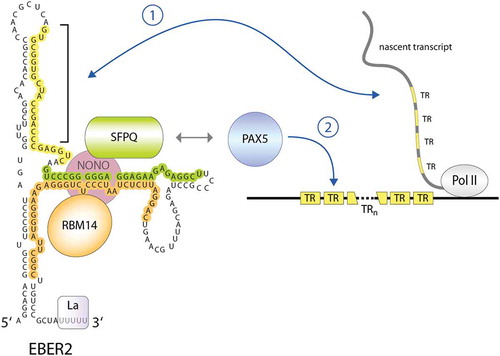
EBER2 acts as a guide RNA to recruit PAX5 to viral target sites
EBER2, unlike EBER1, is amenable to ASO-entailing techniques, as two stretches are accessible for hybridization. Given its nuclear localization, EBER2 was subjected to Capture Hybridization Analysis of RNA Targets (CHART), a method employing ASO selection and comparable to chromatin immunoprecipitation but aimed at identifying RNA binding sites on the genome [Citation55]. Indeed, EBER2 was found to localize to the viral genome, in particular to the TRs [Citation56], which are direct tandem repeat units of approximately 550 base pairs that flank both ends of the linear genome found in virions and are the site of circularization to form the viral episome after its entry into the host cell [Citation4]. The EBER2 binding sites coincide with previously identified binding sites of the B-cell master regulator and transcription factor (TF) PAX5 (Paired box 5) [Citation57,Citation58]. In agreement with chromatin co-localization of EBER2 and PAX5, both factors were shown to interact with each other but indirectly through the bridging factors SFPQ (Splicing factor proline and glutamine rich), NONO (Non-POU domain-containing octamer-binding protein), and RBM14 (RNA binding motif protein 14) [Citation59]; SPFQ and NONO interact with PAX5, while SFPQ and RBM14 bind to separate regions of EBER2 (). The recruitment of this RNP to the TR regions is largely dependent on EBER2 base-pairing with complementary sequences within nascent transcripts originating from the TR regions. While cognate DNA motifs of PAX5 are present in the TR regions, the RNA-RNA interactions involving EBER2 appear to adopt the function of a TF co-factor and enhance the binding affinity of PAX5 to the viral target sites [Citation60]. This recruitment strategy appears to be evolutionarily conserved in related primate lymphocryptoviruses [Citation56]. EBV may have chosen to employ a ncRNA as a TF co-factor, as TF guidance via a ncRNA requires less storage of genetic information than for a corresponding protein-coding gene. For example, a typical DNA binding domain that recognizes 5–10 bp of DNA comprises ~60 amino acids, i.e. 180 bp of DNA, while a ncRNA would require only the same number of complementary nucleotides that participate in base pairing, which would put less strain on the space constraints of the viral capsid. EBER2 was also found to engage in complementary base pairing with other cellular RNAs, but the functional relevance of these RNA-RNA interactions remains yet to be examined [Citation61]. At the TR regions, the EBER2-PAX5 complex has a suppressive role in transcription regulation, as depletion of either component of the RNP results in upregulation of nearby genes [Citation59], such as the latent membrane protein LMP2A. Moreover, depletion of the EBER2 RNP decreases the amount of EBV genomes produced during lytic replication [Citation56]. We have previously speculated that the EBER2 RNP is involved in properly organizing the viral chromatin and that the phenotypes observed upon its depletion are due to viral genome instability. This notion is supported by previous observations in other settings involving genomic repeat regions and TFs of the PAX family, where ncRNAs are also involved in chromatin organization [Citation62]. Based on its requirement for lytic replication, EBER2 may potentially be a therapeutic candidate for impeding EBV proliferation.
Figure 2. EBER2 acts as a guide RNA to recruit the cellular transcription factor PAX5 to the viral TR regions. EBER2 interacts directly with RBM14 and SPFQ (RNA footprints observed in CLIP experiments are indicated in the same colour as the proteins; unpublished results), and both proteins interact with NONO. This EBER2-RNP interacts with PAX5 through the interaction with SFPQ and NONO, and PAX5 is recruited to the TR regions of the EBV genome through RNA-RNA interactions of EBER2 with nascent transcripts originating from this region. The nucleotides of EBER2 engaging in duplex formation are highlighted in yellow
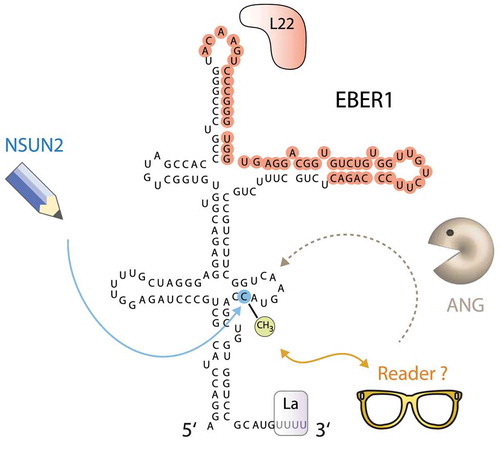
EBER1 carries an RNA modification that affects its stability
Other than the RNA chaperone La, which binds to the characteristic 3′ poly(U) stretch of all RNA polymerase III-transcripts [Citation63,Citation64], the only other known protein interactors of EBER1 are the ribosomal protein L22 and the RNA stability factor AUF1 (AU-rich element binding factor 1), also known as hnRNP D [Citation45,Citation65,Citation66]. Despite the known interacting proteins as well as its astonishing cellular abundance, the molecular mode of action of EBER1 remains elusive. Nonetheless, we recently identified that EBER1 carries an RNA modification that negatively affects its stability.
The study of RNA modifications has recently witnessed a renewed interest from various research fields, as RNA modifications were found to be more widespread and not limited to rRNAs, snRNAs, or tRNAs as previously thought, owing to the development of sensitive tools entailing next-generation sequencing [Citation67,Citation68]. Several modifications, such as N6-methyladenosine, N1-methyladenosine, 5-methylcytosine (m5C), or pseudouridine, have been mapped to mRNAs, which contribute to the regulation of diverse aspects of mRNA processing [Citation69–75]. Notably, a plethora of the writer, reader, and eraser proteins of RNA modifications are de-regulated in disease tissue, thus further adding to the clinical interest in this research area [Citation76].
Based on the high copy number of EBER1, we addressed the question of whether EBER1 may carry RNA modifications that would explain its abundance. Using a mass spectrometry approach, we found that the only modification present in EBER1 is m5C, which stoichiometrically occurs at one modification per RNA molecule [Citation77]. The RNA methyltransferase responsible for the deposition of this methyl mark was identified to be the NSUN2 enzyme of the NOP2/Sun (NSUN) family. Further mapping of the modified nucleotide by bisulphite treatment coupled to next-generation sequencing revealed that only one cytosine (C145) of EBER1 is methylated in nearly all molecules in EBV-infected cells, suggesting that this modification may play an essential part in executing the molecular function of EBER1. While m5C is a common modification in tRNAs and promotes their stability by counteracting the endonucleolytic attack by the RNase Angiogenin [Citation78], m5C modification within EBER1 has surprisingly the opposite effect by enhancing Angiogenin-mediated cleavage (). Depletion of NSUN2 or inhibition of Angiogenin elevates the already high copy number of EBER1 by approximately twofold. Our study thus showed for the first time that m5C modifications can also negatively affect RNA stability and promote degradation. Two general RBPs, ALYREF and YBX1, have been reported to be m5C-specific readers [Citation79,Citation80], but these factors do not interact with EBER1 in a m5C-dependent manner [Citation77], suggesting that other reader proteins of m5C may yet to be identified. As the consequences of m5C modification in RNAs are incompletely understood, further study of this viral ncRNA may provide novel insight into how different outcomes can be elicited depending on RNA context, such as the novel destabilizing effect of m5C. Furthermore, other viral ncRNAs may add to our knowledge of regulation through RNA modification. PAN (polyadenylated nuclear) RNA expressed by KSHV, for example, is a lncRNA, which accumulates to high copy numbers during the lytic cycle to account for ~80% of all nuclear polyadenylated RNAs in the host cell [Citation81,Citation82], and has been reported to be m6A-modified [Citation83–85]; additional ncRNAs expressed by many other viruses [Citation86] may also possibly contain a variety of RNA modifications that help execute their function and shape the host-virus relationship.
Figure 3. EBER1 contains a destabilizing 5-methylcytosine modification. The RNA methyltransferase NSUN2 deposits a single m5C modification at C145 of EBER1. While the reader protein for this methyl mark is unknown, the presence of m5C destabilizes EBER1 by enhancing its degradation by the RNase Angiogenin (ANG). The binding site of L22 as determined by CLIP assays (unpublished result) is highlighted in red; the binding site of AUF1 has not been mapped yet. The RNA chaperone La binds to the terminal 3′ poly(U) stretch
Concluding remarks
EBV, and other members of the Herpesviridae family, express a surprisingly diverse array of ncRNAs, some of which have still evaded elucidation of their function. As in recent years, the continued development of improved technology will further our understanding of the molecular mode of actions of viral ncRNAs and uncover unexpected facets of viral strategies to uphold a proliferative advantage over the host. Moreover, as successful mechanisms in molecular biology are often reused in other settings, the study of viral ncRNAs may shed light onto novel concepts and principles of cellular ncRNAs as well.
Acknowledgments
N.L is supported by the National Institutes of Health [grant number R21 AI151073].
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Epstein MA, Achong BG, Barr YM. Virus particles in cultured Lymphoblasts from Burkitt’s Lymphoma. Lancet. 1964;1:702–703.
- Young LS, Yap LF, Murray PG. Epstein-Barr virus: more than 50 years old and still providing surprises. Nat Rev Cancer. 2016;16:789–802.
- Brown NA, Liu CR, Wang YF, et al. B-cell lymphoproliferation and lymphomagenesis are associated with clonotypic intracellular terminal regions of the Epstein-Barr virus. J Virol. 1988;62:962–969.
- Lindahl T, Adams A, Bjursell G, et al. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976;102:511–530.
- Jankowsky E, Harris ME. Specificity and nonspecificity in RNA-protein interactions. Nat Rev Mol Cell Biol. 2015;16:533–544.
- Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21.
- Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157:77–94.
- Lerner MR, Andrews NC, Miller G, et al. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981;78:805–809.
- Hutzinger R, Feederle R, Mrazek J, et al. Expression and processing of a small nucleolar RNA from the Epstein-Barr virus genome. PLoS Pathog. 2009;5:e1000547.
- Riley KJ, Rabinowitz GS, Yario TA, et al. EBV and human microRNAs co-target oncogenic and apoptotic viral and human genes during latency. Embo J. 2012;31:2207–2221.
- Skalsky RL, Corcoran DL, Gottwein E, et al. The viral and cellular microRNA targetome in lymphoblastoid cell lines. PLoS Pathog. 2012;8:e1002484.
- Kang D, Skalsky RL, Cullen BR. EBV BART MicroRNAs target multiple pro-apoptotic cellular genes to promote epithelial cell survival. PLoS Pathog. 2015;11:e1004979.
- Skalsky RL, Cullen BR. EBV Noncoding RNAs. Curr Top Microbiol Immunol. 2015;391:181–217.
- Tycowski KT, Shu MD, Steitz JA. A small nucleolar RNA is processed from an intron of the human gene encoding ribosomal protein S3. Genes Dev. 1993;7:1176–1190.
- Rodriguez A, Griffiths-Jones S, Ashurst JL, et al. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910.
- Gardner EJ, Nizami ZF, Talbot CC Jr., et al. Stable intronic sequence RNA (sisRNA), a new class of noncoding RNA from the oocyte nucleus of Xenopus tropicalis. Genes Dev. 2012;26:2550–2559.
- Talhouarne GJS, Gall JG. Lariat intronic RNAs in the cytoplasm of vertebrate cells. Proc Natl Acad Sci U S A. 2018;115:E7970–E7.
- Chan SN, Pek JW. Stable intronic sequence RNAs (sisRNAs): an expanding universe. Trends Biochem Sci. 2019;44:258–272.
- Moss WN, Steitz JA. Genome-wide analyses of Epstein-Barr virus reveal conserved RNA structures and a novel stable intronic sequence RNA. BMC Genomics. 2013;14:543.
- Tierney RJ, Kao KY, Nagra JK, et al. Epstein-Barr virus BamHI W repeat number limits EBNA2/EBNA-LP coexpression in newly infected B cells and the efficiency of B-cell transformation: a rationale for the multiple W repeats in wild-type virus strains. J Virol. 2011;85:12362–12375.
- Tompkins VS, Valverde DP, Moss WN. Human regulatory proteins associate with non-coding RNAs from the EBV IR1 region. BMC Res Notes. 2018;11:139.
- Sanger HL, Klotz G, Riesner D, et al. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73:3852–3856.
- Capel B, Swain A, Nicolis S, et al. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell. 1993;73:1019–1030.
- Cocquerelle C, Daubersies P, Majerus MA, et al. Splicing with inverted order of exons occurs proximal to large introns. Embo J. 1992;11:1095–1098.
- Pasman Z, Been MD, Garcia-Blanco MA. Exon circularization in mammalian nuclear extracts. RNA. 1996;2:603–610.
- Szabo L, Salzman J. Detecting circular RNAs: bioinformatic and experimental challenges. Nat Rev Genet. 2016;17:679–692.
- Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453–461.
- Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21:475–490.
- Toptan T, Abere B, Nalesnik MA, et al. Circular DNA tumor viruses make circular RNAs. Proc Natl Acad Sci U S A. 2018;115:E8737–E45.
- Ungerleider N, Concha M, Lin Z, et al. The Epstein Barr virus circRNAome. PLoS Pathog. 2018;14:e1007206.
- Huang JT, Chen JN, Gong LP, et al. Identification of virus-encoded circular RNA. Virology. 2019;529:144–151.
- Ungerleider NA, Jain V, Wang Y, et al. Comparative analysis of gammaherpesvirus circular RNA repertoires: conserved and unique viral circular RNAs. J Virol. 2019;93:93.
- Tagawa T, Gao S, Koparde VN, et al. Discovery of Kaposi’s sarcoma herpesvirus-encoded circular RNAs and a human antiviral circular RNA. Proc Natl Acad Sci U S A. 2018;115:12805–12810.
- Jeck WR, Sorrentino JA, Wang K, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157.
- Legnini I, Di Timoteo G, Rossi F, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66:22–37 e9.
- Pamudurti NR, Bartok O, Jens M, et al. Translation of CircRNAs. Mol Cell. 2017;66:9–21 e7.
- Li X, Liu CX, Xue W, et al. Coordinated circRNA biogenesis and function with NF90/NF110 in viral infection. Mol Cell. 2017;67:214–27 e7.
- Conn SJ, Pillman KA, Toubia J, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134.
- Liang D, Wilusz JE. Short intronic repeat sequences facilitate circular RNA production. Genes Dev. 2014;28:2233–2247.
- Slack FJ, Chinnaiyan AM. The role of non-coding RNAs in oncology. Cell. 2019;179:1033–1055.
- Rosa MD, Gottlieb E, Lerner MR, et al. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol Cell Biol. 1981;1:785–796.
- Howe JG, Steitz JA. Localization of Epstein-Barr virus-encoded small RNAs by in situ hybridization. Proc Natl Acad Sci U S A. 1986;83:9006–9010.
- Fok V, Friend K, Steitz JA. Epstein-Barr virus noncoding RNAs are confined to the nucleus, whereas their partner, the human La protein, undergoes nucleocytoplasmic shuttling. J Cell Biol. 2006;173:319–325.
- Gulley ML, Tang W. Laboratory assays for Epstein-Barr virus-related disease. J Mol Diagn. 2008;10:279–292.
- Lee N, Pimienta G, Steitz JA. AUF1/hnRNP D is a novel protein partner of the EBER1 noncoding RNA of Epstein-Barr virus. RNA. 2012;18:2073–2082.
- Yajima M, Kanda T, Takada K. Critical role of Epstein-Barr Virus (EBV)-encoded RNA in efficient EBV-induced B-lymphocyte growth transformation. J Virol. 2005;79:4298–4307.
- Gregorovic G, Bosshard R, Karstegl CE, et al. Cellular gene expression that correlates with EBER expression in Epstein-Barr Virus-infected lymphoblastoid cell lines. J Virol. 2011;85:3535–3545.
- Swaminathan S, Tomkinson B, Kieff E. Recombinant Epstein-Barr virus with small RNA (EBER) genes deleted transforms lymphocytes and replicates in vitro. Proc Natl Acad Sci U S A. 1991;88:1546–1550.
- Xu M, Yao Y, Chen H, et al. Genome sequencing analysis identifies Epstein-Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat Genet. 2019;51:1131–1136.
- Bowden RJ, Simas JP, Davis AJ, et al. Murine gammaherpesvirus 68 encodes tRNA-like sequences which are expressed during latency. J Gen Virol. 1997;78(Pt 7):1675–1687.
- Bullard WL, Kara M, Gay LA, et al. Identification of murine gammaherpesvirus 68 miRNA-mRNA hybrids reveals miRNA target conservation among gammaherpesviruses including host translation and protein modification machinery. PLoS Pathog. 2019;15:e1007843.
- Wang Y, Feldman ER, Bullard WL, et al. Targets EWSR1 (Ewing Sarcoma Breakpoint Region 1) In vivo to promote latent infection of germinal center B cells. mBio. 2019;10.
- Feldman ER, Kara M, Oko LM, et al. A gammaherpesvirus noncoding RNA is essential for hematogenous dissemination and establishment of peripheral latency. mSphere. 2016;1:1.
- Hoffman BA, Wang Y, Feldman ER, et al. Epstein-Barr virus EBER1 and murine gammaherpesvirus TMER4 share conserved in vivo function to promote B cell egress and dissemination. Proc Natl Acad Sci U S A. 2019;116:25392–25394.
- Simon MD, Wang CI, Kharchenko PV, et al. The genomic binding sites of a noncoding RNA. Proc Natl Acad Sci U S A. 2011;108:20497–20502.
- Lee N, Moss WN, Yario TA, et al. EBV noncoding RNA binds nascent RNA to drive host PAX5 to viral DNA. Cell. 2015;160:607–618.
- Medvedovic J, Ebert A, Tagoh H, et al. Pax5: a master regulator of B cell development and leukemogenesis. Adv Immunol. 2011;111:179–206.
- Arvey A, Tempera I, Tsai K, et al. An atlas of the Epstein-Barr virus transcriptome and epigenome reveals host-virus regulatory interactions. Cell Host Microbe. 2012;12:233–245.
- Lee N, Yario TA, Gao JS, et al. EBV noncoding RNA EBER2 interacts with host RNA-binding proteins to regulate viral gene expression. Proc Natl Acad Sci U S A. 2016;113:3221–3226.
- Lee N, Steitz JA. Noncoding RNA-guided recruitment of transcription factors: A prevalent but undocumented mechanism? Bioessays. 2015;37:936–941.
- Nanni AV, Lee N. Identification of host RNAs that interact with EBV noncoding RNA EBER2. RNA Biol. 2018;15:1181–1191.
- Bulut-Karslioglu A, Perrera V, Scaranaro M, et al. A transcription factor-based mechanism for mouse heterochromatin formation. Nat Struct Mol Biol. 2012;19:1023–1030.
- Teplova M, Yuan YR, Phan AT, et al. Structural basis for recognition and sequestration of UUU(OH) 3ʹ temini of nascent RNA polymerase III transcripts by La, a rheumatic disease autoantigen. Mol Cell. 2006;21:75–85.
- Wolin SL, Cedervall T. The La protein. Annu Rev Biochem. 2002;71:375–403.
- Toczyski DP, Matera AG, Ward DC, et al. The Epstein-Barr virus (EBV) small RNA EBER1 binds and relocalizes ribosomal protein L22 in EBV-infected human B lymphocytes. Proc Natl Acad Sci U S A. 1994;91:3463–3467.
- Toczyski DP, Steitz JA. EAP, a highly conserved cellular protein associated with Epstein-Barr virus small RNAs (EBERs). Embo J. 1991;10:459–466.
- Roundtree IA, Evans ME, Pan T, et al. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200.
- Bohnsack MT, Sloan KE. Modifications in small nuclear RNAs and their roles in spliceosome assembly and function. Biol Chem. 2018;399:1265–1276.
- Squires JE, Patel HR, Nousch M, et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–5033.
- Safra M, Sas-Chen A, Nir R, et al. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017;551:251–255.
- Li X, Xiong X, Zhang M, et al. Base-resolution mapping reveals distinct m(1)A methylome in nuclear- and mitochondrial-encoded transcripts. Mol Cell. 2017;68:993–1005 e9.
- Carlile TM, Rojas-Duran MF, Zinshteyn B, et al. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature. 2014;515:143–146.
- Schwartz S, Bernstein DA, Mumbach MR, et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell. 2014;159:148–162.
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206.
- Meyer KD, Saletore Y, Zumbo P, et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3ʹ UTRs and near stop codons. Cell. 2012;149:1635–1646.
- Delaunay S, Frye M. RNA modifications regulating cell fate in cancer. Nat Cell Biol. 2019;21:552–559.
- Henry BA, Kanarek JP, Kotter A, et al. 5-methylcytosine modification of an Epstein-Barr virus noncoding RNA decreases its stability. RNA. 2020;26:1038–1048.
- Blanco S, Dietmann S, Flores JV, et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. Embo J. 2014;33:2020–2039.
- Yang X, Yang Y, Sun BF, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;27:606–625.
- Yang Y, Wang L, Han X, et al. RNA 5-methylcytosine facilitates the maternal-to-zygotic transition by preventing maternal mRNA decay. Mol Cell. 2019;75:1188–202 e11.
- Zhong W, Wang H, Herndier B, et al. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc Natl Acad Sci U S A. 1996;93:6641–6646.
- Sun R, Lin SF, Gradoville L, et al. Polyadenylylated nuclear RNA encoded by Kaposi sarcoma-associated herpesvirus. Proc Natl Acad Sci U S A. 1996;93:11883–11888.
- Tan B, Liu H, Zhang S, et al. Viral and cellular N(6)-methyladenosine and N(6),2ʹ-O-dimethyladenosine epitranscriptomes in the KSHV life cycle. Nat Microbiol. 2018;3:108–120.
- Baquero-Perez B, Antanaviciute A, Yonchev ID, et al. The Tudor SND1 protein is an m(6)A RNA reader essential for replication of Kaposi’s sarcoma-associated herpesvirus. Elife. 2019;8:8.
- Hesser CR, Karijolich J, Dominissini D, et al. N6-methyladenosine modification and the YTHDF2 reader protein play cell type specific roles in lytic viral gene expression during Kaposi’s sarcoma-associated herpesvirus infection. PLoS Pathog. 2018;14(4):e1006995.
- Tycowski KT, Guo YE, Lee N, et al. Viral noncoding RNAs: more surprises. Genes Dev. 2015;29:567–584.
- Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768.
